Method for improving thermal stability of liquid fructosaminase
A fructose amino acid, thermal stability technology, applied in the directions of enzyme stabilization, biochemical equipment and methods, enzymes, etc., can solve the problems of high viscosity of determination reagents, poor thermal stability of liquid glycated amino acid oxidase, etc., and achieves improved method, The effect of improved thermal stability and easy handling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Prepare 100ml of pH7.5 tris-hydrochloric acid (Tris-HCL) buffer solution, the concentration of Tris-HCL contained is 30mmol / L, the concentration of fructose amino acid oxidase (derived from recombinant E.coli) 1KU / L, the concentration of sodium azide is 1g / L;
[0021] (2) Add 0.2 g of 1-butylsulfonic acid-3-methyltrifluoromethanesulfonate ([ BSMIM]OTf), 1g trehalose, 0.05ml TritonX-100, stir to dissolve.
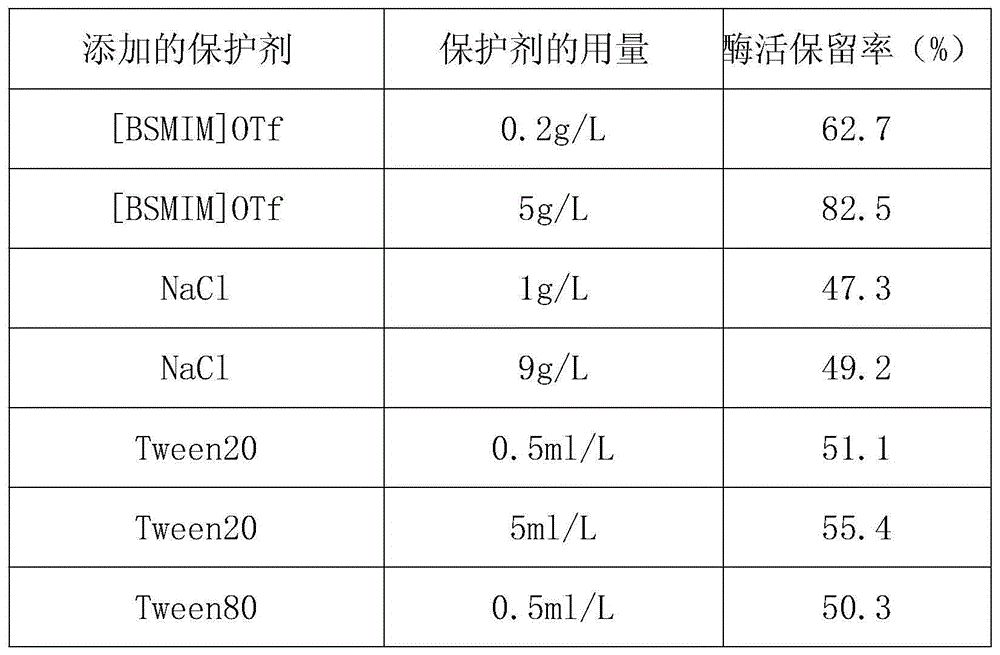
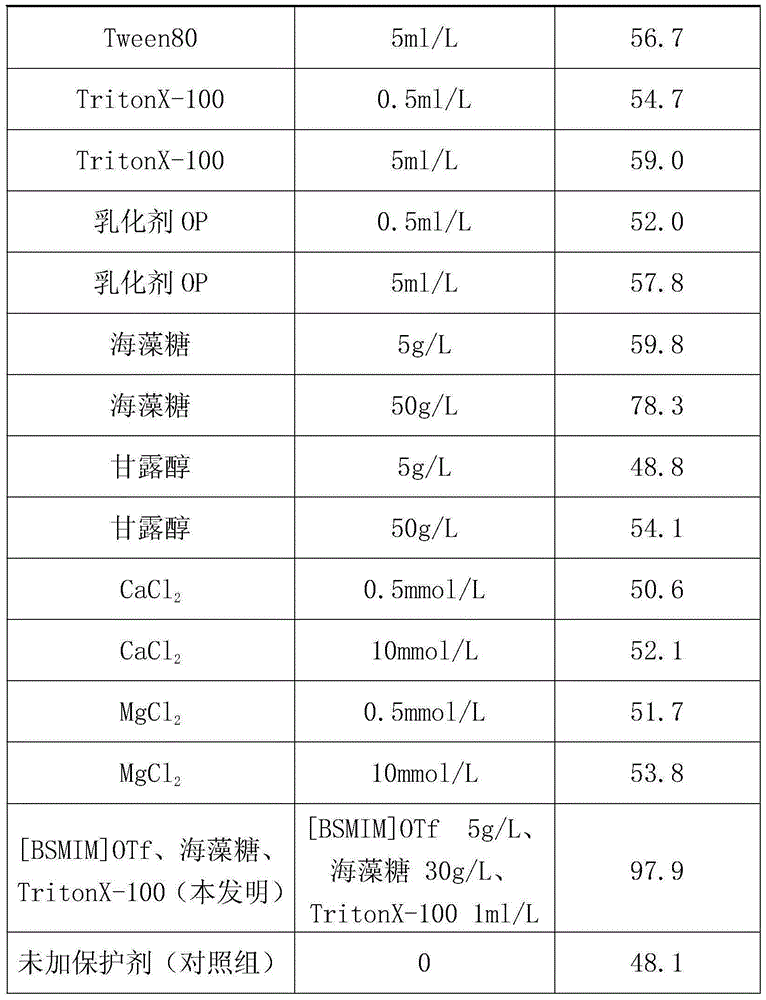
[0022] The solution obtained in step (2) was placed in a water bath at 37° C. for 30 minutes, and then the enzyme retention rate was measured. The enzyme activity retention rate (%) placed at 4°C without heat treatment and without protective agent was taken as 100%, and treated under the same experimental conditions without protective agent as a control group, and the enzyme activity retention rate (%) was calculated and compared. The enzyme activity retention rates measured by the inventive group and the control group are 98.2% and 45.7% respectively. It can be...
Embodiment 2
[0024] (1) Prepare 100ml of pH8.0 tris-hydrochloric acid (Tris-HCL) buffer solution, the concentration of Tris-HCL contained is 65mmol / L, the concentration of fructose amino acid oxidase (derived from recombinant E.coli) 5.5KU / L, the concentration of sodium azide is 1g / L;
[0025] (2) Add 0.6 g of 1-butylsulfonic acid-3-methyltrifluoromethanesulfonate ([ BSMIM]OTf), 3g trehalose, 0.3ml TritonX-100, stir to dissolve.
[0026] The solution obtained in step (2) was placed in a water bath at 37° C. for 30 minutes, and then the enzyme retention rate was measured. The enzyme activity retention rate (%) placed at 4°C without heat treatment and without protective agent was taken as 100%, and treated under the same experimental conditions without protective agent as a control group, and the enzyme activity retention rate (%) was calculated and compared. The enzyme activity retention rates measured by the inventive group and the control group are 98.5% and 47.4% respectively. It can b...
Embodiment 3
[0028] (1) Prepare 100ml of pH8.5 tris-hydrochloric acid (Tris-HCL) buffer solution, the concentration of Tris-HCL contained is 100mmol / L, the concentration of fructose amino acid oxidase (derived from recombinant E.coli) 10KU / L, the concentration of sodium azide is 1g / L;
[0029] (2) Add 1 g of 1-butylsulfonic acid-3-methyltrifluoromethanesulfonate ([BSMIM ]OTf), 5g trehalose, 0.5ml TritonX-100, stir to dissolve.
[0030]The solution obtained in step (2) was placed in a water bath at 37° C. for 30 minutes, and then the enzyme retention rate was measured. The enzyme activity retention rate (%) placed at 4°C without heat treatment and without protective agent was taken as 100%, and treated under the same experimental conditions without protective agent as a control group, and the enzyme activity retention rate (%) was calculated and compared. The enzyme activity retention rates measured by the invention group and the control group are 98.0% and 46.3% respectively. It can be s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com