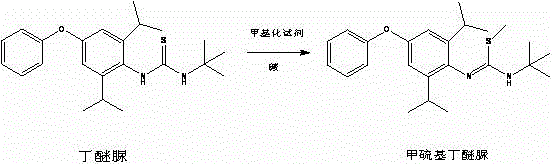
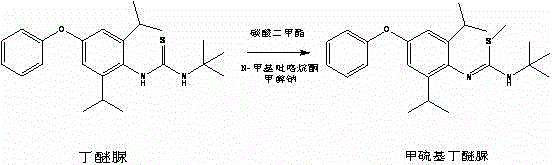
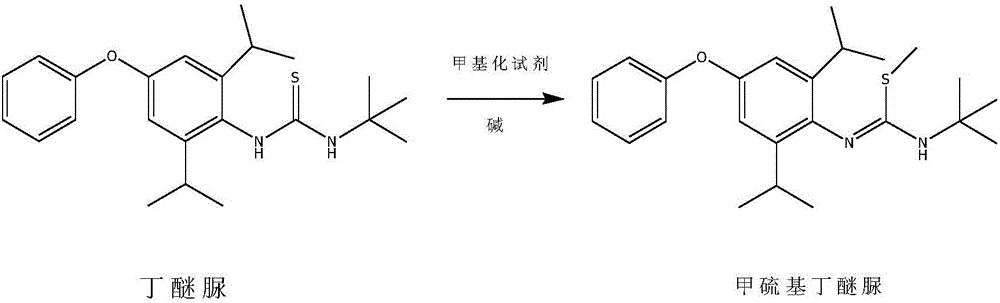
Method for preparing methylmercaptodiafenthiuron
A technology of methyl thiobutyl ether and methylation, which is applied in the direction of organic chemistry, can solve the problems of toxicity and high production cost, and achieve the effect of mild reaction conditions, high cost and high content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025] In a 5L autoclave, 770g (2 moles) of diafenthiuron and 1.5L of methyl ethyl ketone were sequentially added, 134.4g (2.4 moles) of potassium hydroxide were added, 106g (2.1 moles) of methyl chloride was introduced, and after sealing, the temperature was raised to 70°C , React for 4 hours. After the reaction, the solvent was recovered under negative pressure, and 1L of water was added to the remaining materials, stirred at room temperature for 1 hour, filtered, and dried to obtain a white powder of methylthio diafenthiuron, 799.1g, content 97.6%, melting point 88-91°C. The rate is 98.0%.
Embodiment 2
[0027]
[0028] In a 5L autoclave, 770g (2 moles) of diafenthiuron and 1.8L of toluene were sequentially added, 268.8g (2.4 moles) of potassium tert-butoxide were added, and after stirring for 1 hour, 209g (2.2 moles) of methyl bromide was introduced. After sealing, The temperature was raised to 60°C and reacted for 4 hours. After the reaction, the solvent was recovered under negative pressure, and 1L of water was added to the remaining materials, stirred at room temperature for 1 hour, filtered, and dried to obtain a white powder of methionine, 792.6g, content 98.1%, melting point 89-91℃, The rate is 97.7%.
Embodiment 3
[0030]
[0031] Add diafenthiuron 770g (2 moles) and 1.5L N-methylpyrrolidone in a 3L reaction flask, add 129.6g (2.4 moles) of sodium methoxide and 270g (3 moles) of dimethyl carbonate, and heat to 180°C. React for 3 hours. After the reaction, the solvent was recovered under negative pressure, and 1.2L of water was added to the remaining materials, stirred at room temperature for 1 hour, filtered, and dried to obtain white powder methionine, 744.6g, content 97.1%, melting point 87-91°C, The yield was 90.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com