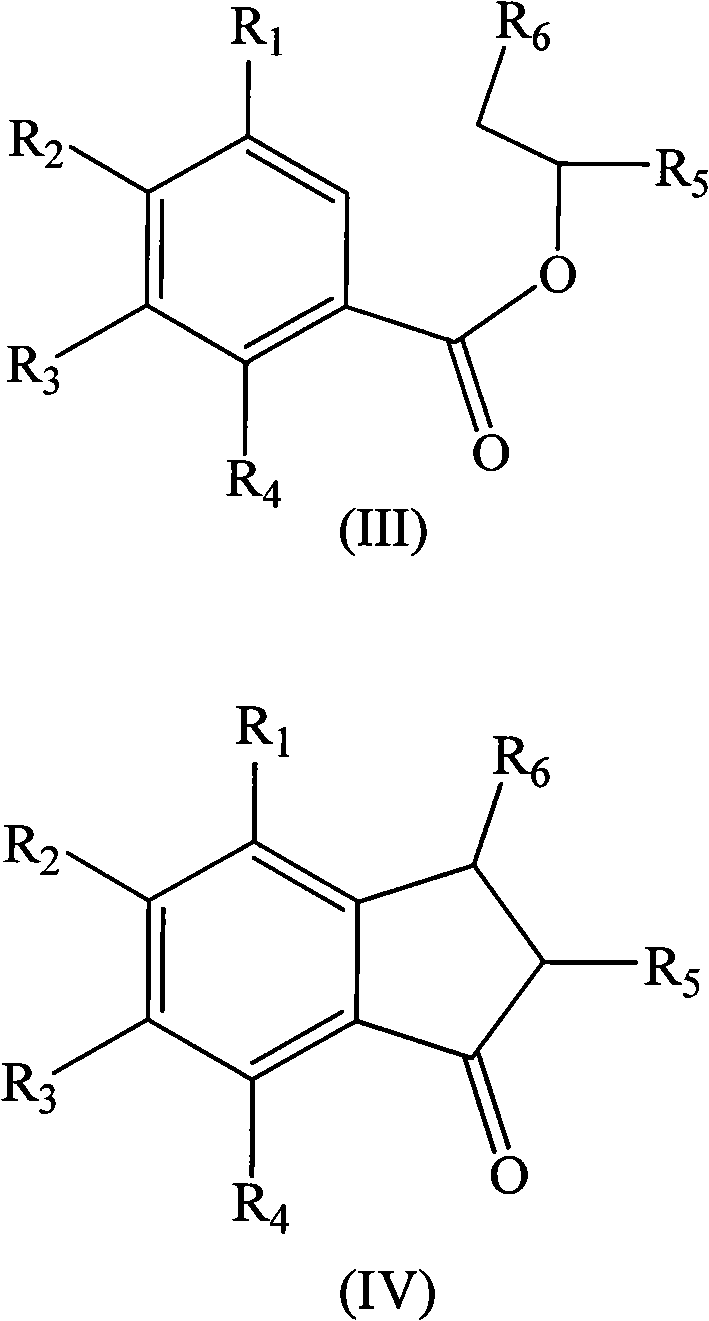
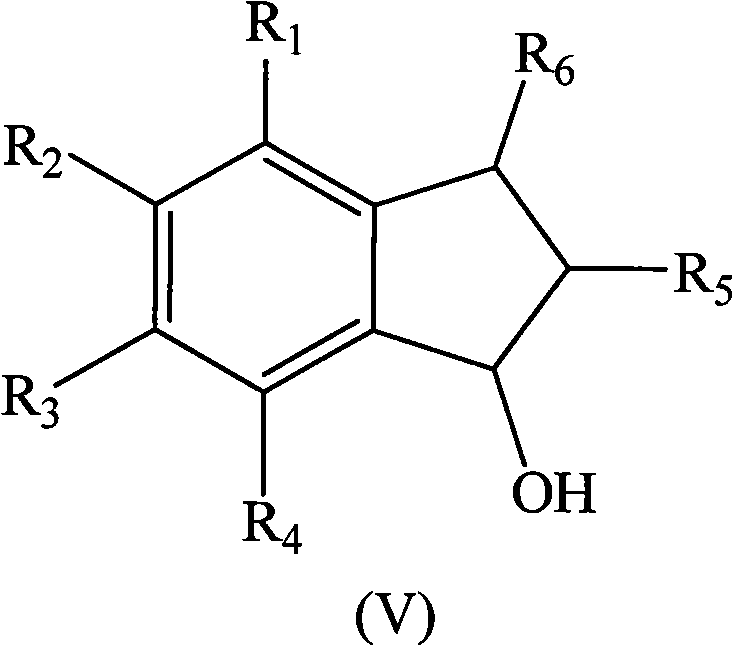
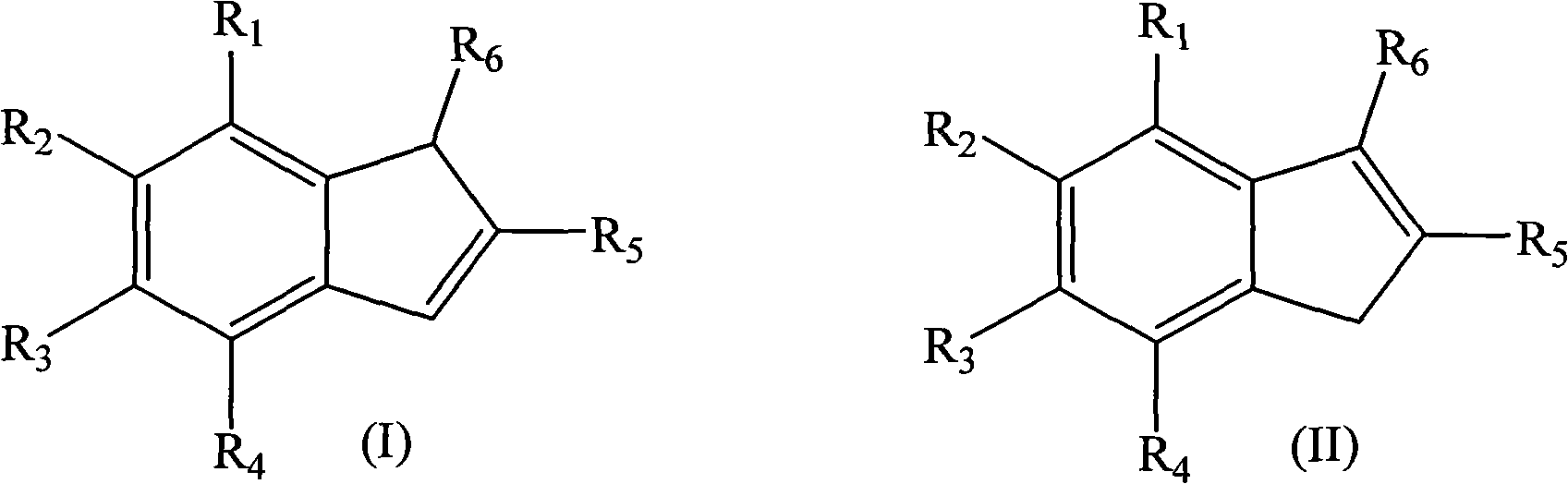
Method for preparing indene compounds
An ester compound and compound technology, applied in the field of preparation of organic compounds, can solve the problems of high cost and time-consuming, and achieve the effects of saving cost, being suitable for mass production, and simplifying the synthesis route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Synthesis of 2,3-dihydro-1-indanone:
[0048] Under the protection of nitrogen, add 85 grams of dipolyphosphoric acid to a one-liter round-bottomed flask equipped with mechanical stirring. After heating to 70 ° C, add 15 grams (0.1 mol) of ethyl benzoate and 2.7 grams of (0.02mol) a homogeneous solution made of anhydrous aluminum trichloride. After stirring for half an hour, the reaction mixture was cooled to below 20°C, and 100 grams of ice water was slowly added to the reaction system, fully stirred and cooled to 50°C-60°C At ℃, add 100 milliliters of n-heptane to extract the mixture, let stand to fully separate the layers, collect the organic layer, wash with saturated sodium bicarbonate solution until alkaline, then dry the organic layer with anhydrous sodium sulfate and remove the solvent to obtain the organic product 10.1 grams, determined by gas chromatography, wherein the 2,3-dihydro-1-indanone content reached 75%. The product was collected after vacuum distill...
Embodiment 2
[0052] The dipolyphosphoric acid in Example 1 was changed to polyphosphoric acid, and the quality and other conditions remained unchanged. After the reaction, 7.5 grams of 2,3-dihydro-1-indanone could be obtained. It can be used in the synthesis of indenoid compounds in the next step.
Embodiment 3
[0054] 85 grams of dipolyphosphoric acid in Example 1 was changed to 45 grams of trifluoromethanesulfonic acid, the reaction temperature was raised to 90°C, and other conditions were unchanged, and 5.8 grams of 2,3-dihydro-1-indanone were obtained after the reaction . It can be used in the synthesis of indenoid compounds in the next step.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com