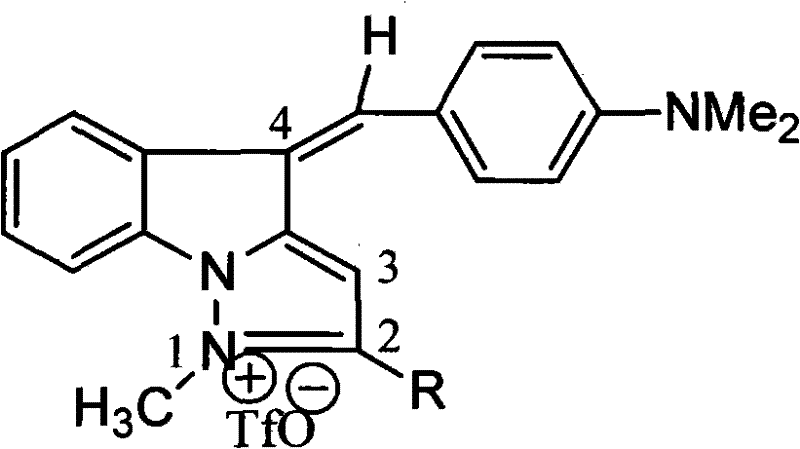
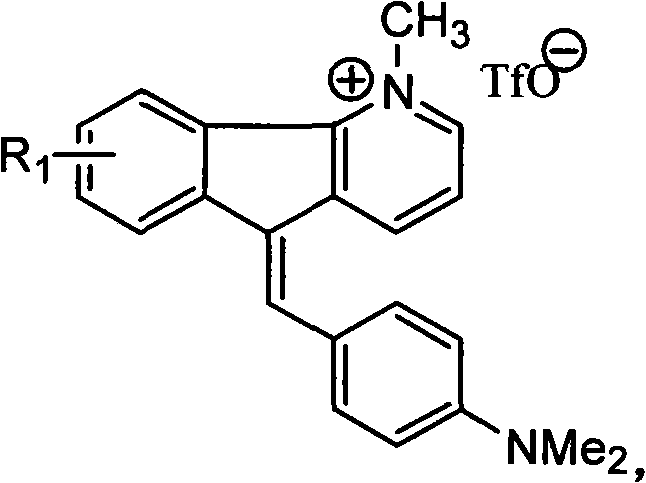
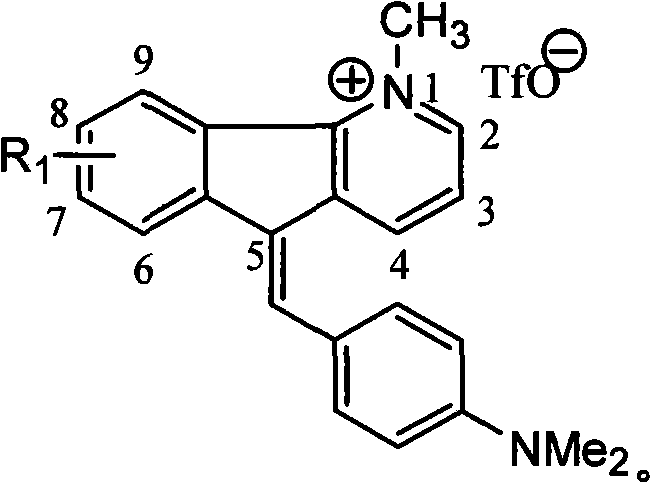
1-methyl-5h-indene (1,2-b) pyridine trifluoromethanesulfonic salt-5-(4-dimethylamino group) benzylidene derivative and preparation method thereof
A technology of pyridine trifluoromethanesulfonate and dimethylamino, which is applied in the field of preparation of antitumor drugs, can solve the problems of cumbersome synthesis steps and difficult substituents, and achieve the effects of simple process, strong inhibitory effect and good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Preparation of 1-methyl-5-(4-dimethylaminobenzene)-5H-inden[1,2-b]pyridine trifluoromethanesulfonate
[0029] (1) Preparation of enamine: Dissolve 1-indanone (5.28g, 0.04mol) in 25mL benzene, add morpholine (9mL, 0.1mol) and catalytic amount of toluene-4-sulfonic acid, stir and reflux until oil-water separation The instrument no longer observes the formation of water. Distilled under reduced pressure to obtain an amber oily product;
[0030] (2) Condensation and cyclization: 3-bromopropylamine hydrobromide (2.19g, 0.01mol) was dissolved in 10mL of dry DMF, and the oil obtained in step (1) (2.21g, 0.011mol) was added, and dissolved in 100- 110°C, stirred and refluxed for 4h. After the reaction, the reaction solution was poured into 5% hydrochloric acid solution (15mL), and extracted with ether to remove non-alkaline substances; an excess of 50% sodium hydroxide solution was added to the aqueous layer, extracted three times with ether, and the ether layer was...
Embodiment 2
[0035] Example 2: Preparation of 1-methyl-8-fluoro-5-(4-dimethylaminobenzene)-5H-indene[1,2-b]pyridine trifluoromethanesulfonate
[0036] (1)-(3) method is the same as embodiment one
[0037] (4) Methylation reaction: Dissolve 8-fluoro-5H-inden[1,2-b]pyridine (0.16 g, 0.9 mmol) in 5 mL of dry CH 2 Cl 2 Medium, N 2 Methyl trifluoromethanesulfonate (0.21 mL, 1.8 mmol) was added under protection, stirred at room temperature for 24 h, and the reaction was tracked by TLC. After the reaction, evaporated to dryness, separated and purified by column chromatography, CH 2 Cl 2 -CH 3 Gradient elution with OH (100:1~50:1) gave white solid 1-methyl-8-fluoro-5H-indene[1,2-b]pyridine trifluoromethanesulfonate (0.26g, 91%);
[0038] (5) Coupling reaction: 1-methyl-8-fluoro-5H-inden[1,2-b]pyridine trifluoromethanesulfonate (0.26g, 0.82mmol) and p-dimethylaminobenzaldehyde (0.18 g, 1.21mmol) was refluxed in 50mL glacial acetic acid for 2 days. Distillation under reduced pressure, separati...
Embodiment 3
[0040] Example 3: Preparation of 1,8-dimethyl-5-(4-dimethylaminobenzene)-5H-indene[1,2-b]pyridine trifluoromethanesulfonate
[0041] (1)-(3) method is the same as embodiment one
[0042] (4) Methylation reaction: Dissolve 8-methyl-5H-inden[1,2-b]pyridine (0.16 g, 0.9 mmol) in 5 mL of dry CH 2 Cl 2 Medium, N 2 Methyl trifluoromethanesulfonate (0.21 mL, 1.8 mmol) was added under protection, stirred at room temperature for 20 h, and the reaction was tracked by TLC. After the reaction, evaporated to dryness, separated and purified by column chromatography, CH 2 Cl 2 -CH 3 OH (100:1~50:1) gradient elution gave white solid 1,8-dimethyl-5H-indene[1,2-b]pyridine trifluoromethanesulfonate (0.29g, 97%);
[0043] (5) Coupling reaction: 1,8-dimethyl-5H-inden[1,2-b]pyridine trifluoromethanesulfonate (0.29g, 0.88mmol) and p-dimethylaminobenzaldehyde (0.18g , 1.21mmol) was refluxed in 50mL glacial acetic acid for 2 days. Distillation under reduced pressure, separation and purificatio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com