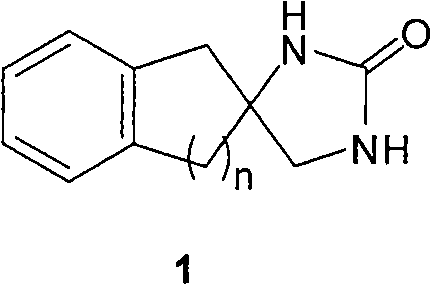
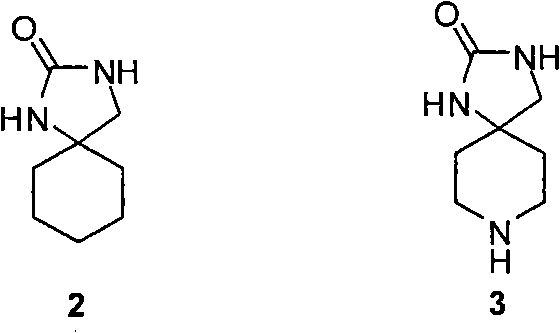Synthesizing method of aromatic ring ureas loop-coil medicament template
A synthesis method and an aromatic ring technology, applied in the direction of organic chemistry, etc., can solve the problems of unsuitable large-scale derivative synthesis, long reaction steps, low efficiency, etc., and achieve the effects of few reaction steps, mild conditions and simple process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]
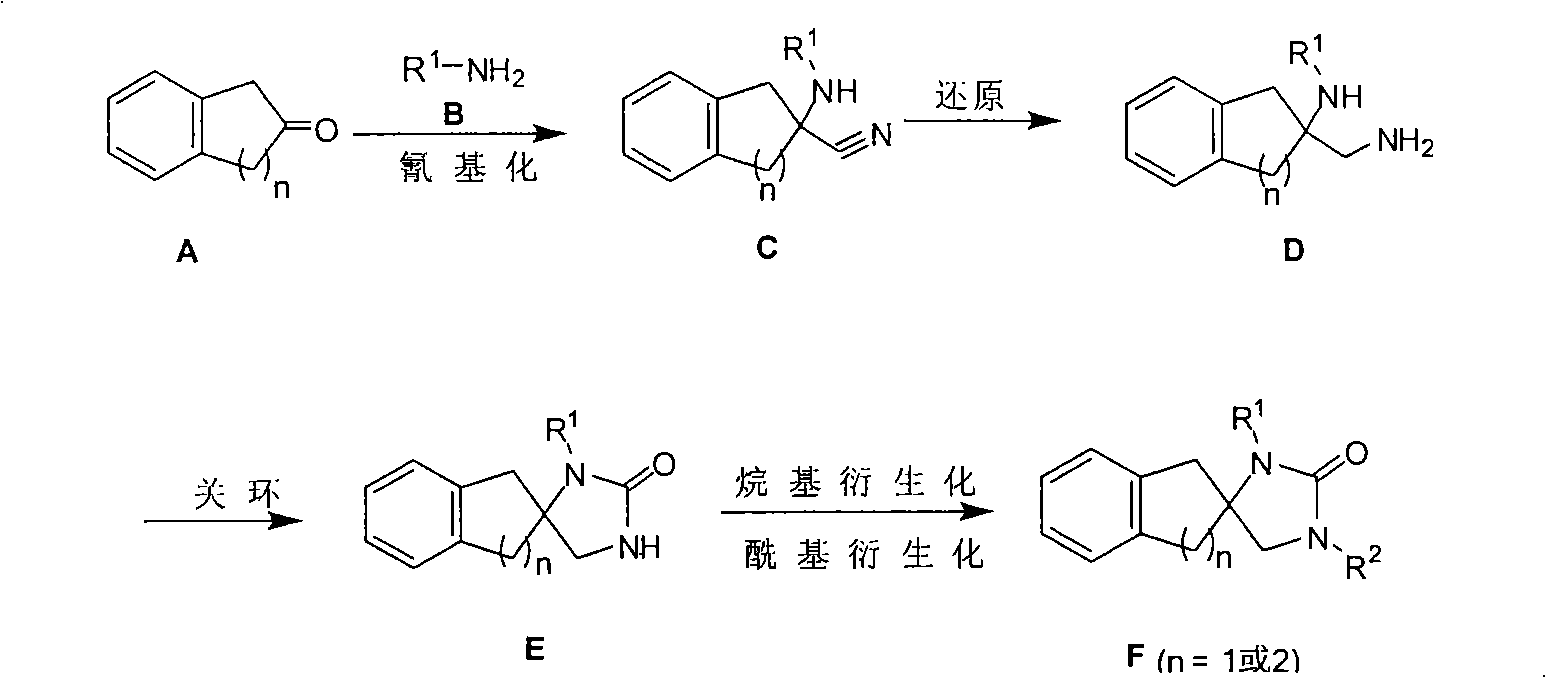
[0044] 1. Synthesis of 2-(propylamine)-2,3-dihydro-1H-indene-2-carbonitrile
[0045] 2-Indanone (4 g, 30.3 mmol) and propylamine hydrochloride (3.1 g, 32.6 mmol) were dissolved in water (60 mL) and stirred at room temperature for 60 minutes; then sodium cyanide (1.62 g, 33.1 mmol) was added, and continued Stir at room temperature for 15 hours. The reaction system was extracted with dichloromethane (120 mL), washed with saturated brine (3×100 mL), and the organic phase was washed with Na 2 SO 4 Dry and remove the solvent to obtain 5.6 g of crude 2-(propylamine)-2,3-dihydro-1H-indene-2-carbonitrile, MS(m / z): 201(M+1); directly applied to without purification Next step.
[0046] 2. Synthesis of 2-(aminomethyl)-N-propyl-2,3-dihydro-1H-indene-2-amine
[0047] 5.6 g (purity 30%, 12.6 mmol) of the crude 2-(propylamine)-2,3-dihydro-1H-indene-2-carbonitrile obtained in step 1 was dissolved in 150 mL of anhydrous ether, and cooled in an ice bath to Below 5 degrees, add ...
Embodiment 2
[0052]
[0053] 1. Synthesis of 2-(benzylamine)-2,3-dihydro-1H-indene-2-carbonitrile
[0054] 2-Indanone (3.58 g, 27 mmol) and benzylamine hydrochloride (4.27 g, 29.7 mmol) were dissolved in water (350 mL) and stirred at room temperature for 60 minutes; then sodium cyanide (1.46 g, 29.7 mmol) was added, Stirring was continued at room temperature for 15 hours. The reaction system was extracted with dichloromethane (1000 mL), washed with saturated brine (3×100 mL), and the organic phase was washed with Na 2 SO 4 Dry and remove the solvent to obtain 7g crude product of 2-(benzylamine)-2,3-dihydro-1H-indene-2-carbonitrile, MS (m / z): 249 (M+1); directly applied to without purification Next step.
[0055] 2. Synthesis of 2-(aminomethyl)-N-benzyl-2,3-dihydro-1H-inden-2-amine
[0056] 7 g (purity 31%, 12.1 mmol) of the crude 2-(benzylamine)-2,3-dihydro-1H-indene-2-carbonitrile obtained in step 1 was dissolved in 200 mL of anhydrous ether, and cooled in an ice bath to Below 5 d...
Embodiment 3
[0061]
[0062] 1. Synthesis of 2-(aniline)-2,3-dihydro-1H-indene-2-carbonitrile
[0063] 2-Indanone (10 g, 0.075 mol) and aniline hydrochloride (7.67 g, 82.5 mmol) were dissolved in water (120 mL), and stirred at room temperature for 30 minutes; then sodium cyanide (4.04 g, 82.5 mmol) was added, and continued Stir at room temperature for 15 hours. The reaction system was extracted with dichloromethane (250 mL), washed with saturated brine, and the organic phase was washed with Na 2 SO 4 Dry and remove the solvent to obtain 2-(isopropylamine)-2,3-dihydro-1H-indene-2-carbonitrile crude product 14.71g, MS(m / z): 235(M+1); used directly without purification in the next step.
[0064] 2. Synthesis of 2-(aminomethyl)-N-phenyl-2,3-dihydro-1H-inden-2-amine
[0065] 14.71 g (purity 70%, 63 mmol) of the crude 2-(aniline)-2,3-dihydro-1H-indene-2-carbonitrile obtained in step 1 was dissolved in 250 mL of anhydrous ether, and cooled to 5 in an ice bath. Below the temperature, add l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com