Halogenated 1,2,3-triazole carbene, and preparation method and application thereof
A technology of triazole carbene and triazole carbene, applied in the field of organic catalysis, can solve problems such as limiting the development of triazole carbene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
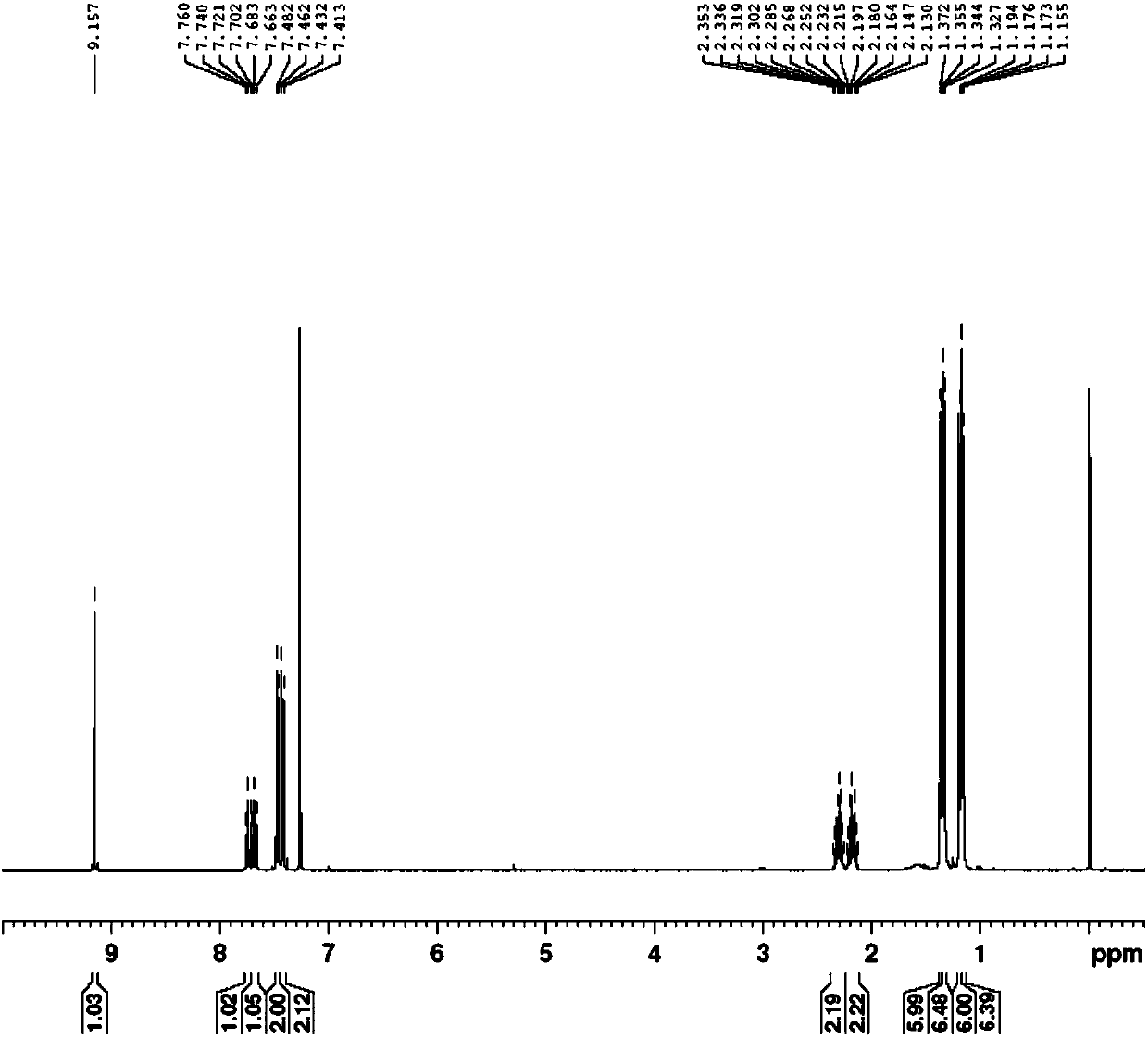
[0101] Preparation of 1,3-bis(2,6-diisopropyl-benzene)-5-chloro-1,2,3-triazolecarbene
[0102] 1) Preparation of 1,3-bis(2,6-diisopropyl-benzene)-5-chloro-1,2,3-triazole salt
[0103] In a Shrek bottle at -78°C under nitrogen protection, 3.6 g (10 mmol) of 1,3-bis(2,6-diisopropyl-benzene)-triazene, 2 g (11 mmol) of potassium hexafluorophosphate, and ), 30ml of dichloromethane, 1mL (mmol) of 1,1-dichloroethylene and 1.7mL (15mmol) of tert-butyl hypohalite were added in the dark. Allow to warm to room temperature freely. After that, water was added for washing, and after washing with water three times, the organic phase was dried. The organic solvent was removed by rotary evaporation, and the obtained solid was washed three times with ether. After washing off most of the black matter, it was recrystallized with dichloromethane and ether to obtain 1,3-bis(2,6-diisopropyl-benzene )-5-chloro-1,2,3-triazole salt. After weighing, 1,3-bis(2,6-diisopropyl-benzene)-5-chloro-1,2,3-tr...
Embodiment 2
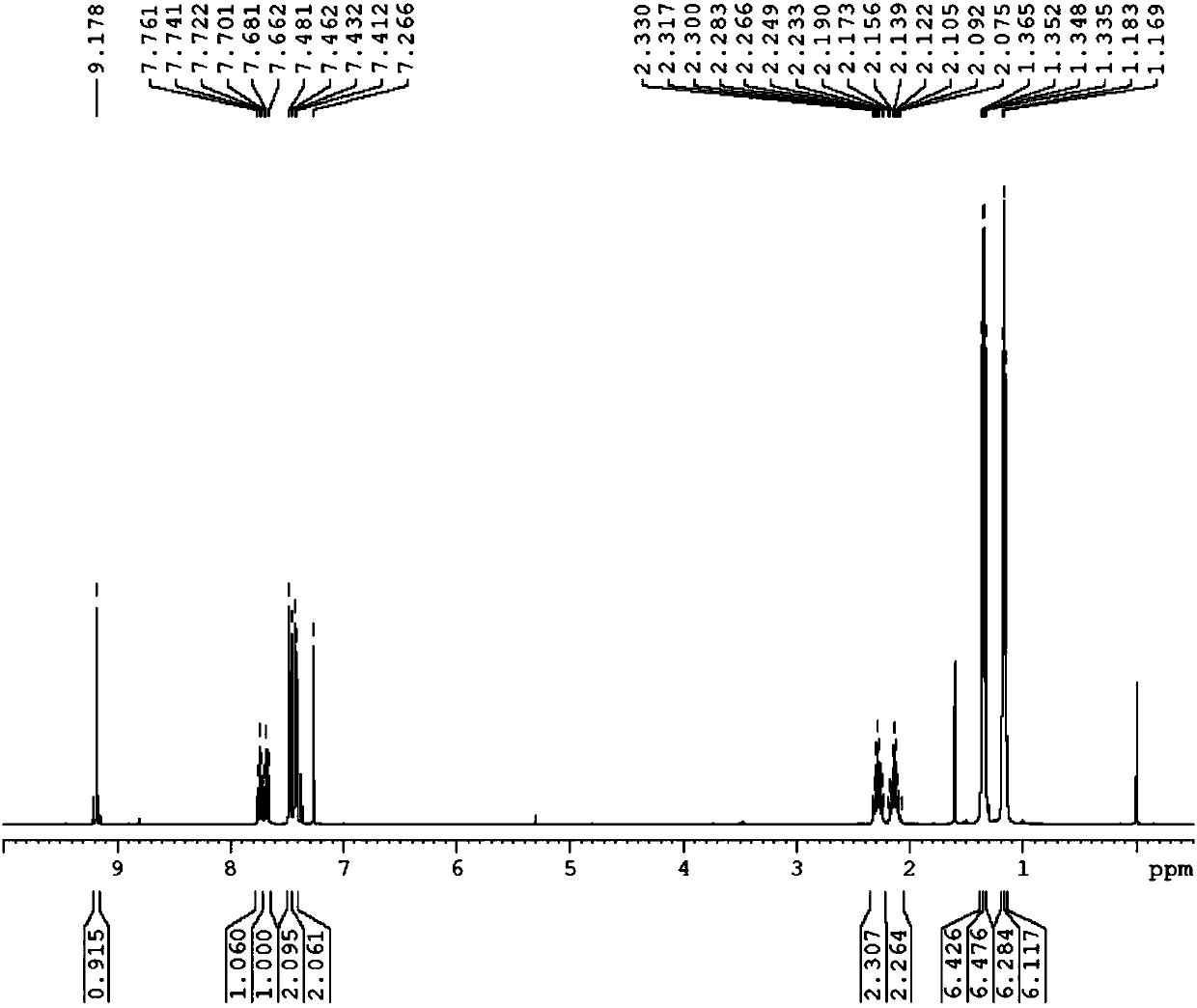
[0109] Preparation of 1,3-bis(2,6-diisopropyl-phenyl)-5-bromo-1,2,3-triazolecarbene
[0110] 1) Preparation of 1,3-bis(2,6-diisopropyl-benzene)-5-bromo-1,2,3-triazole salt
[0111] In a Shrek bottle at -78°C under nitrogen protection, 3.6 g (10 mmol) of 1,3-bis(2,6-diisopropyl-benzene)-triazene, 2 g (11 mmol) of potassium hexafluorophosphate, and ), 30 mL of dichloromethane, 0.8 mL (10 mmol) of 1,2-dibromoethylene and 1.7 mL (15 mmol) of tert-butyl hypohalite were added in the dark. Let it warm up to room temperature naturally. After that, water was added for washing, and after washing with water three times, the organic phase was dried. The organic solvent was removed by rotary evaporation, and the obtained solid was washed three times with ether. After washing off most of the black matter, it was recrystallized with dichloromethane and ether to obtain 1,3-bis(2,6-diisopropyl-benzene )-5-bromo-1,2,3-triazole salt. After weighing, 1,3-bis(2,6-diisopropyl-benzene)-5-bromo-1...
Embodiment 3
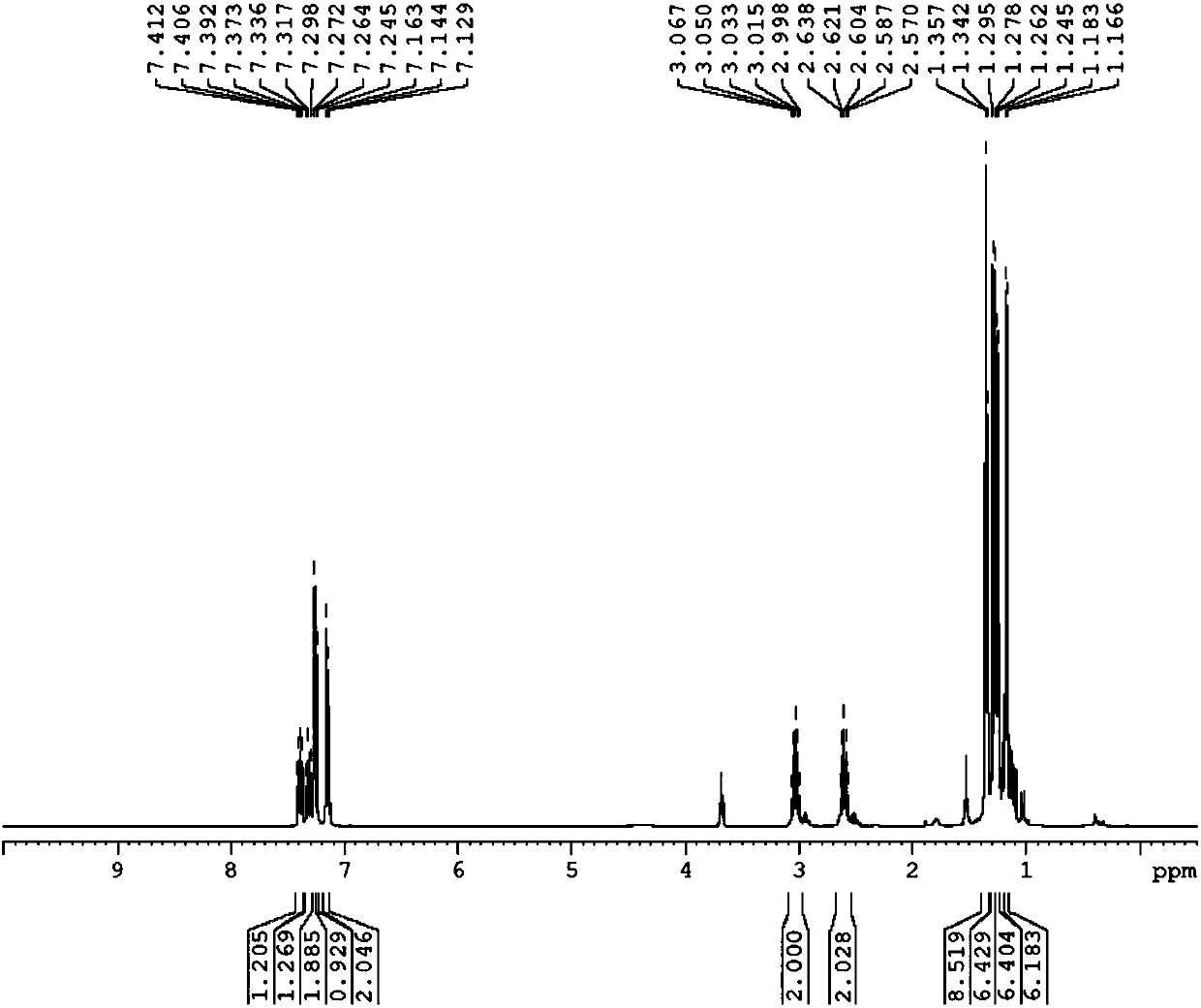
[0119] Preparation of 1,3-bis(2,6-diisopropyl-phenyl)-5-fluoro-1,2,3-triazolecarbene
[0120] 1) Preparation of 1,3-bis(2,6-diisopropyl-benzene)-5-fluoro-1,2,3-triazole salt
[0121] In a Shrek bottle at -78°C under nitrogen protection, 3.6 g (10 mmol) of 1,3-bis(2,6-diisopropyl-benzene)-triazene, 2 g (11 mmol) of potassium hexafluorophosphate, and ), 30 mL of dichloromethane, 0.8 mL (10 mmol) of 1,2-difluoroethylene and 1.7 mL (15 mmol) of tert-butyl hypohalite were added in the dark. Let it warm up to room temperature naturally. After that, water was added for washing, and after washing with water three times, the organic phase was dried. The organic solvent was removed by rotary evaporation, and the obtained solid was washed three times with ether. After washing off most of the black matter, it was recrystallized with dichloromethane and ether to obtain 1,3-bis(2,6-diisopropyl-benzene )-5-fluoro-1,2,3-triazole salt. After weighing, 1,3-bis(2,6-diisopropyl-benzene)-5-flu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com