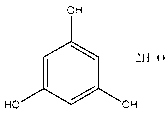
Production process of medicinal phloroglucinol
A kind of phloroglucinol, pharmaceutical grade technology, applied in the production technology field of pharmaceutical grade phloroglucinol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 2, the synthesis of phloroglucinol
[0033] 1. Mix 15g of 1,3,5-trimethoxybenzene and 1000ml of 35% concentrated hydrochloric acid into a three-necked flask, add 50ml of H 3 PO 4 , add 0.5g platinum dioxide at the same time, stir at room temperature for 4h, place in ice bath, add 600g Na in batches while stirring 2 CO 3 Neutralize, adjust the pH to 2-3 and then filter. The filtrate was extracted three times with 600ml of methyl tert-butyl ether, the extract was dried over anhydrous sodium sulfate, filtered, and the methyl tert-butyl ether was evaporated to obtain a yellow solid phloroglucinol crude product with a purity of 96.5%. The yield was 86.7%, mp205~210℃.
Embodiment 2
[0034] 2. Take purified water 10 times the amount of the crude product, add crude phloroglucinol, heat to 80°C, stir to dissolve. Add 0.50% activated carbon and keep warm for 45 minutes. The filtrate is firstly filtered through a 0.45 μm filter membrane, and then finely filtered through a 0.22 μm filter membrane to obtain a fine filtrate.
[0035] 3. Pass the finely filtered filtrate through the hollow fiber ultrafiltration membrane. The ultrafiltration membrane adopts a hollow fiber ultrafiltration polysulfone membrane, so that the inlet pressure of the ultrafiltration membrane is controlled at 1.75MPa, and the hollow fiber material line velocity is 1m / s , to obtain the ultrafiltrate.
[0036] 4. Cool the ultrafiltrate to room temperature, crystallize for 4 hours, centrifuge, take the white crystals and dry them in vacuum (-0.085~-0.080MPa, 40~45℃) for 3 hours to obtain pharmaceutical grade phloroglucinol with a purity of 99.9 % (HPLC method).
[0037] Embodiment 3, the synth...
Embodiment 3
[0039] 2. Take purified water 10 times the amount of the crude product, then add the crude product phloroglucinol, heat to 80°C, stir to dissolve. Add 0.50% activated carbon and keep warm for 45 minutes. The filtrate is firstly filtered through a 0.45 μm filter membrane, and then finely filtered through a 0.22 μm filter membrane to obtain a fine filtrate.
[0040] 3. Pass the finely filtered filtrate through the hollow fiber ultrafiltration membrane, the ultrafiltration membrane adopts the hollow fiber ultrafiltration polysulfone membrane, so that the inlet pressure of the ultrafiltration membrane is controlled at 2MPa, and the hollow fiber material line velocity is 1.5m / s , to obtain the ultrafiltrate.
[0041] 4. Cool the ultrafiltrate to room temperature, crystallize for 4 hours, centrifuge, take the white crystals and dry them in vacuum (-0.085~-0.080MPa, 40~45℃) for 3 hours to obtain pharmaceutical grade phloroglucinol with a purity of 99.8 % (HPLC method).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com