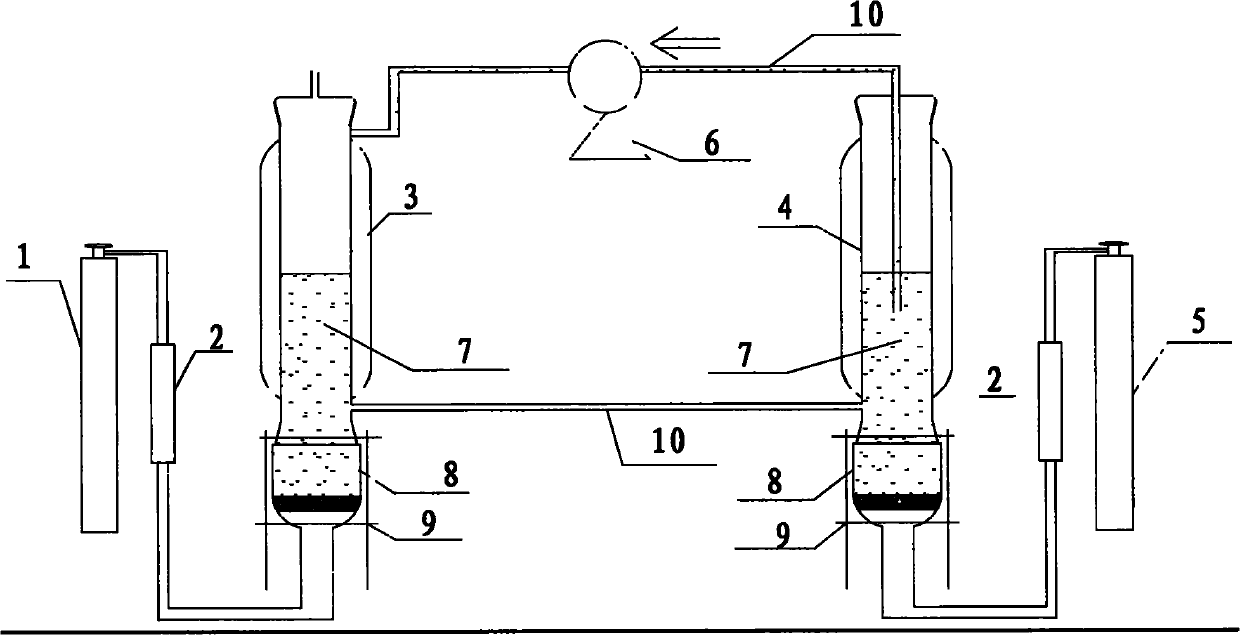
Method for non-aqueous phase wet oxidation of hydrogen sulfide
A wet oxidation and hydrogen sulfide technology, applied in the preparation/purification of sulfur, can solve the problems of harsh operating conditions, complex process, complicated process operation, etc., and achieve the effect of rapid and efficient oxidation removal without secondary pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0026] Embodiment: 2: 1 iron-based ionic liquid oxidation hydrogen sulfide
[0027] First, ferric trichloride hexahydrate and 1-methyl-3-butyl imidazole chloride with a molar ratio of 2:1 were fully stirred and mixed overnight in the air, and the water phase was removed by centrifugation to obtain a hydrophobic oil phase, which was Iron-based ionic liquid.
[0028] Inject a total of 450ml of iron-based ionic liquid into the oxidation reactor and regeneration reactor, keep 200ml of iron-based ionic liquid in the oxidation reactor, turn on the peristaltic pump to circulate the ionic liquid in the oxidation reactor and regeneration reactor, and adjust the flow rate The flow rate that makes liquid flow is 21.3ml / min, regulates reaction temperature through the water bath wall and is 50 ℃, with the flow rate of 40ml / min the hydrogen sulfide standard gas that concentration is 1.01% is passed in the desulfurizer, simultaneously with the flow rate of 50ml / min The flow rate is to feed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com