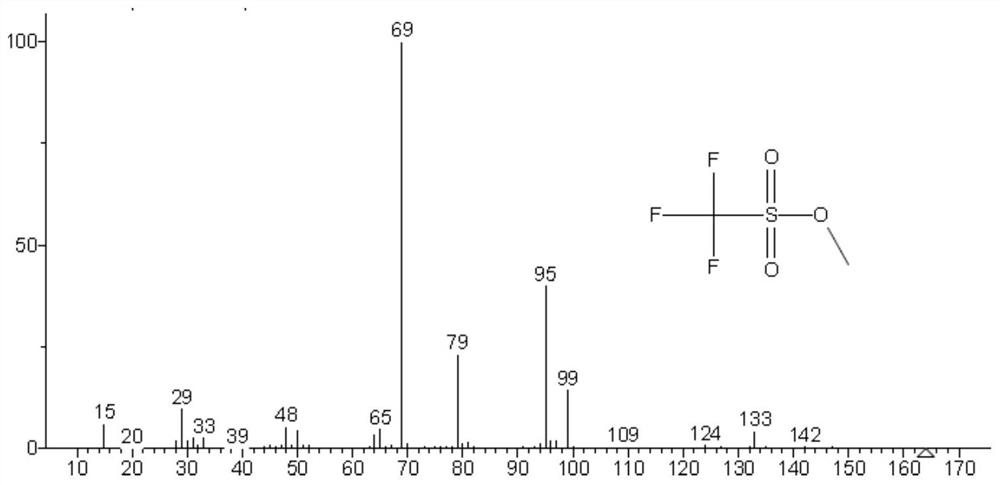
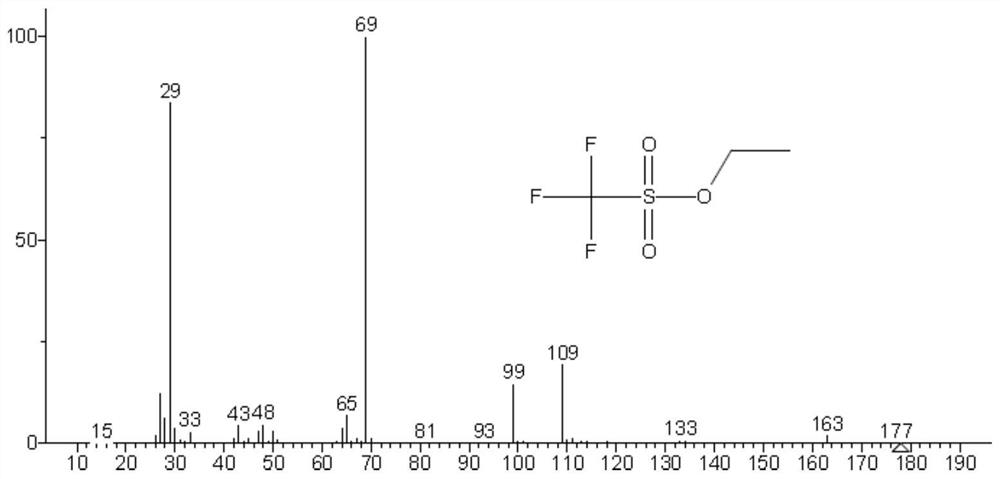
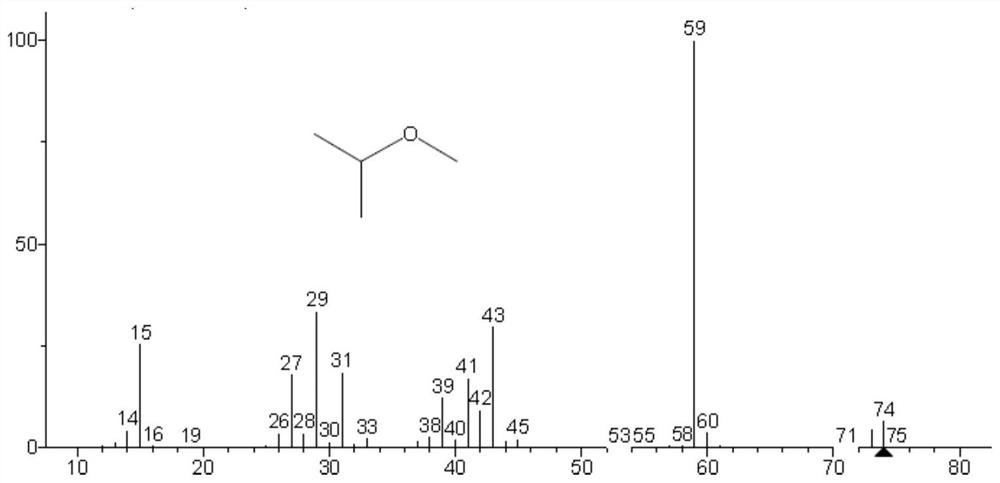
Method for detecting methyl trifluoromethanesulfonate and ethyl trifluoromethanesulfonate residues
A technology of ethyl trifluoromethanesulfonate and methyl trifluoromethanesulfonate, which is applied in the field of analytical chemistry, can solve problems such as poor detection accuracy, and achieve the effects of stable baseline, avoiding interference, and strong selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The specific research of methyl trifluoromethanesulfonate and ethyl trifluoromethanesulfonate residual determination in embodiment 1 crude drug:
[0042] Accurately weigh about 100mg of methyl trifluoromethanesulfonate and ethyl trifluoromethanesulfonate into a 100mL volumetric flask containing about 10mL of isopropanol (IPA), shake well after distilling to the mark with isopropanol, respectively Labeled methyl triflate-IPA solution, ethyl triflate-IPA solution. Methyl trifluoromethanesulfonate and ethyl trifluoromethanesulfonate are respectively reacted with isopropanol in a solution of isopropanol, and the reaction is as follows:
[0043]
[0044] The methyl trifluoromethanesulfonate-IPA solution and the ethyl trifluoromethanesulfonate-IPA solution were analyzed by GC-MS respectively, and the chromatographic conditions and mass spectrometry conditions were as follows:
[0045] The chromatographic conditions are:
[0046] The chromatographic column adopts DB-FFAP ca...
Embodiment 2
[0056] Sensitivity, precision, linearity, accuracy and stability research of methyl trifluoromethanesulfonate and ethyl trifluoromethanesulfonate residual determination in embodiment 2 crude drug:
[0057] Accurately weigh about 100mg of methyl trifluoromethanesulfonate and ethyl trifluoromethanesulfonate into a 100mL volumetric flask containing about 10mL of isopropanol, shake up to the mark with isopropanol, and precisely pipette 0.25 Put mL of this solution into a 100mL volumetric flask, dilute to the mark with isopropanol, shake well, and mark it as the reference substance stock solution.
[0058] The chromatographic conditions and mass spectrometry conditions of the GC-MS analysis method are as follows:
[0059] The chromatographic conditions are:
[0060] The chromatographic column adopts DB-FFAP capillary gas chromatography column, the column length is 30m, the inner diameter is 0.32mm, and the film thickness is 1.0μm;
[0061] Helium was used as the carrier gas, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Column length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Film thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com