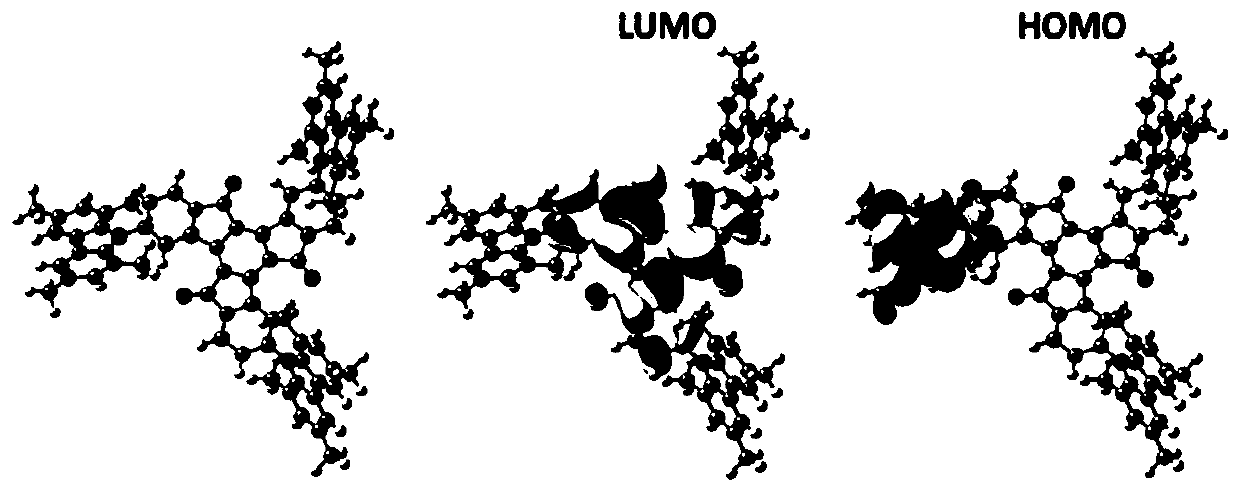
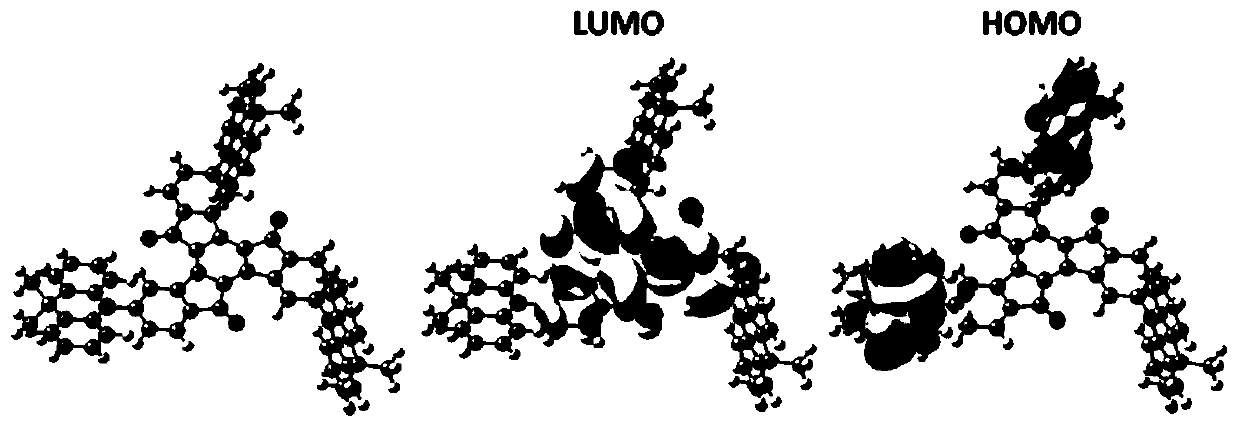
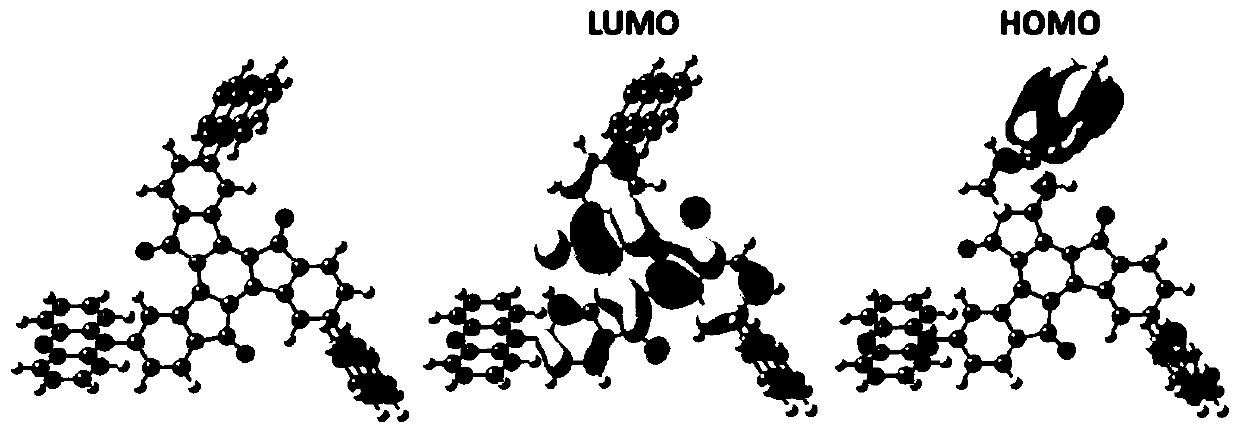
Electroluminescent materials using truxenone as electron acceptor and application of electroluminescent materials
A technology of tri-indanone and luminescent materials, which can be applied in the directions of luminescent materials, electric solid-state devices, circuits, etc., can solve the problems of high cost and limited development.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] Dissolve 5-Br-1-indanone (2.00g, 9.5mmol) in 30ml of chloroform, and slowly add liquid bromine (3.04g, 19.0mmol) dropwise under vigorous stirring, and continue stirring for 1h, then continuously introduce Nitrogen for 1 h, after the reaction, the residual solvent was removed in vacuo, and the solid crude product was recrystallized with a small amount of methanol to obtain the light yellow intermediate 2,2,5-tribromo-1-indanone N (2.91 g, 83%).
[0029] 2,2,5-Tribromo-1-indanone (3.00g, 8.1mmol) was kept in a 25ml round bottom flask at a high temperature of 220°C and continuously stirred until no gas escaped, the mixture was cooled to room temperature and dispersed in 25ml di In methyl chloride, it was filtered after being treated with supernatant for 5 minutes. The crude product was washed twice with 25 ml of dichloromethane, dried and recrystallized with nitrobenzene to obtain light yellow intermediate M (1.51 g, 30%).
[0030]
[0031] Intermediate M (2...
Embodiment 2
[0033]
[0034]Intermediate M (2.00g, 3.2mmol) was added to a three-necked flask, and then 1,3,6,9-tetramethylcarbazole (0.858g, 3.84mmol) and palladium acetate (36.2mg, 0.161mmol) were added , Potassium tert-butoxide (0.723g, 6.44mmol), tri-tert-butylphosphine tetrafluoroborate (140.2mg, 0.483mmol), and then add 30mL of toluene as a solvent, under the protection of nitrogen, heat to 110°C and react for 24h. After the reaction, the reaction solution was poured into saturated sodium chloride solution, extracted with dichloromethane (3 × 20mL) to combine the organic layers, and dried with anhydrous magnesium sulfate, the solvent was removed under reduced pressure, and the crude product was separated by column chromatography to obtain Target product (TR2) as yellow solid. Yield 59%, MALDI-TOF-MS: 1048.2994 [M + ].
Embodiment 3
[0036]
[0037] The obtained intermediate M (2.00g, 3.2mmol) was added to a three-necked flask, and then 3,6-di-tert-butylcarbazole (1.073g, 3.84mmol), palladium acetate (36.2mg, 0.161mmol) were added, Potassium tert-butoxide (0.723g, 6.44mmol), tri-tert-butylphosphine tetrafluoroborate (140.2mg, 0.483mmol), and 30mL of toluene were added as a solvent, heated to 110°C under nitrogen protection, and reacted for 24h. After the reaction, the reaction solution was poured into saturated sodium chloride solution, extracted with dichloromethane (3×15mL) and dried with anhydrous magnesium sulfate, the solvent was removed under reduced pressure, and the crude product was separated by column chromatography to obtain the target product as a yellow solid (TR3). Yield 64%, MALDI-TOF-MS: 1216.6226 [M + ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com