3-sulfonylation-indenone compound and preparation method thereof
A technology of indanone and sulfonylation is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., to achieve the effects of high yield, favorable for industrial production and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
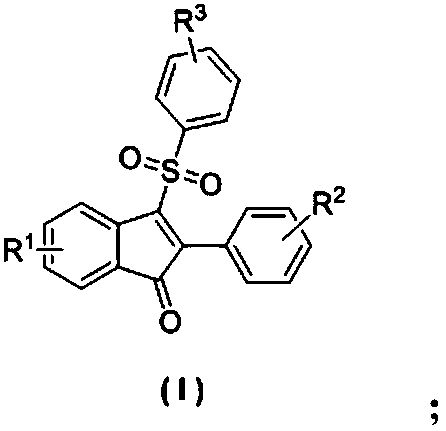
[0027] The structural formula of 3-sulfonylated-indanone compound is as follows:
[0028]
[0029] The preparation steps are as follows:
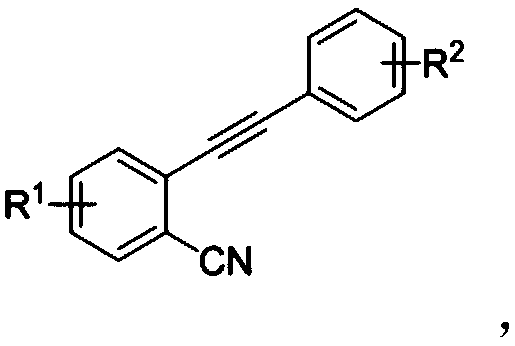
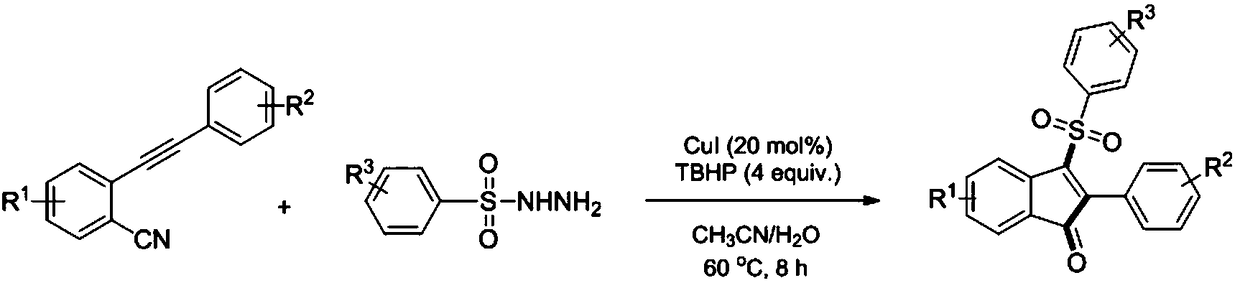
[0030] Dissolve 2-(phenylethynyl)benzonitrile (0.2mmol) and p-toluenesulfonyl hydrazide (0.4mmol) in a mixed solution of 1.5mL acetone and 0.5mL water, then add cuprous iodide (0.04 mmol) and tert-butyl hydroperoxide (0.8 mmol), reacted at 45°C for 12 hours. After the reaction, it was extracted, dried, and the solvent was evaporated under reduced pressure, and the residue was separated by silica gel column chromatography (petroleum ether:ethyl acetate=10:1) to obtain a red solid.
[0031] Yield 72%. 1 H NMR (400MHz, CDCl 3 ,δ)7.98(d,J=7.6Hz,1H),7.58(d,J=7.2Hz,1H),7.56-7.47(m,3H),7.45-7.29(m,4H),7.29-7.19(m ,2H),7.14(d,J=8.1Hz,2H),2.35(s,3H); 13 C NMR (100MHz, CDCl 3 ,δ)194.3,151.3,145.2,141.22,139.9,136.8,135.0,130.4,129.6,129.5,128.8,127.9,127.8,127.6,124.4,123.9,21.6; HRMS Calcd for C 22 h 17 o 3 S[M+H] + :m / z 361.0898,Found:...
Embodiment 2
[0033] The structural formula of 3-sulfonylated-indanone compound is as follows:
[0034]
[0035] The preparation method is as follows:
[0036] Dissolve 5-methyl-2-(phenylethynyl)benzonitrile (0.2mmol) and p-toluenesulfonyl hydrazide (0.4mmol) in a mixed solution of 1.5mL acetonitrile and 0.5mL water, then add iodine Cuprous chloride (0.04mmol) and tert-butyl hydroperoxide (0.8mmol) were reacted at 80°C for 6 hours. After the reaction was completed, it was extracted, dried, and the solvent was evaporated under reduced pressure, and the residue was separated by silica gel column chromatography (petroleum ether:ethyl acetate=10:1) to obtain a yellow solid.
[0037] Yield: 65%. 1 H NMR (400MHz, CDCl 3 ,δ)7.83(d,J=7.6Hz,1H),7.53(d,J=8.0Hz,2H),7.41-7.33(m,4H),7.29-7.23(m,3H),7.13(d,J =8.0Hz,1H),2.36(s,3H),2.34(s,3H); 13 C NMR (100MHz, CDCl 3 ,δ) 194.6, 153.1, 151.5, 145.1, 140.0, 139.3, 138.4, 136.9, 135.0, 130.4, 129.6, 129.3, 129.1, 128.0, 127.8, 127.6, 125.4, 123.7, 2...
Embodiment 3
[0039] The structural formula of 3-sulfonylated-indanone compound is as follows:
[0040]
[0041] The preparation steps are as follows:
[0042] Dissolve 2-(p-tolylethynyl)benzonitrile (0.2mmol) and p-toluenesulfonyl hydrazide (0.4mmol) in a mixed solution of 1.5mL acetonitrile and 0.5mL water, then add cuprous iodide ( 0.04mmol) and tert-butyl hydroperoxide (0.8mmol), react at 60°C for 10 hours. After the reaction, it was extracted, dried, and the solvent was evaporated under reduced pressure, and the residue was separated by silica gel column chromatography (petroleum ether:ethyl acetate=10:1) to obtain a red solid.
[0043] Yield: 76%. 1 H NMR (400MHz, CDCl 3,δ)7.93(d,J=7.6Hz,1H),7.65-7.52(m,3H),7.50-7.46(m,1H),7.29(t,J=8.0Hz,1H),7.20-7.18(m ,4H),7.15(d,J=8.4Hz,2H),2.39(s,3H),2.34(s,3H); 13 C NMR (100MHz, CDCl 3 ,δ)194.5,150.5,145.2,141.3,140.2,139.8,137.0,135.0,130.5,129.6,129.3,128.8,128.4,127.8,124.9,124.3,123.7,21.6,21.5; HRMS Calcd for C 23 h 19 o 3 S[M+H]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com