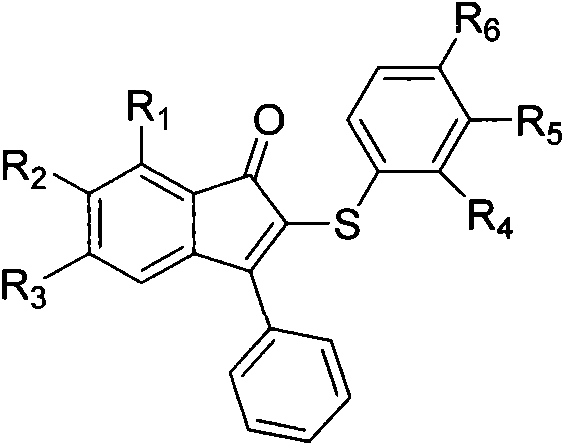
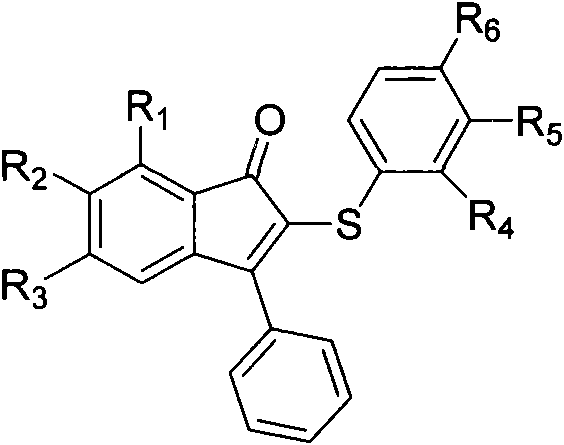
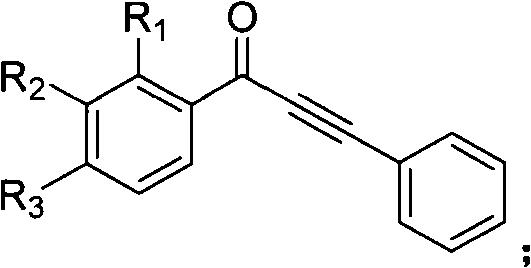
Method for synthesizing indenone-2-diphenyl sulfide derivatives
A technology for phenylene sulfide and derivatives, which is applied in the field of organic synthesis, can solve the problems of unable to synthesize indanone-2-phenylene sulfide derivatives, unable to synthesize indanone derivatives, expensive catalysts, etc., and achieves mild conditions and easy operation. Simple, Inexpensive Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment one: the present embodiment is 3-phenylindanone-2-phenylene sulfide (such as figure 1 , R 1 =R 2 =R 3 =R 4 =R 5 =R 6 =H) synthetic; With 1,3-diphenyl propargyl ketone, thiophenol as raw material, its reaction formula is as follows:
[0044]
[0045] Experimental steps:
[0046] (1) Put 0.206 g (1 mmol) of 1,3-diphenylpropynyl ketone and 0.81 g (3 mmol) manganese acetate in a round bottom flask, add 20 mL of acetonitrile, heat to reflux, then add 1.5 mL of thiophenol ( 1.5mmol), TLC tracking reaction until the end. Cool to room temperature, filter;
[0047] (2) rotary evaporation removes acetonitrile;
[0048] (3) The concentrated residue was separated by chromatographic column to obtain the target product (yield 81%).
[0049] Product data: 1 H NMR (400MHz, CDCl 3 ): δ7.61(d, 2H, J=5.5Hz, ArH), 7.55-7.50(m, 4H, ArH), 7.41-7.38(t, 1H, J=7.4Hz, ArH), 7.32-7.28(m , 3H, ArH), 7.23-7.20(t, 3H, J=7.1Hz, ArH), 7.20-7.13(t, 1H, J=7.0Hz, ArH). 13 C NM...
Embodiment 2
[0050] Embodiment Two: This embodiment is the synthesis of 3-phenyl-7-fluoroindanone-2-phenylene sulfide; with 1-(2-fluorophenyl)-3-phenylpropynyl ketone and thiophenol as Raw material, its reaction formula is as follows:
[0051]
[0052] Experimental steps:
[0053] (1) Put 1mmol of 1-(2-fluorophenyl)-3-phenylpropynone and 1.5mmol of manganese acetate in a round bottom flask, add 20mL of ethanol, heat to 30°C, then add 2mL of thiophenol (2mmol), TLC followed the reaction until the end. Cool to room temperature and filter.
[0054] (2) rotary evaporation removes ethanol;
[0055] (3) The concentrated residue was separated by chromatographic column to obtain the target product (yield 46%).
[0056] Product data: 1 H NMR (300MHz, CDCl 3 ): δ7.54 (s, 2H, ArH), 7.50 (d, 3H, J=2.4Hz, ArH), 7.39-7.37 (m, 1H, ArH), 7.28-7.26 (m, 2H, ArH), 7.21 -7.18(m, 3H, ArH), 7.01-6.93(m, 2H, ArH). 13 C NMR (75MHz, CDCl 3 ): 189.5, 160.8-160.7 ( 1 J F-C =4.4Hz), 160.1, 156.5, 146.8,...
Embodiment 3
[0057] Embodiment Three: This embodiment is the synthesis of 3-phenyl-5-chloroindanone-2-phenylene sulfide; with 1-(4-chlorophenyl)-3-phenylpropynyl ketone and thiophenol as Raw material, its reaction formula is as follows:
[0058]
[0059] (1) Put 1 mmol of 1-(4-chlorophenyl)-3-phenylpropynone and 0.41 g (1.5 mmol) of manganese acetate in a round bottom flask, add 20 mL of formic acid, heat to reflux, and then add phenylsulfide Phenol 1.5mL (1.5mmol), TLC tracking reaction until the end. Cool to room temperature and filter.
[0060] (2) rotary evaporation removes formic acid;
[0061] (3) The concentrated residue was separated by chromatographic column to obtain the target product (yield 78%).
[0062] Product data: 1 H NMR (300MHz, CDCl 3 ): δ7.56-7.53 (m, 2H, ArH), 7.51-7.49 (dd, 3H, J 1 =1.9Hz,J 2 =5.0Hz, ArH), 7.44(d, 1H, J=7.7Hz, ArH), 7.29-7.23(m, 3H, ArH), 7.21(s, 1H, ArH), 7.17-7.15(m, 3H, ArH ). 13 C NMR (100MHz, CDCl 3 ): 193.7, 160.0, 139.9, 134.9, 13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com