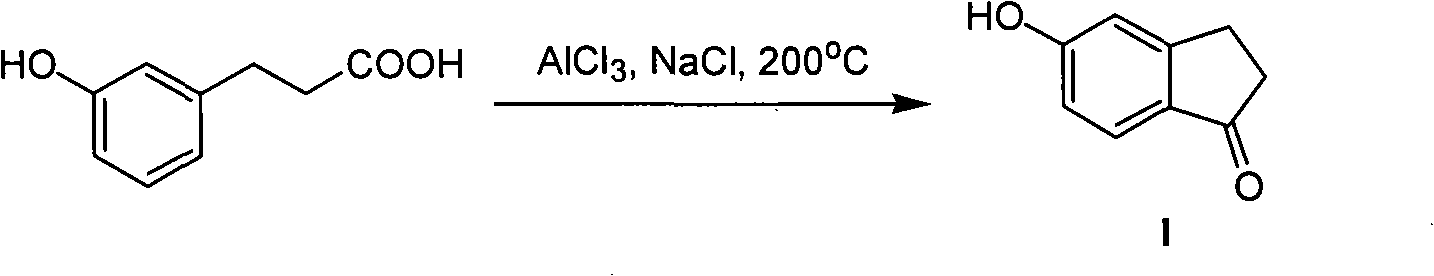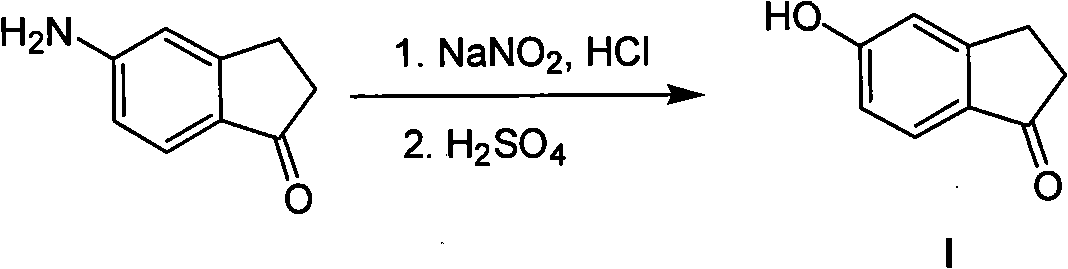Synthesis method of 5- hydroxide radical-1-indenone
A synthetic method, the technology of indanone, applied in a new synthetic field, can solve the problems of difficult post-processing, harsh reaction conditions, difficult to obtain raw materials, etc., and achieve the effects of easy operation, rapid preparation, and easy scale-up
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
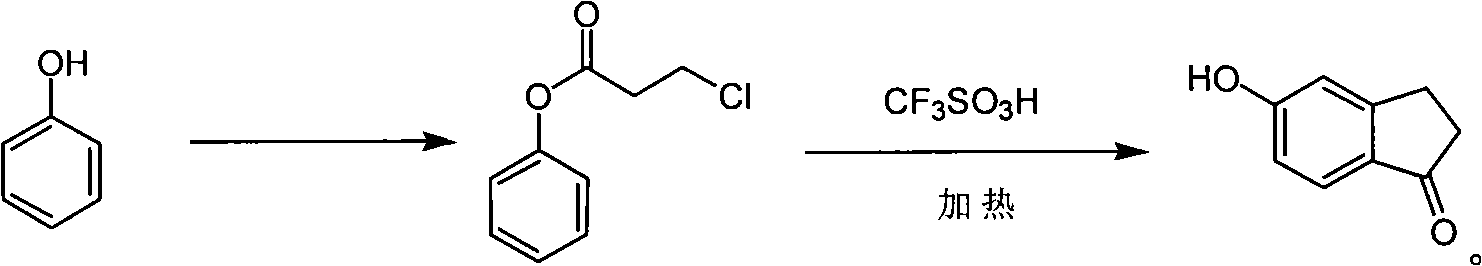
[0020] 1. Synthesis of phenyl 3-chloropropionate
[0021]
[0022] Phenol (9.4g, 100mmol) was dissolved in 3-chloropropionyl chloride (15g, 120mmol), and the reaction mixture was stirred at 100°C for 12h under nitrogen protection, and detected by TLC until the phenol reaction was complete. The reactant was cooled to room temperature, poured into 120 mL of ice water, extracted with ethyl acetate (120 mL×3), combined the organic phases, washed with 1M NaOH, water and saturated brine, dried over anhydrous sodium sulfate, filtered to remove the desiccant, and reduced Concentrate under reduced pressure to obtain phenyl 3-chloropropionate (16.6g, yield 90%) 1 H NMR (CDCl 3 ): 3.03(t, 2H), 3.85(t, 2H), 7.09-7.12(m, 2H), 7.22-7.26(m, 1H), 7.36-7.40(m, 2H).
[0023] 2. Synthesis of 5-hydroxy-1-indanone
[0024]
[0025] Phenyl 3-chloropropionate (1.85g, 10mmol) was dissolved in trifluoromethanesulfonic acid (4.42mL, 50mmol), and the reaction mixture was placed in an oil bath w...
Embodiment 2
[0028] Synthesis of 5-Hydroxy-1-indanone
[0029]
[0030] Phenyl 3-chloropropionate (1.85g, 10mmol) was dissolved in trifluoromethanesulfonic acid (8.84mL, 100mmol), and the reaction mixture was placed in an oil bath whose temperature had reached 170°C under nitrogen protection and stirred for 2h. The reactant was cooled to room temperature, poured into 20 mL of ice water, extracted with ethyl acetate (20 mL×3), combined the organic phases, washed with saturated sodium bicarbonate, water and saturated brine, dried over anhydrous sodium sulfate, and filtered to remove the desiccant. Concentration under reduced pressure gave 5-hydroxy-1-indanone (710 mg, yield 48%) HPLC (method: 0-60 / 6minute): Rt = 3.03.
Embodiment 3
[0032] Synthesis of 5-Hydroxy-1-indanone
[0033]
[0034] Phenyl 3-chloropropionate (1.85g, 10mmol) was dissolved in trifluoromethanesulfonic acid (17.68mL, 200mmol), and the reaction mixture was placed in an oil bath whose temperature had reached 170°C under nitrogen protection and stirred for 2h. The reactant was cooled to room temperature, poured into 20 mL of ice water, extracted with ethyl acetate (20 mL×3), combined the organic phases, washed with saturated sodium bicarbonate, water and saturated brine, dried over anhydrous sodium sulfate, and filtered to remove the desiccant. Concentration under reduced pressure gave 5-hydroxy-1-indanone (740 mg, yield 50%) HPLC (method: 0-60 / 6minute): Rt=3.03
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com