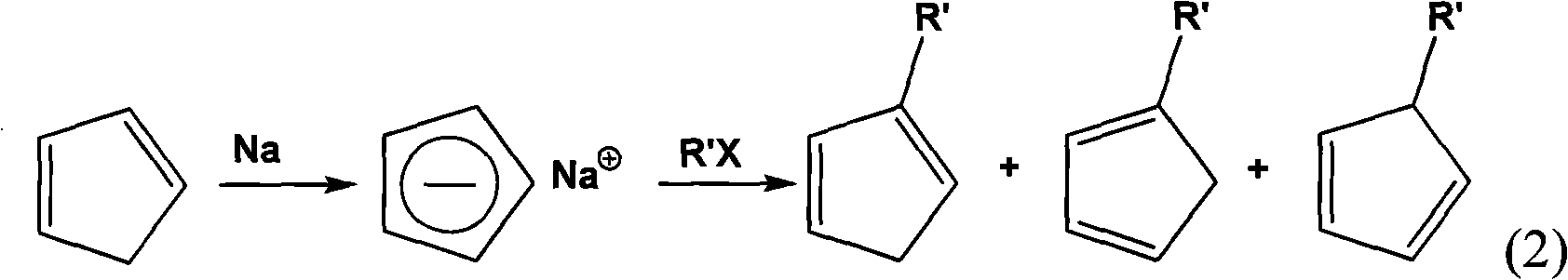
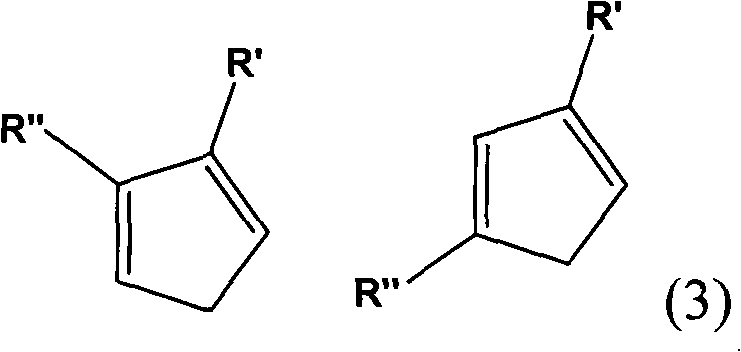
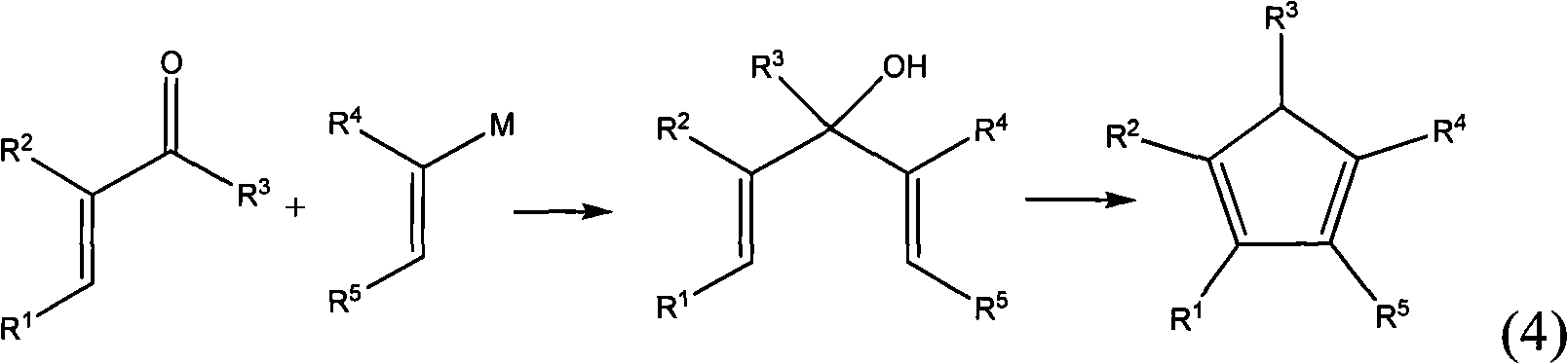
Method for preparing multi-substituted cyclopentadiene and substituted indene
A cyclopentadiene and multi-substituted technology is applied in the field of synthesis of multi-substituted cyclopentadiene and substituted indene, which can solve problems such as poor selectivity of target products, and achieve the effects of increased ratio, high conversion rate and improved controllability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of 1-methyl-3-n-butylcyclopentadiene
[0038] Put the fully dried 250 ml three-necked flask into a stirring magnet, place it in an oil bath, vacuum, and flush with nitrogen, repeated three times. Add 20 ml of dry tetrahydrofuran that has been dewatered and deoxygenated, add 2.7 g of magnesium, and start stirring. A solution of n-butyl bromide in tetrahydrofuran (13.7 g of n-butyl bromide, 25 ml of tetrahydrofuran) is added dropwise, and the rate of dropping is preferably such that the reaction system slightly boils. After the dripping, the reaction system was refluxed for 1 hour in an oil bath at 80°C. The oil bath was changed to an ice water bath, the reaction system was cooled to 0°C, and 9.6 g methylcyclopentenone / 50 ml tetrahydrofuran was slowly added dropwise. After the addition is complete, stir at room temperature overnight. 3.2 g of anhydrous methanol was slowly added dropwise at room temperature. After the addition is complete, change the...
Embodiment 2
[0039] Example 2 Preparation of 1-methyl-3-n-butylcyclopentadiene
[0040] The implementation process is the same as in Example 1. The difference is that the reagent containing active hydrogen atoms is 4.6 grams of absolute ethanol.
Embodiment 3
[0041] Example 3 Preparation of 1,2,3,4,5-pentamethylcyclopentadiene
[0042] The fully dried 250 ml three-necked flask is placed in a stirring magnet, placed in an ice bath, vacuumed and flushed with nitrogen, repeated three times. Add 23 mL of Grignard reagent (methylmagnesium chloride, 22% tetrahydrofuran solution) and start stirring. 9.4 g of tetramethylcyclopentenone / 50 ml of tetrahydrofuran was slowly added dropwise. After the addition is complete, stir at room temperature overnight. 1.2 g of deionized water was slowly added dropwise at room temperature. After the addition is complete, change the ice water bath to an oil bath, reflux for 1.5 hours, cool, treat with dilute hydrochloric acid, separate the oil phase, and extract the water phase twice with 10 ml of ether. Combine the organic phases. Dry with anhydrous sodium sulfate, filter, and remove the solvent with a rotary evaporator.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com