3-phenyl glutaric acid compound, preparation method and purpose thereof
A technology for phenylglutaric acid and compounds, which is applied in the application field of preparing pharmaceutical intermediates 1-indanone-3-acetic acid compounds (II), can solve high production costs, many synthesis steps, and cumbersome post-processing processes And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation of 3-(2,2-diethoxyethoxy)benzaldehyde (2a)
[0042] Add 12.21 g (0.1 mol) of 3-hydroxybenzaldehyde, 21.68 g (0.11 mol) of bromoacetal, anhydrous K 2 CO 3 16.56 g (0.12 mol) and N,N - 80 ml of dimethylformamide, heated to 120°C and stirred for 10 h, after the reaction was completed, filtered, the filtrate was evaporated to remove the solvent under reduced pressure, the residual oil was dissolved in 150 ml of chloroform, and successively washed with 10% aqueous sodium hydroxide solution 20 ml, washed with 25 ml of saturated NaCl aqueous solution, and the organic layer was washed with anhydrous NaCl 2 SO 4 Dry, filter, and evaporate the solvent under reduced pressure to obtain 22.87 g of 3-(2,2-diethoxyethoxy)benzaldehyde, with a yield of 96.0%; HR-TOFMS (+Q) m / z : 239.1280 ([C 13 h 18 o 4 +H] + Calculated: 239.1283).
Embodiment 2
[0043] Example 2 Preparation of 4-chloro-3-(2,2-diethoxyethoxy)benzaldehyde (2b)
[0044] Operation process is with embodiment 1, just 3-hydroxybenzaldehyde is replaced with 4-chloro-3-hydroxybenzaldehyde, N,N -Dimethylformamide was replaced by acetone, anhydrous K 2 CO 3 Replaced with sodium ethoxide, heated and refluxed and stirred for 20 h, followed by post-treatment in Example 1 to obtain 4-chloro-3-(2,2-diethoxyethoxy)benzaldehyde with a yield of 95.0%; HR-TOFMS ( +Q) m / z : 273.0898 ([C 13 h 17 ClO 4 +H] + Calculated: 273.0894).
Embodiment 3~15
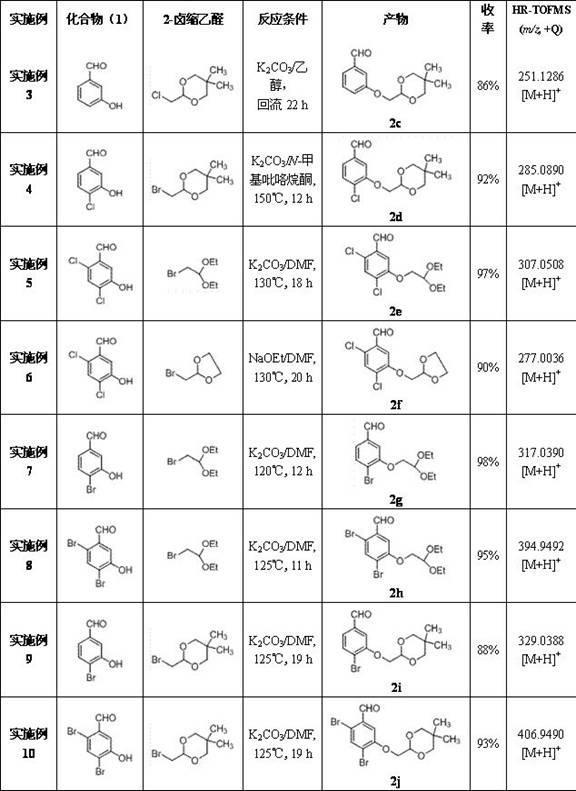
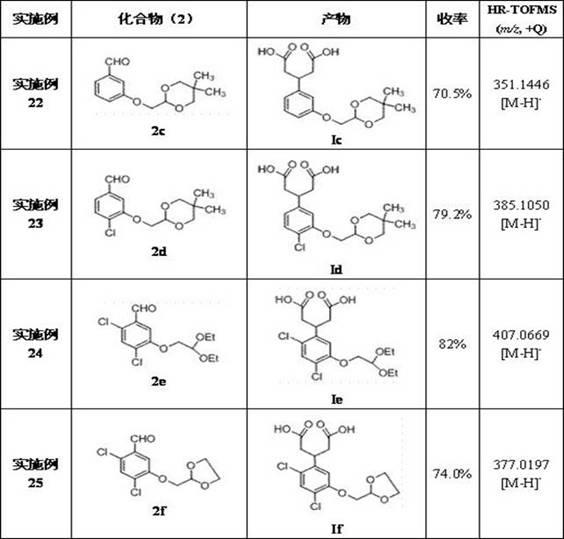
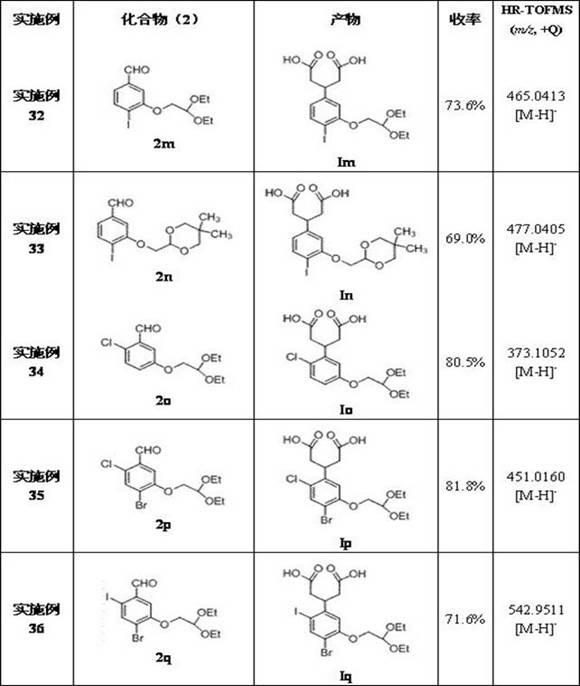
[0045] Examples 3-15 Preparation of 3-(2-oxyethoxy)benzaldehyde compounds (2c~2o)
[0046] Operation process is with embodiment 1, just 3-hydroxybenzaldehyde is used corresponding compound ( 1 ) to replace bromoacetal with the corresponding 2-haloacetal, and change the reaction conditions accordingly to obtain 3-(2-oxyethoxy)benzaldehyde compounds ( 2c~2h ), and its chemical structure is as follows:
[0047]
[0048]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com