Method for preparing poly (conjugated diene) by using pyridine imine iron catalyst and application of poly (conjugated diene)
A technology of polyconjugated diene and pyridinium iron, which is applied in the application field of polyconjugated diene, can solve the problems such as unsatisfactory strength, and achieve the effects of increasing crosslinking density, good biocompatibility and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: The method for preparing polyconjugated diene with pyridineimide iron catalyst in this embodiment is carried out according to the following steps:
[0026] Under anhydrous and anoxic conditions, pyridineimine hydrazine catalyst A (10 μmol, 1 equiv, 5.20 mg), cocatalyst MAO (5 mmol, 500 equiv, 3.33 mL), β-farnesene monomer solution (20 mmol, 2000 equiv, 5.1 mL) was added to 5 mL of toluene, and after polymerization at 25°C for 90 min, 1 mL of 2,6-di-tert-butyl-4-methylphenol in ethanol (2,6-di-tert-butyl-4-methylphenol) was added. 1 wt% phenol) and 10 mL of a mixed solution of methanol and concentrated hydrochloric acid (v / v = 50:1) to quench the reaction, pour off the clear liquid, wash three times with ethanol, and then dry at 40 °C for 24 h to constant weight to obtain Poly-beta-farnesene.
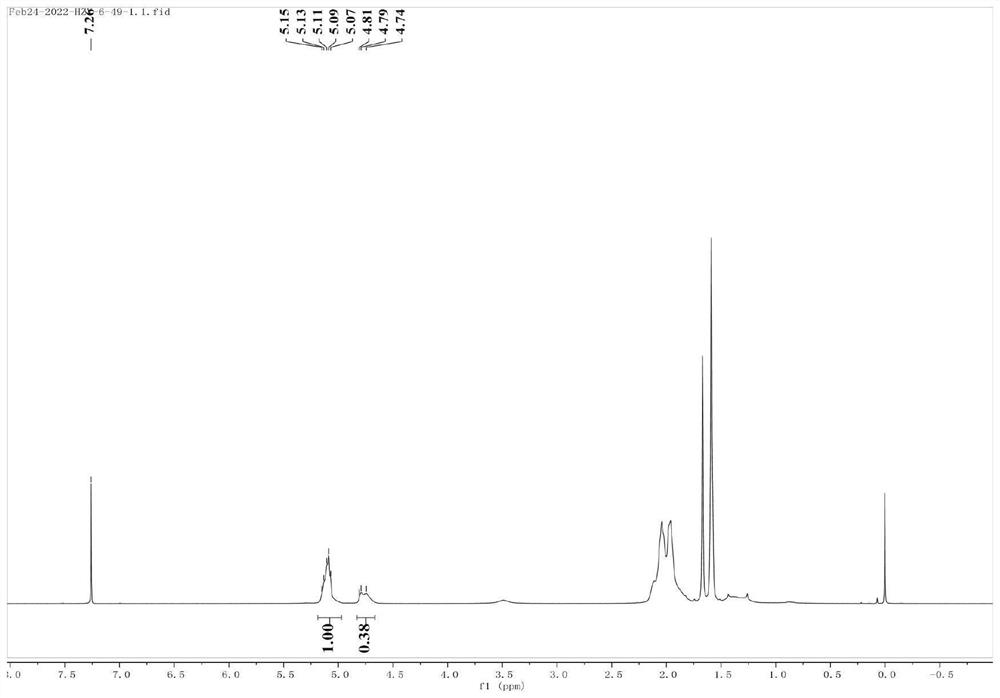
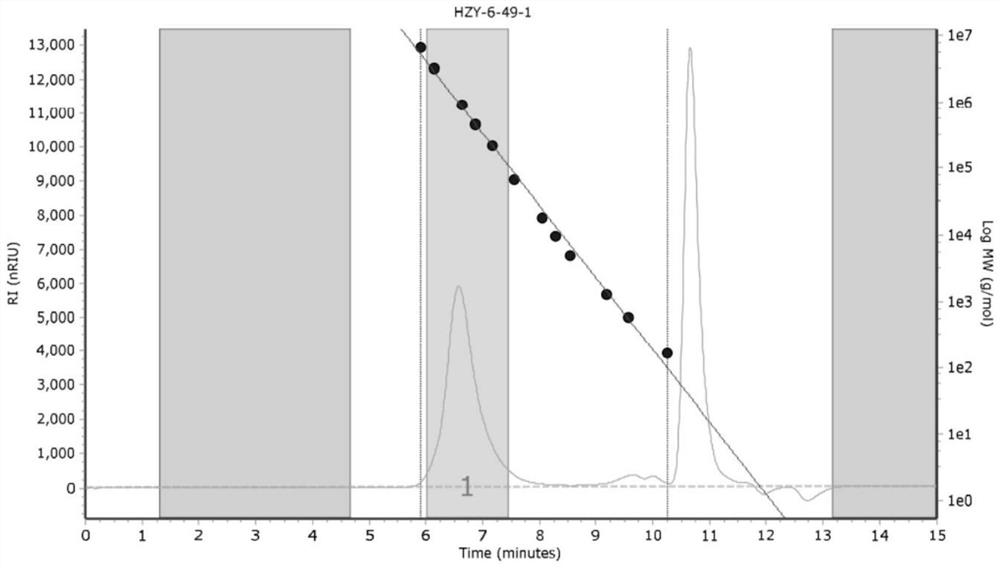
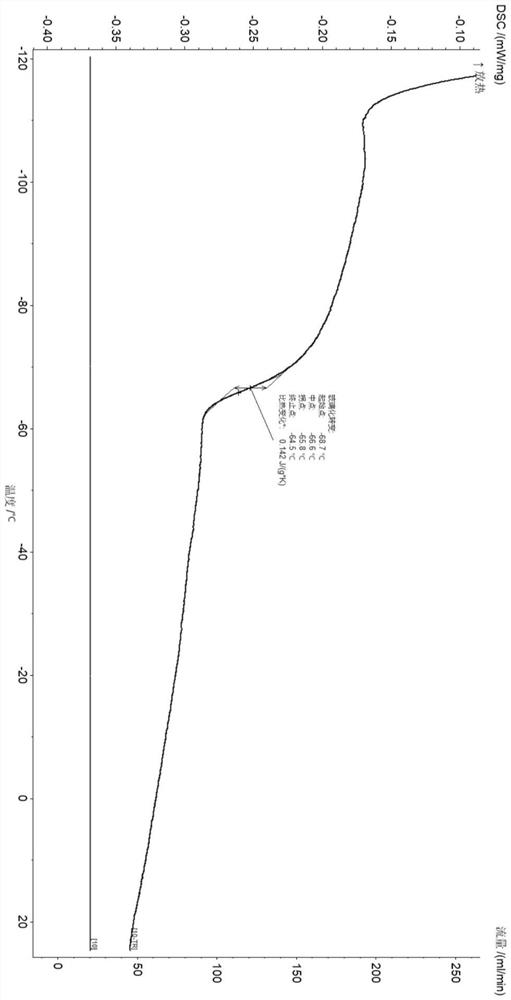
[0027] Results: The yield of poly-β-farnesene in this example was >99%, and the microstructural composition of the polymer was: 52% 1,4-polyβ-farnesene and 48% 3,4-...
Embodiment 2
[0030] Embodiment 2: The method for preparing polyconjugated diene with pyridineimide iron catalyst in this embodiment is carried out according to the following steps:
[0031] Under anhydrous and oxygen-free conditions, pyridineimine hydrazine catalyst B (10 μmol, 1 equiv, 5.48 mg), cocatalyst MAO (5 mmol, 500 equiv, 3.33 mL), β-farnesene monomer solution (20 mmol, 2000 equiv, 5.1 mL) was added to 5 mL of toluene, and after polymerization at 25°C for 90 min, 1 mL of 2,6-di-tert-butyl-4-methylphenol in ethanol (2,6-di-tert-butyl-4-methylphenol) was added. 1 wt% phenol) and 10 mL of a mixed solution of methanol and concentrated hydrochloric acid (v / v = 50:1) to quench the reaction, pour off the clear liquid, wash three times with ethanol, and then dry at 40 °C for 24 h to constant weight to obtain Poly-beta-farnesene.
[0032] Results: The yield of poly-β-farnesene in this example was >99%, and the microstructure selectivity of the polymer was: 58% for 1,4-polyβ-farnesene and ...
Embodiment 3
[0033] Embodiment 3: The method for preparing polyconjugated diene with pyridineimide iron catalyst in this embodiment is carried out according to the following steps:
[0034] Under anhydrous and oxygen-free conditions, pyridineimine hydrazine catalyst C (10 μmol, 1 equiv, 5.48 mg), cocatalyst MAO (5 mmol, 500 equiv, 3.33 mL), β-farnesene monomer solution (20 mmol, 2000 equiv, 5.1 mL) was added to 5 mL of toluene, and after polymerization at 25°C for 90 min, 1 mL of 2,6-di-tert-butyl-4-methylphenol in ethanol (2,6-di-tert-butyl-4-methylphenol) was added. 1 wt% phenol) and 10 mL of a mixed solution of methanol and concentrated hydrochloric acid (v / v = 50:1) to quench the reaction, pour off the clear liquid, wash three times with ethanol, and then dry at 40 °C for 24 h to constant weight to obtain Poly-beta-farnesene.
[0035] Results: The yield of poly-β-farnesene in this example was 90%, and the microstructure selectivity of the polymer was: 50% of 1,4-poly-β-farnesene and 5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com