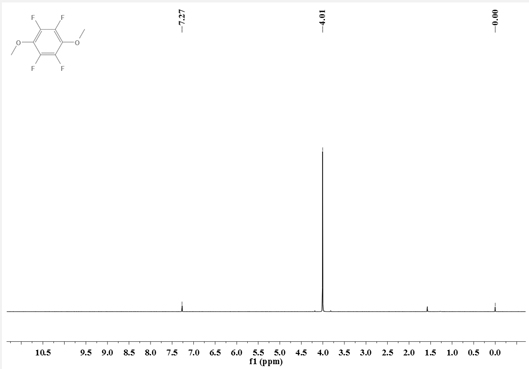
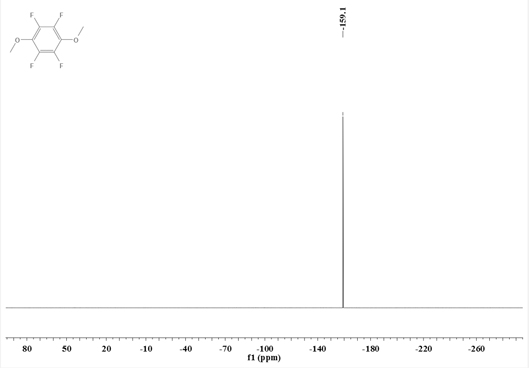
Synthesis method of 1, 4-dimethoxy tetrafluorobenzene
A technology of dimethoxytetrafluorobenzene and dimethylpentafluoroaniline, which is applied in the field of synthesis of 1,4-dimethoxytetrafluorobenzene, can solve the problems of long reaction time, unfavorable industrial production, low yield, etc. problem, to achieve the effects of simple post-processing, improved anti-overcharge performance, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Add N,N-dimethylpentafluoroaniline (211.1g, 1.0mol) into a 2L three-necked flask equipped with a stirrer, a thermometer, and a condenser tube, and add a 30% methanol solution of sodium methoxide (198g, containing Sodium methoxide (1.1mol) was added dropwise to the reaction system—N,N-dimethylpentafluoroaniline. After the dropwise addition, the reflux reaction was carried out for 6 hours. After the reaction was completed, the reaction solution was filtered, and 200g of ethyl acetate was added to obtain an organic Washing with 200g of deionized water, liquid separation, washing and liquid separation cycle 3 times, the obtained organic phase was decompressed and desolvated by a rotary evaporator, and then rectified to obtain 178.3g of intermediate I.
[0040] Intermediate I (133.9g, 0.6mol) and acetonitrile (300g) were respectively added to a 1L three-necked flask equipped with a stirrer, a thermometer, and a condenser, and methyl trifluoromethanesulfonate (108.3g, 0.66mol)...
Embodiment 2
[0044] Add N,N-dimethylpentafluoroaniline (211.1g, 1.0mol) into a 2L three-necked flask equipped with a stirrer, a thermometer, and a condenser tube, and add a 30% methanol solution of sodium methoxide (234g, containing The mixed solution of sodium methoxide (1.3mol) and 200g of acetonitrile was added dropwise to the reaction system. After the dropwise addition, the reflux reaction was carried out for 8h. After the reaction was completed, the reaction solution was filtered, and 200g of ethyl acetate was added. Washing and liquid separation were performed 3 times in total. The obtained organic phase was decompressed by a rotary evaporator to remove the solvent, and then rectified to obtain 190.5 g of intermediate I.
[0045] Intermediate I (133.9g, 0.6mol) and acetonitrile (300g) were respectively added to a 1L three-necked flask equipped with a stirrer, a thermometer, and a condenser, and methyl trifluoromethanesulfonate (128.0g, 0.78mol) was added dropwise, After the dropwise...
Embodiment 3
[0048] Add N,N-dimethylpentafluoroaniline (211.1g, 1.0mol) into a 2L three-necked flask equipped with a stirrer, a thermometer, and a condenser tube, and add a 30% methanol solution of sodium methoxide (234g, containing The mixed solution of sodium methoxide (1.3mol) and 200g of acetonitrile was added dropwise to the reaction system. After the dropwise addition, the reflux reaction was carried out for 8h. After the reaction was completed, the reaction solution was filtered, and 200g of ethyl acetate was added. Washing, liquid separation, a total of 3 times, the obtained organic phase was decompressed by a rotary evaporator to remove the solvent, and then rectified to obtain intermediate I190.5g.
[0049] Intermediate I (133.9g, 0.6mol) and acetonitrile (300g) were respectively added to a 1L three-necked flask equipped with a stirrer, a thermometer, and a condenser, and methyl trifluoromethanesulfonate (128.0g, 0.78mol) was added dropwise, After the dropwise addition, the tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com