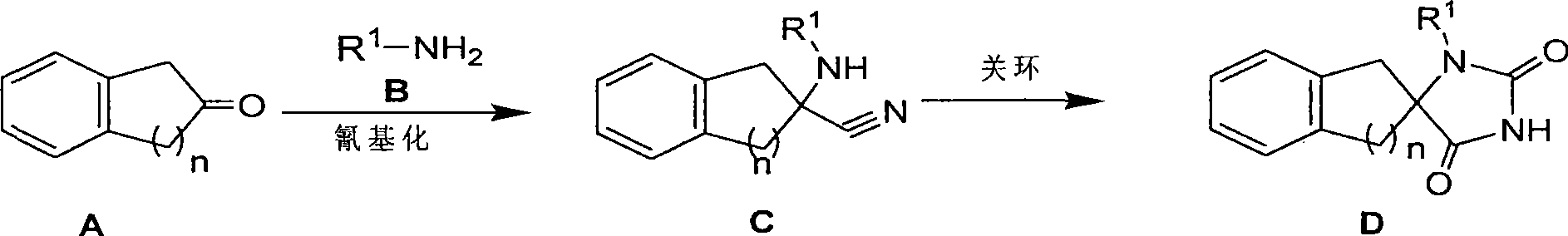
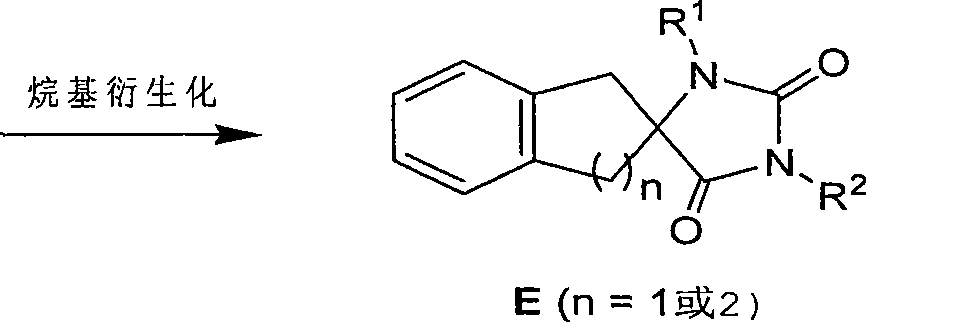
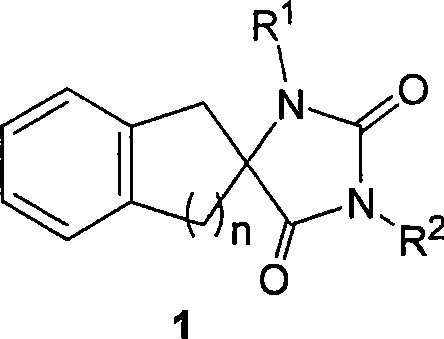
Synthetic method for aromatic ring bisamide spiro drug template
A technology for the synthesis of cyclobisamide spiro compounds, which is applied in the field of synthesis of aromatic ring bisamide spiro drug templates, can solve the problems of cumbersome methods, lack of feasibility and selectivity of aromatic ring bisamide spiro compounds, etc. Achieve the effect of simple process, few reaction steps and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
[0042] 1. Synthesis of 2-(propylamine)-2,3-dihydro-1H-indene-2-carbonitrile
[0043]Dissolve 2 indanoxine (10g, 0.076mol) and propylamine hydrochloride (8g, 0.084mol) in water (120mL) and stir at room temperature for 30 minutes; then add sodium cyanide (4.1g, 0.084mol) and continue to Stir for 15 hours. The reaction system was extracted with dichloromethane (200mL), washed with saturated brine, and the organic phase was washed with Na 2 SO 4 Drying, removal of solvent to obtain 2-(propylamine)-2,3-dihydro-1H-indene-2-carbonitrile crude product 10g, MS (m / z): 201 (M+1); without purification, directly used in the following step.
[0044] 2. Synthesis of 3-propyl-1′,3′-dihydrospiro[imidazoline-4,2′-indene]-2,5-dione
[0045] Dissolve 4 g (purity 50%, 0.01 mol) of 2-(propylamine)-2,3-dihydro-1H-indene-2-carbonitrile crude product obtained in step 1 in 200 mL of dichloromethane, and add isocyanate dropwise at room temperature Dichloromethane solution (50mL) of sulf...
Embodiment 2
[0051]
[0052] 1. Synthesis of 2-(cyclopropylamine)-2,3-dihydro-1H-indene-2-carbonitrile
[0053] 2-Indanone (10g, 0.076mol) and cyclopropylamine hydrochloride (7.8g, 0.084mol) were dissolved in water (120mL), stirred at room temperature for 30 minutes; then sodium cyanide (4.1g, 0.084mol) was added, Stirring was continued at room temperature for 15 hours. The reaction system was extracted with dichloromethane (200mL), washed with saturated brine, and the organic phase was washed with Na 2 SO 4 Drying and removal of the solvent gave 11 g of crude product 2-(cyclopropylamine)-2,3-dihydro-1H-indene-2-carbonitrile, MS (m / z): 199 (M+1); directly applied to Next step.
[0054] 2. Synthesis of 3-cyclopropyl-1′,3′-dihydrospiro[imidazoline-4,2′-indene]-2,5-dione
[0055] Dissolve 3.5 g (purity 45%, 0.008 mol) of 2-(cyclopropylamine)-2,3-dihydro-1H-indene-2-carbonitrile crude product obtained in step 1 in 150 mL of dichloromethane, drop it at room temperature Add a dichloromet...
Embodiment 3
[0058]
[0059] 1. Synthesis of 2-(benzylamine)-2,3-dihydro-1H-indene-2-carbonitrile
[0060] 2-Indanone (3g, 0.023mol) and benzylamine hydrochloride (3.6g, 0.025mol) were dissolved in water (60mL), stirred at room temperature for 30 minutes; then sodium cyanide (1.35g, 0.025mol) was added, Stirring was continued at room temperature for 15 hours. The reaction system was extracted with dichloromethane (120mL), washed with saturated brine, and the organic phase was washed with Na 2 SO 4 Drying and removal of the solvent gave 5 g of crude product 2-(benzylamine)-2,3-dihydro-1H-indene-2-carbonitrile, MS (m / z): 249 (M+1); directly applied to Next step.
[0061] 2. Synthesis of 3-benzyl-1′,3′-dihydrospiro[imidazoline-4,2′-indene]-2,5-dione
[0062] 3 g (purity 55%, 0.007 mol) of 2-(benzylamine)-2,3-dihydro-1H-indene-2-carbonitrile crude product obtained in step 1 were dissolved in 150 mL of dichloromethane, and added dropwise at room temperature Isocyanate sulfonyl chloride ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com