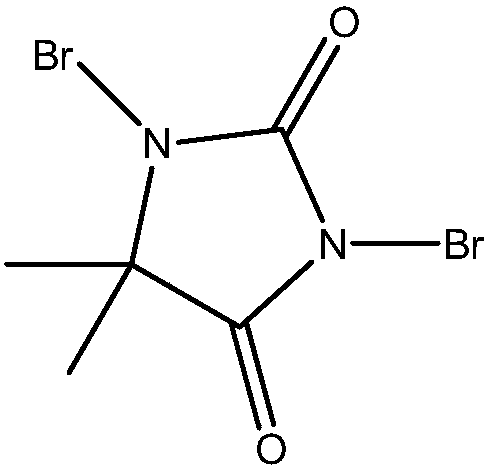
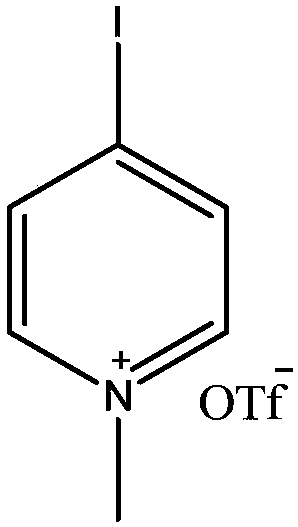
Preparation method for synthesizing O-glycoside based on catalytic activation of thioglycoside by 4-iodopyridin-N-methyltrifluoromethanesulfonate
A technology of methyl trifluoromethanesulfonate and synthesis method, which is applied in the field of preparation based on 4-iodopyridine-N-methyl trifluoromethanesulfonate catalyzing and activating thioglycoside to synthesize O-glycoside, can solve the problem of unsuitable The problems of large industrial production, high price and high toxicity have achieved good application prospects, convenient operation and low toxicity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
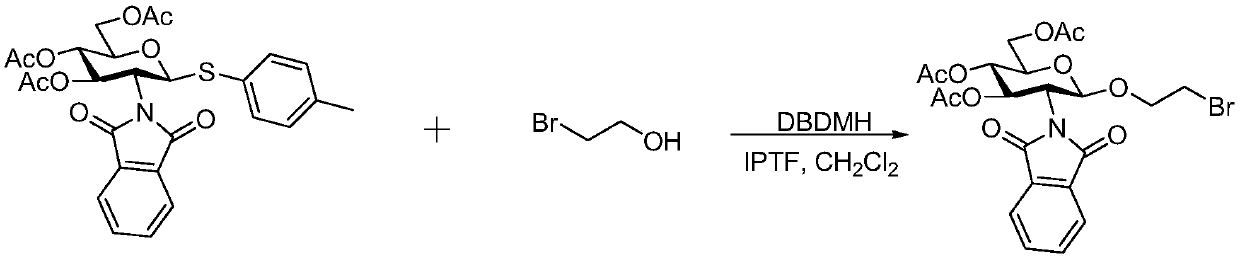
[0013] Example 1 (preparation of 2'-bromoethyl 3,4,6-tri-O-acetyl-2-deoxy-2-phthaloyl-β-D-glucoside)
[0014]
[0015] Yield 95% (514 mg); 1 H NMR (400MHz, CDCl 3 )δ7.83(dd,J=5.4,3.1Hz,2H),7.71(dd,J=5.5,3.1Hz,2H),5.79(dd,J=10.7,9.1Hz,1H),5.41(d,J =8.5Hz, 1H), 5.16(dd, J=10.1, 9.2Hz, 1H), 4.36-4.27(m, 2H), 4.17(dd, J=12.3, 2.4Hz, 1H), 4.09(dt, J= 11.2,5.6Hz,1H),3.87(m,1H),3.75(m,1H),3.37-3.25(m,2H),2.10(s,3H),2.02(s,3H),1.85(s,3H ). 13 C NMR (100MHz, CDCl 3 )δ170.6, 170.1, 169.4, 134.2, 131.5, 123.5, 98.2, 72.0, 70.7, 68.9, 68.7, 61.9, 54.5, 50.3, 20.7, 20.6, 20.4.
example 2
[0016] Example 2[6-O-(2,3,4,-tri-O-acetyl-β-D-rhamnosyl)-(1→6)-1,2:3,4-di-O- isopropyl-α-D-galactoside)]
[0017]
[0018] Yield 90% (743 mg); 1 H NMR (400MHz, CDCl 3 )δ5.48(d,J=5.0Hz,1H),5.27-5.23(m,2H), 5.07-5.00(m,1H),4.78(s,1H),4.60(dd,J=7.9,2.4Hz ,1H),4.30-4.26(m,2H),4.02-3.96(m, 2H),3.83(dd,J=9.6,7.3Hz,1H),3.56(dd,J=9.6,6.2Hz,1H), 2.12(s,3H),2.01(s,3H),1.96(s,3H),1.53(s,3H),1.41(s,3H),1.31(s,6H),1.18(d,J=6.3Hz ,3H). 13 C NMR (100MHz, CDCl 3 ) δ169.9, 109.1, 108.7, 97.2, 96.1, 71.1, 70.7, 70.6, 70.6, 69.8, 69.2 66.3, 66.2, 65.3, 26.1, 25.9, 24.9, 24.4, 20.9, 20.7, 20.7, 17.2.
example 3
[0019] Example 3 (Epiandrosterone 2,3,4,6-tetra-O-benzoyl-β-D-galactoside)
[0020]
[0021] Yield 97% (842 mg); 1 H NMR (400MHz, CDCl 3 )δ8.10(dd,J=8.2,1.1Hz,2H),8.03(dd,J=8.3,1.1Hz,2H),7.96(dd,J=8.3,1.1Hz,2H),7.78(dd,J =8.3,1.1Hz,2H),72.64-7.55(m,2H),7.53(d,J=12.4Hz,1H),7.48(d,J=1.5Hz,2H),7.46-7.42(m,3H) ,7.41-7.39(m,1H),7.37(d,J=7.8Hz,1H),7.23(t,J=7.9Hz,2H),5.97(d,J=4.2Hz,1H),5.76(dd, J=10.4,7.9Hz,1H),5.59 (dd,J=10.4,3.5Hz,1H),4.91(d,J=7.9Hz,1H),4.68(dd,J=11.2,6.9Hz,1H), 4.42(dd, J=11.3, 6.4Hz, 1H), 4.32(t, J=6.7Hz, 1H), 3.62(m, 1H), 2.42(dd, J=19.3, 8.4Hz, 1H), 2.04(s , 1H), 1.90(d, J=17.9Hz, 2H), 1.79(d, J=11.5Hz, 2H), 1.66-1.55(m, 3H), 1.54-1.46(m, 3H), 1.26-1.15( m,5H),1.01(dd,J=14.0,4.1Hz,1H),0.92-0.86(m,1H),0.83(s,3H),0.72(s,3H), 0.65-0.58(m,1H) . 13 C NMR (100MHz, CDCl 3 )δ221.2,166.0,165.7,165.6,165.2,133.5,133.2, 133.1,130.1,129.8,129.7,129.6,129.5,129.0,128.8,128.6,128.4,128.3,128.2 100.6,80.2,71.9, 71.2,70.1,68.1, 62.1, 54.4, 51.4, 47.8, 44.7, 36.8, 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com