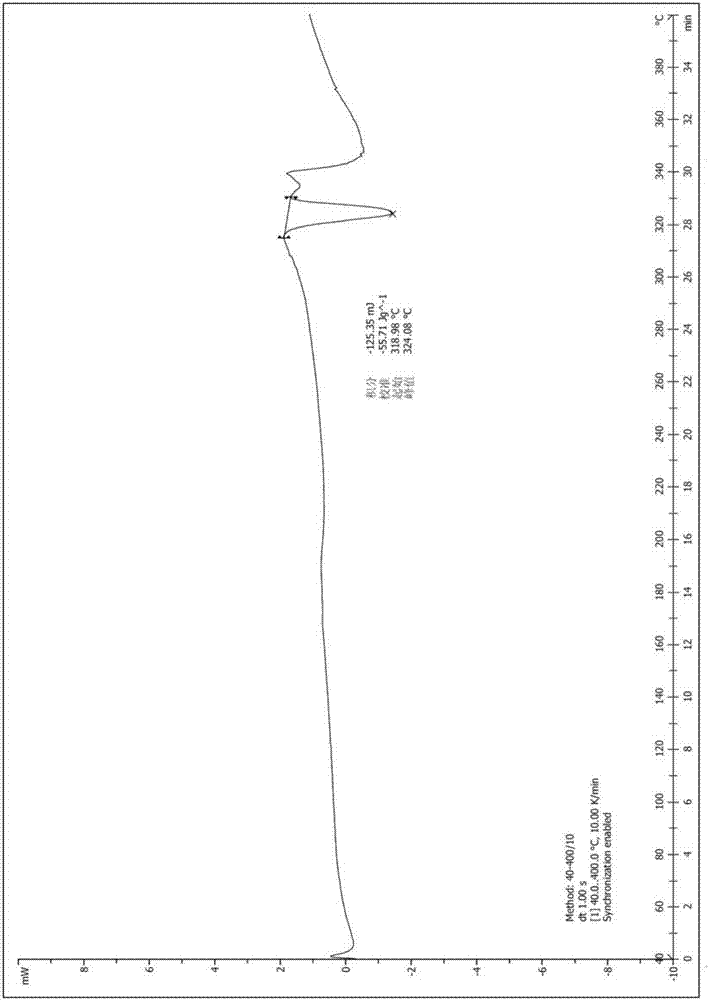
Patents
Literature
82 results about "Anastrozole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
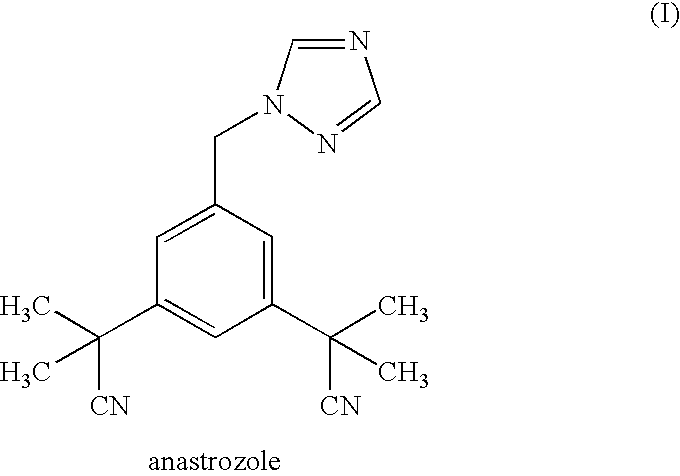
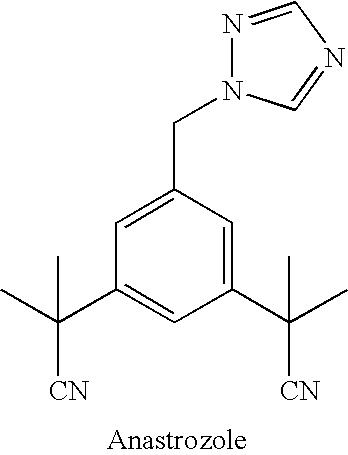
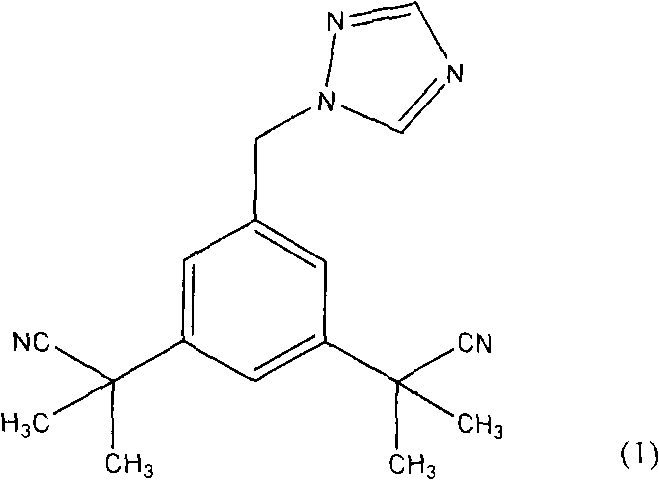
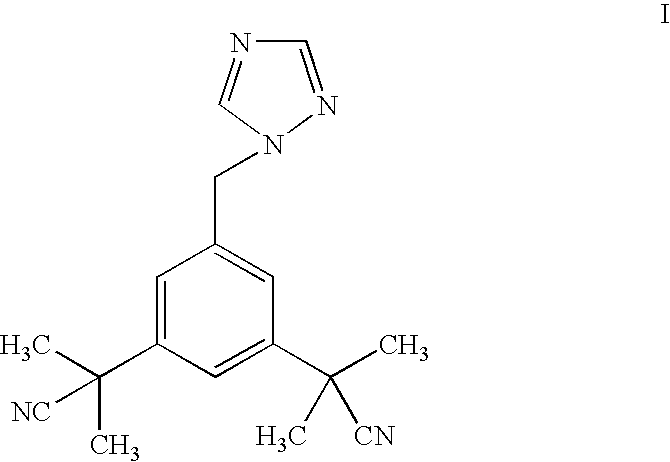
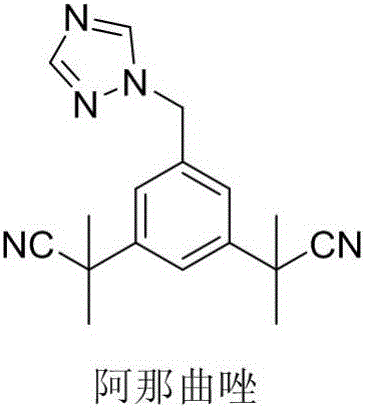
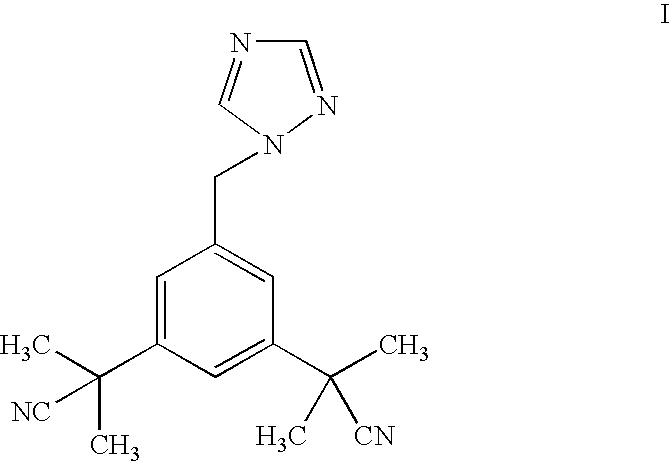
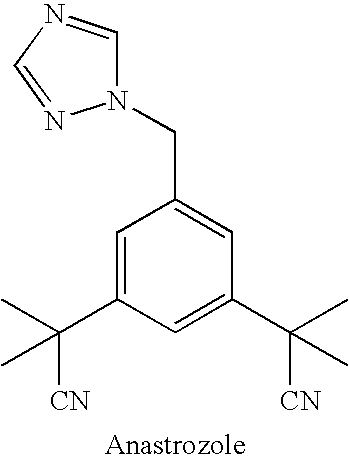
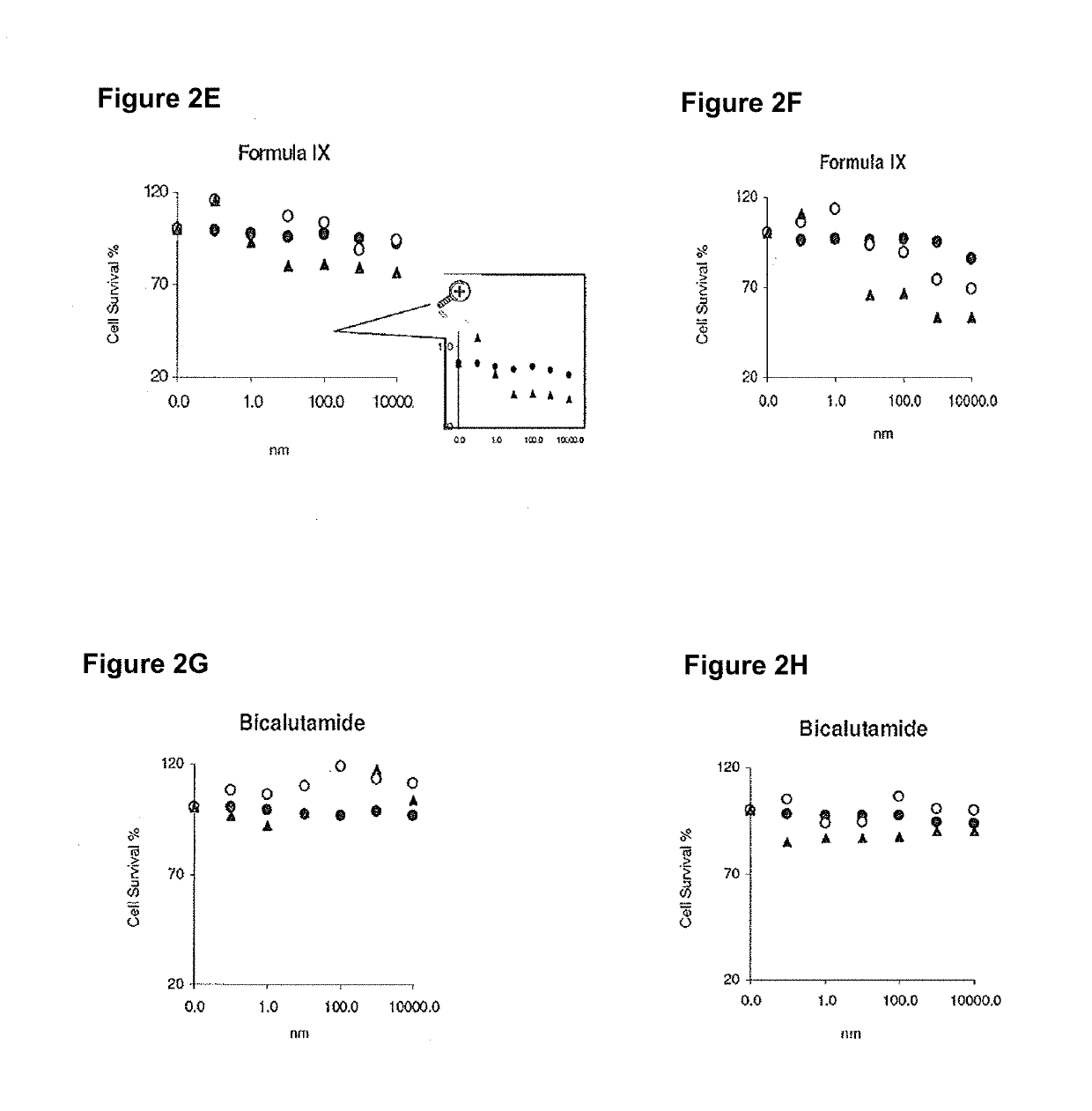
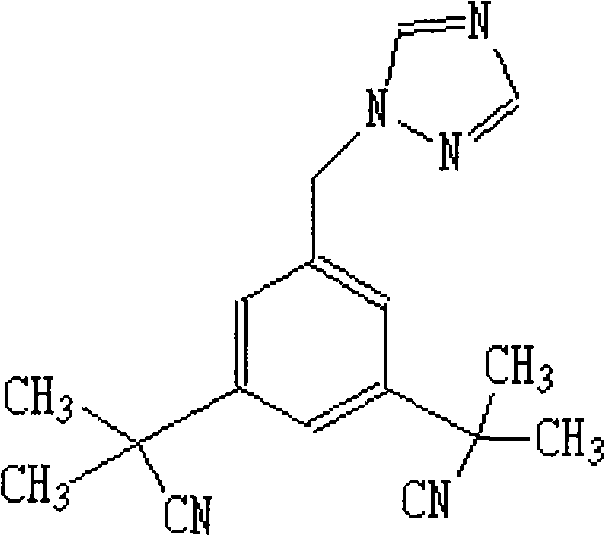
Anastrozole is used to treat breast cancer in women after menopause.
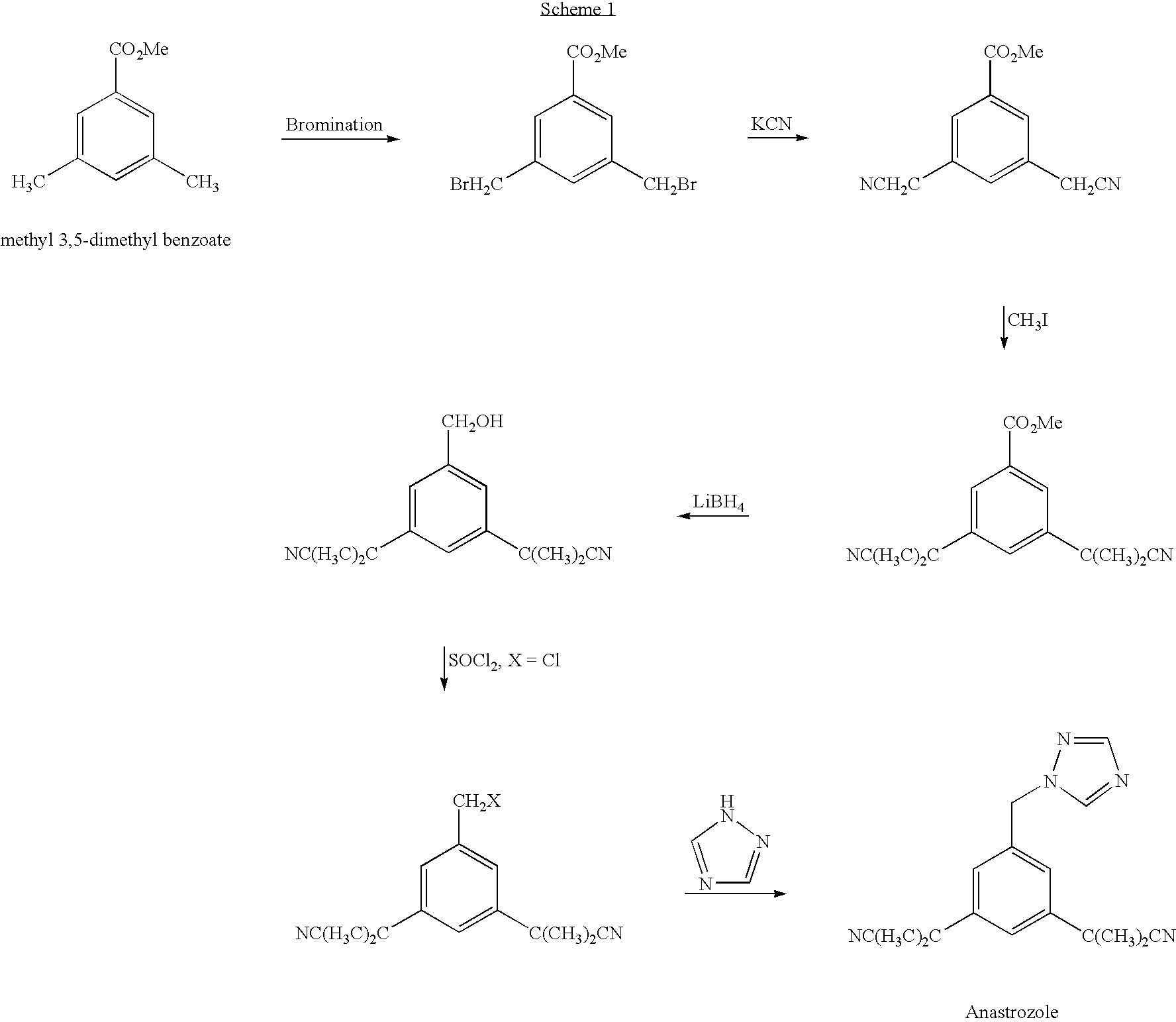
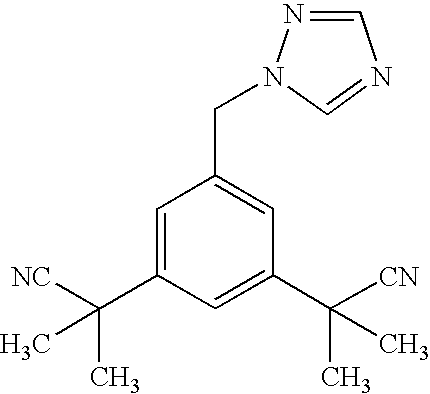
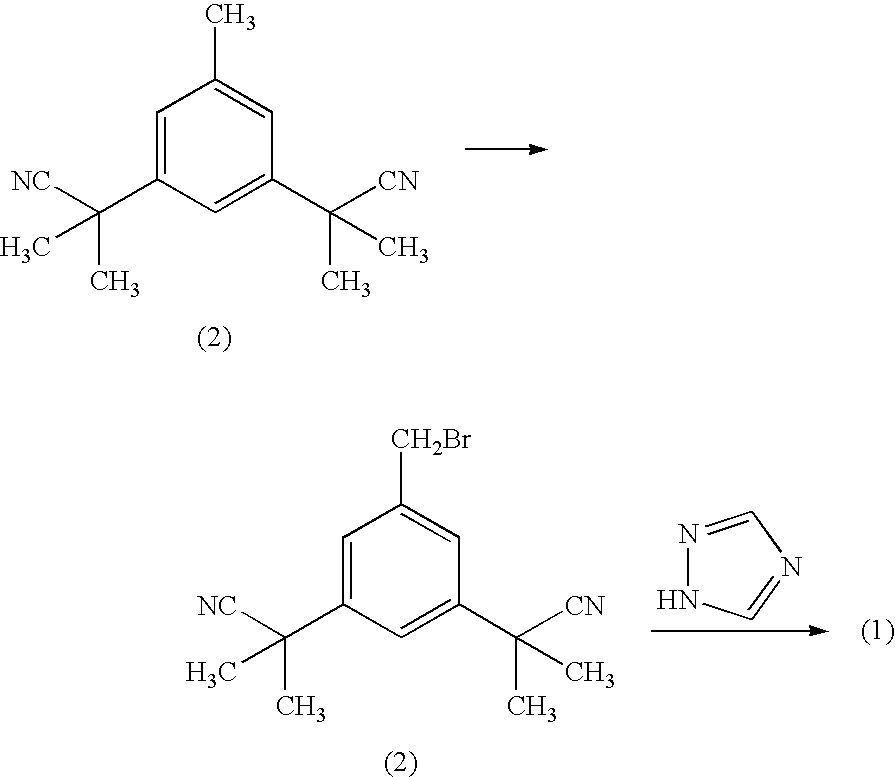
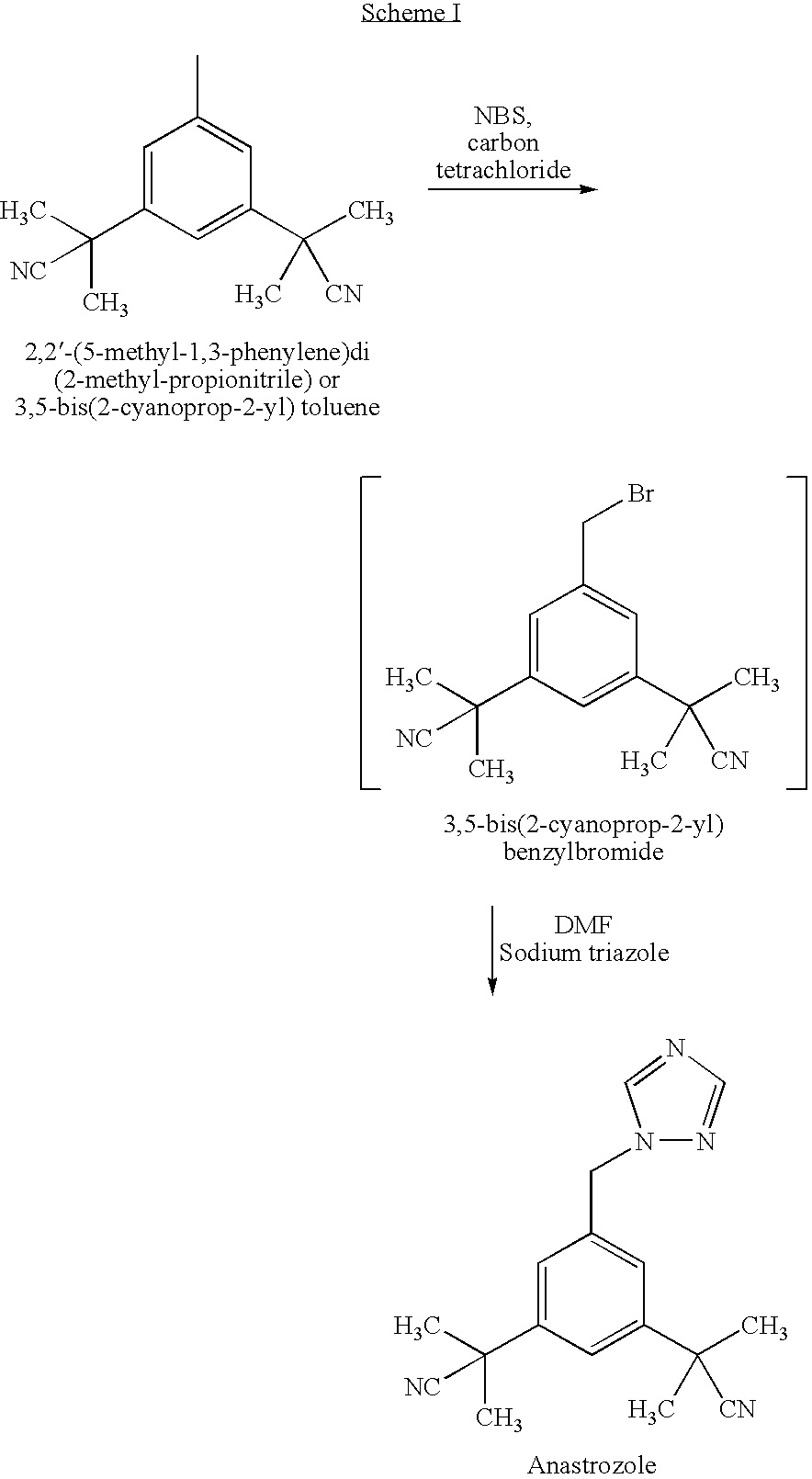
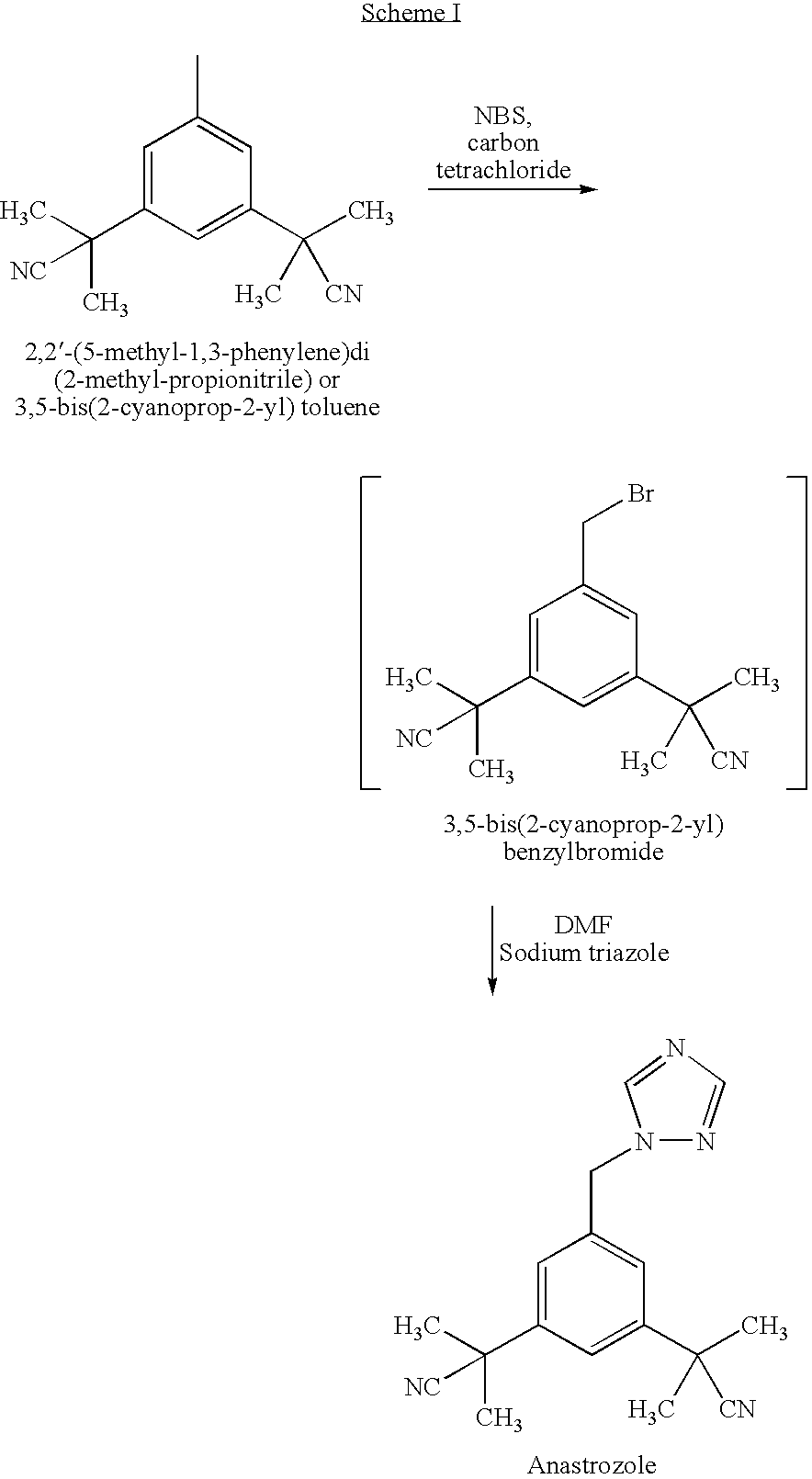
Novel processes for preparing substantially pure anastrozole
InactiveUS20060035950A1Straightforward and more purificationReduce in quantityBiocideCarboxylic acid nitrile preparationAnastrozoleSolvent
The present invention provides novel processes for purifying anastrozole, devoid of using liquid chromatography. The purification processes are via the isolated anastrozole salt forms, either by crystallization or by selective acidic extractions, and optionally in both cases, converting the purified anastrozole salt to anastrozole base. Also provided is an improved process for the synthesis of anastrozole, which is obtained by alkylating the isolated and purified starting material 3,5-bis(2-cyanoprop-2-yl)benzylbromide, the process being devoid of using toxic, hazardous and environmental unfriendly solvents and reagents.
Owner:CHEMAGIS
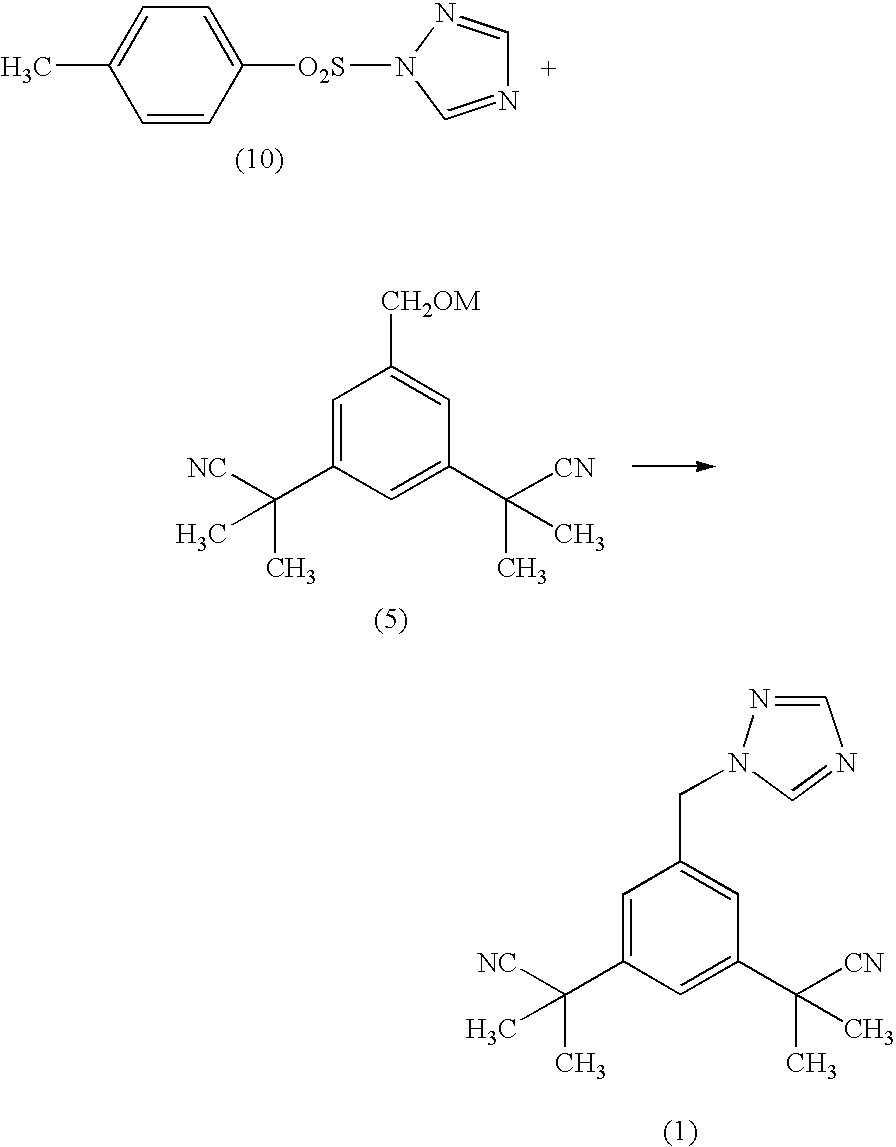
Process for making anastrozole
InactiveUS20080076933A1High purityEconomically and ecologically advantageousOrganic chemistryAntineoplastic agentsAnastrozoleStereochemistry
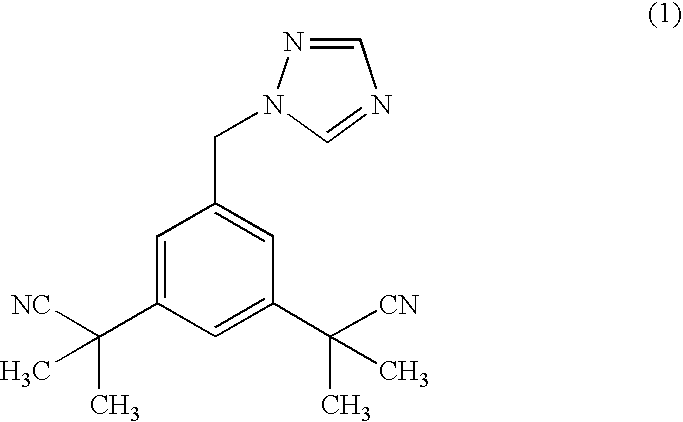
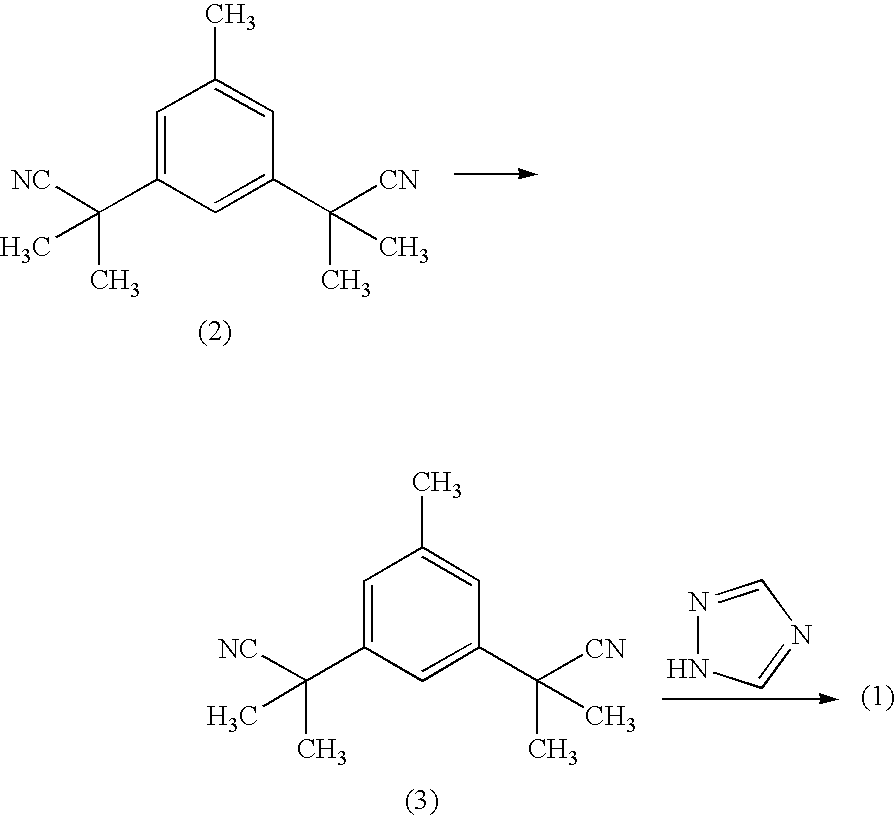
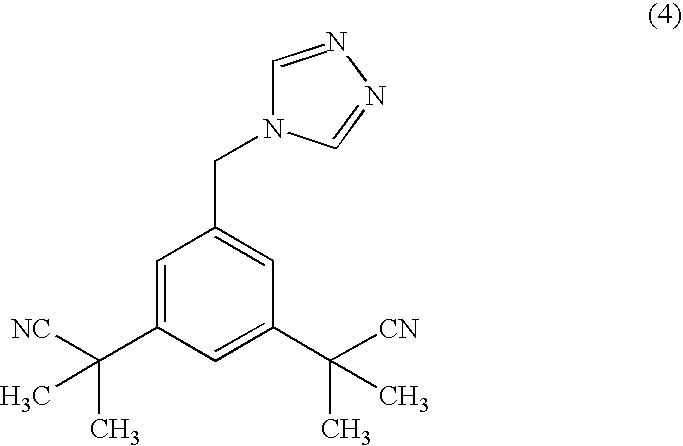
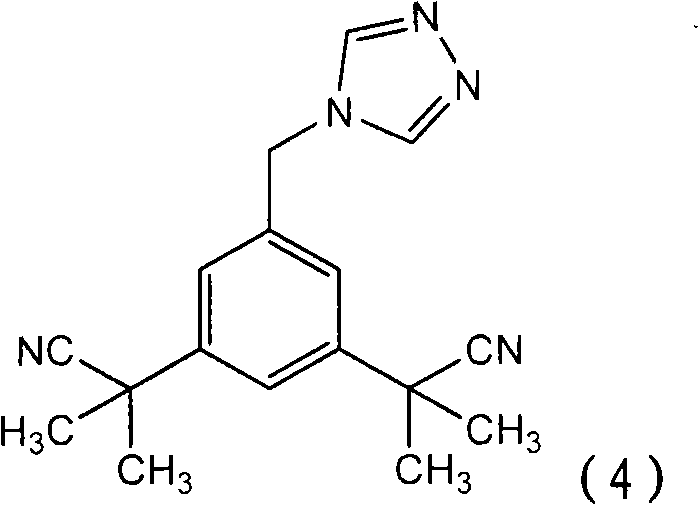
A process for making anastrozole using a 1-substituted triazole can reduce formation of the undesired iso-anastrozole. A typical process is represented by wherein compound (10) is a 1-substituted triazole and compound (1) is anastrozole.
Owner:SYNTHON BV
Process for Preparation of Anastrozole
Owner:FORMOSA LAB
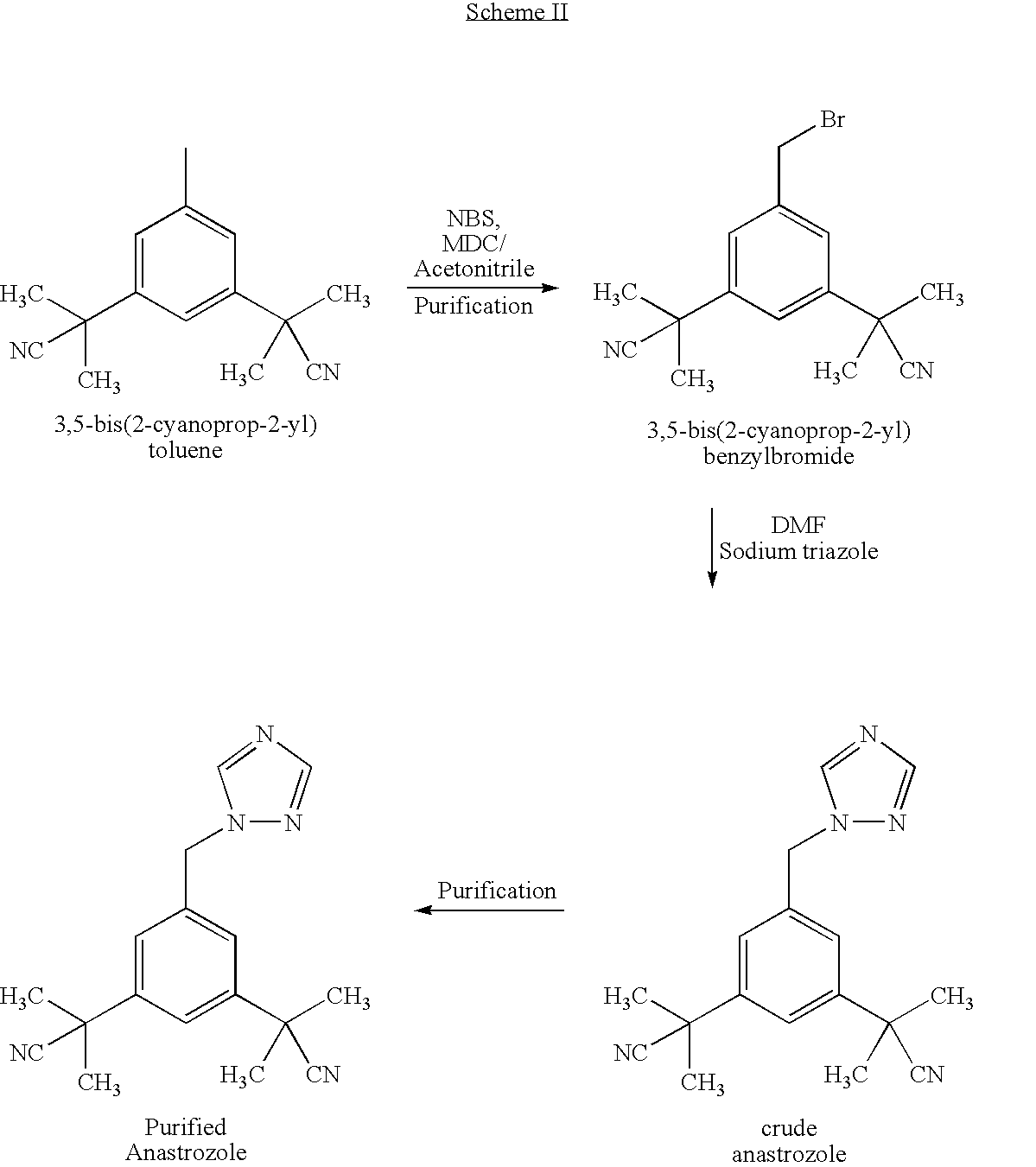
Process for purification of anastrozole
InactiveUS20070281982A1Good water solubilitySignificant purityBiocideOrganic chemistryAnastrozoleAlcohol
Anastrozole can be purified by crystallization from an aqueous-based solvent system. The aqueous-based solvent system can contain dilute acid, or an alcohol or both.
Owner:SYNTHON BV
Anastrozole dispersed tablet formulation
InactiveCN1634042APromote absorptionImprove Medication AdherenceOrganic active ingredientsPill deliveryClinical efficacyClinical trial
The invention belongs to medical technology, in particular to a dispersible tablet form of anastrozole. Through a large number of clinical investigations, we have overcome the technical prejudice that anastrozole does not need to be developed into dispersible tablets in the prior art, and obtained the present invention. Clinical experiments have confirmed that the adverse reactions of the present invention are significantly reduced, and the compliance of medication is significantly improved. Obtained unexpected clinical efficacy. It has the characteristics of low production cost, stable preparation, fast absorption and high bioavailability. The invention is suitable for treating advanced breast cancer of postmenopausal women, and provides a good therapeutic drug dosage form for breast cancer patients.
Owner:LUNAN PHARMA GROUP CORPORATION
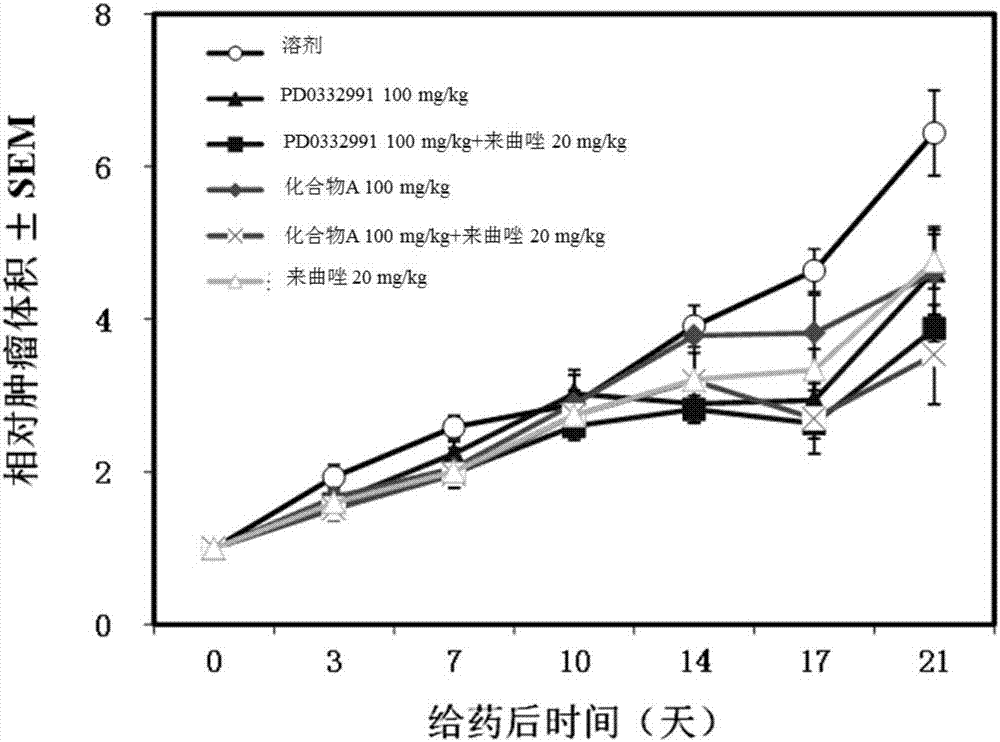
Applications of combined CDK4/6 inhibitor and aromatase inhibitor in preparing medicines for treating breast cancer
The invention relates to applications of combined CDK4 / 6 inhibitor and aromatase inhibitor in preparing medicines for treating breast cancer. Specifically, the CDK4 / 6 inhibitor is a compound A or medicinal salt of the compound A, and the aromatase inhibitor is selected from one or more of formestane, exemestane, fadrozole, letrozole, vorozole and anastrozole.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Fluorouracil containing anti-cancer sustained-release injection
InactiveCN101234084AEasy to operateGood repeatabilityOrganic active ingredientsPharmaceutical delivery mechanismPolyethylene glycolSuspending Agents
The invention relates to anticancer sustained release injection which comprises sustained release microspheres and menstruum, wherein, the sustained release microspheres comprise anticancer active components and sustained release auxiliary material; the menstruum is special menstruum that contains suspending agent. The anticancer active components are fotemustine, nimustine, carmustine or combination of bendamustine and mitozolomide, docetaxel, etoposide, teniposide, vinblastine, anastrozole, tamoxifen, fluorouracil or mitomycin C; the sustained release auxiliary material is polylactic acid and polylactic acid copolymer, polyethylene glycol and polylactic acid copolymer of polyethylene glycol, terminal carboxyl group polylactic acid copolymer, EVAc, fatty acid and decanedioic acid copolymer, etc.; viscosity of the suspending agent is 100cp-3,000cp (at 25 DEG C-30 DEG C), and the suspending agent is selected from sodium carboxymethylcellulose, etc. The sustained release microspheres can also be made into sustained release implant; the injection or implant is injected or placed in or around tumor so as to reduce general reaction of the drug and selectively improve and keep local concentration for about 30-50 days. The anticancer sustained release injection can be used solely and can also promote anti-tumor effects of non-operative treatments, such as chemotherapy and / or radiotherapy, etc.
Owner:JINAN SHUAIHUA PHARMA TECH
Process for purification of anastrozole
Anastrozole can be purified by crystallization from an aqueous-based solvent system. The aqueous-based solvent system can contain dilute acid, or an alcohol or both.
Owner:SYNTHON BV
Pharmaceutical formulation comprising bicalutamide
Owner:ASTRAZENCA UK LTD
Oral medicinal composition containing anastrozole and preparation technology thereof
Disclosed is a prescription composition of a drug compound containing granules for oral administration and the preparation process, characterized in that the granule contains anastrozole, filler and other pharmaceutical excipient. The anastrozole is produced into suitable preparation with good bioavailability and drug stability in the preparation process.
Owner:HAINAN SHENGKE LIFE SCI RES INST
Mixed granulating equipment and application of mixed granulating equipment for preparing solid preparation
InactiveCN104367478AReasonable particle size distributionSimple structurePharmaceutical product form changeAnastrozoleAgricultural engineering
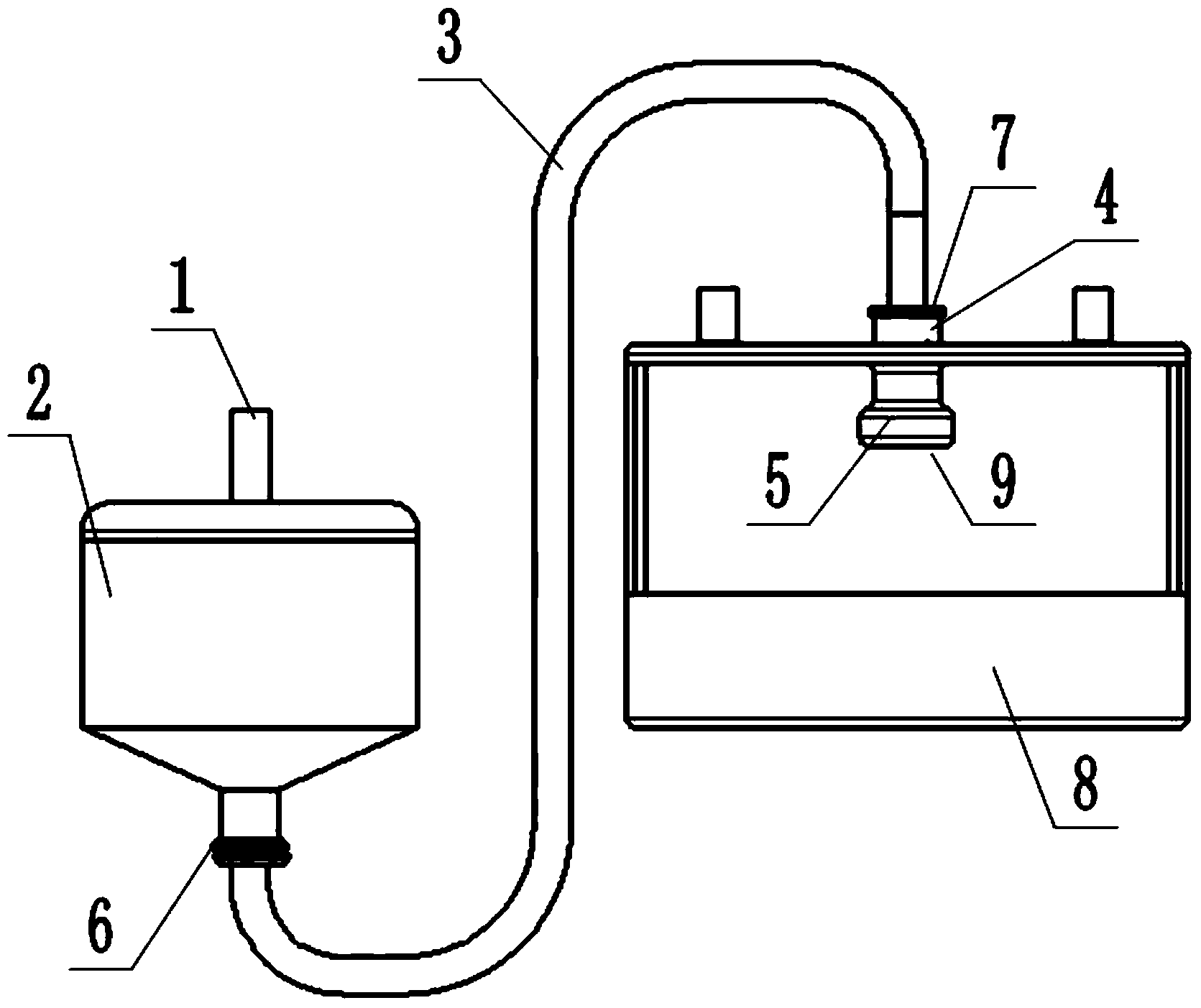
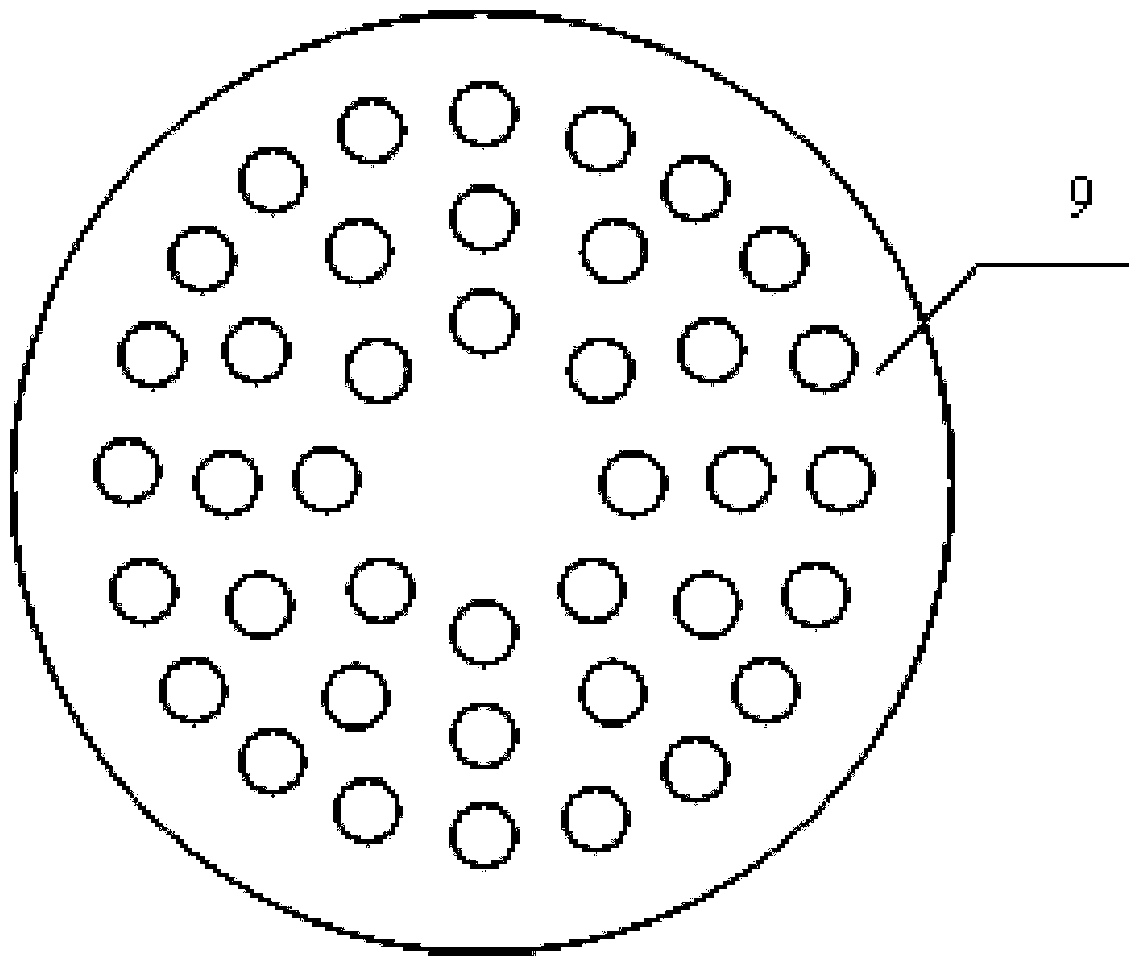
The invention discloses granulating equipment. The granulating equipment comprises a pressure tank and a mixed granulating machine, the pressure tank is arranged on the left side of the mixed granulating machine, the upper portion of the pressure tank is provided with a compressed air connector, the bottom of the pressure tank is provided with a connection hose, and the connection hose is used for being connected with the pressure tank and the mixed granulating machine. The granulating equipment further comprises a sprinkler, the top of the sprinkler is provided with holes, and the sprinkler is arranged on the upper half portion of the mixed granulating machine and is connected with the connection hose through a connection pipe. The granulating equipment is suitable for preparing solid preparations like capecitabine tablets, moxifloxacin hydrochloride tablets, Letrozol tablets, anastrozole tablets, repaglinide tablets, acarbose chewable tablets and tacrolimus capsules. The granulating equipment is easy and convenient to operate; the prepared particles are good in mobility and compressibility; the manufactured preparations are smooth and attractive in appearance and free of spots on surfaces, meanwhile, the quality indexes like disintegration time, the dissolution rate and the content uniformity all accord with or exceed inner quality standards, and finally the solid preparations with good quality can be obtained.
Owner:HANGZHOU ZHONGMEI HUADONG PHARMA
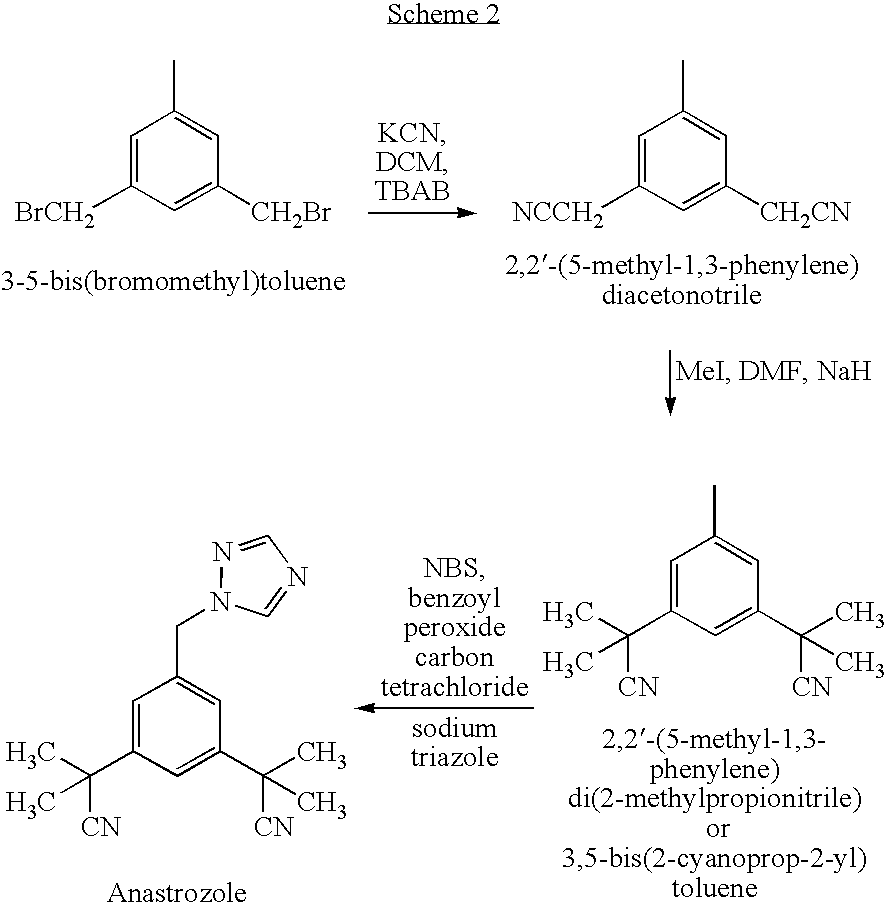
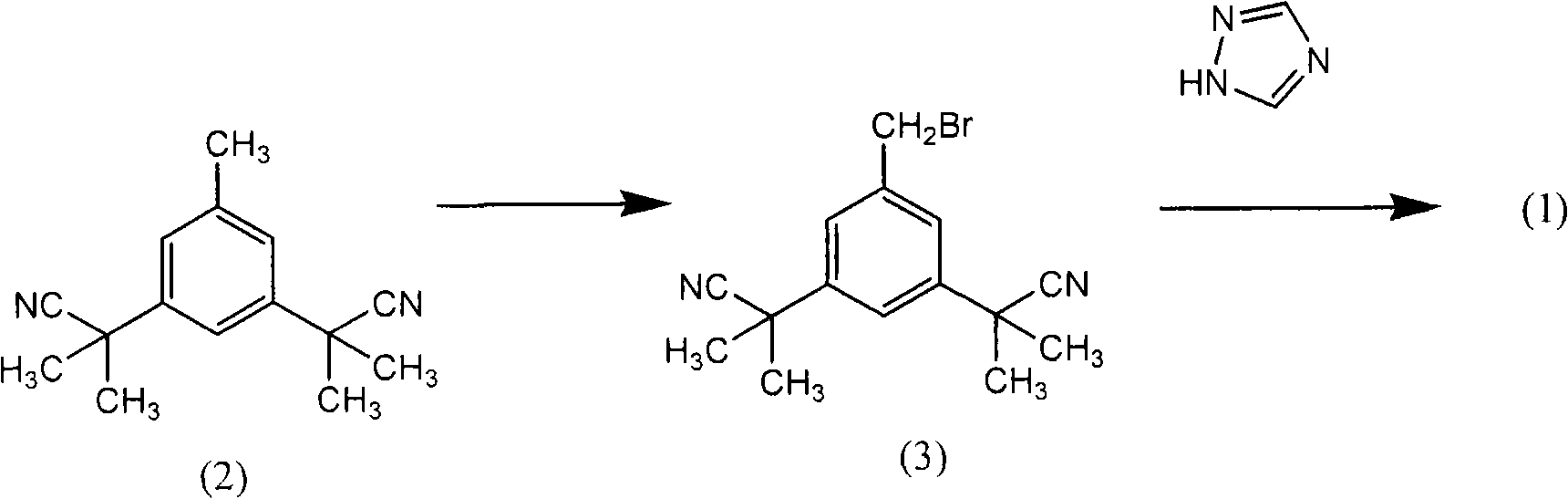
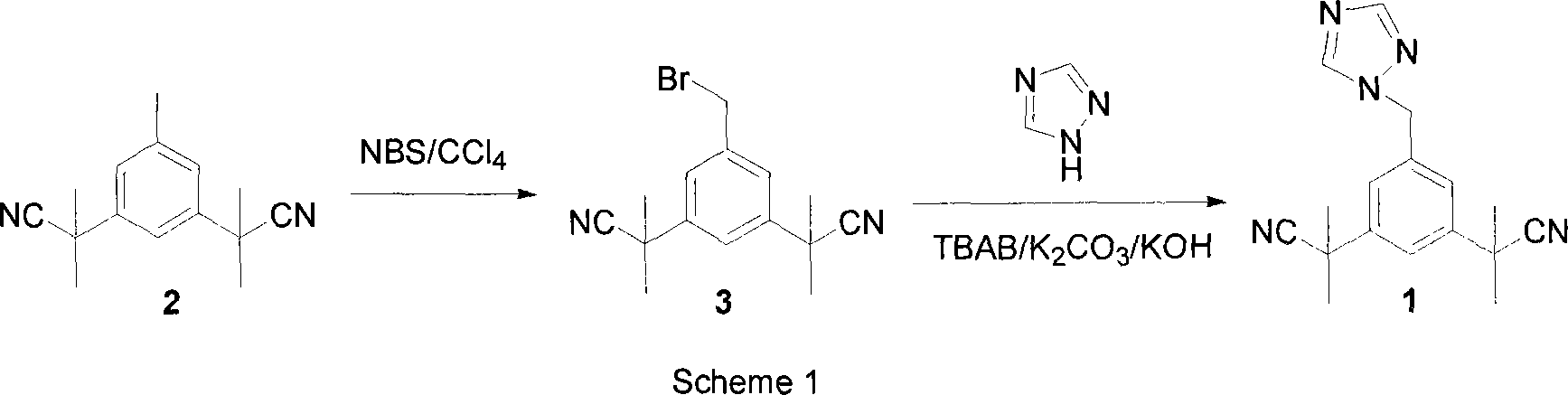
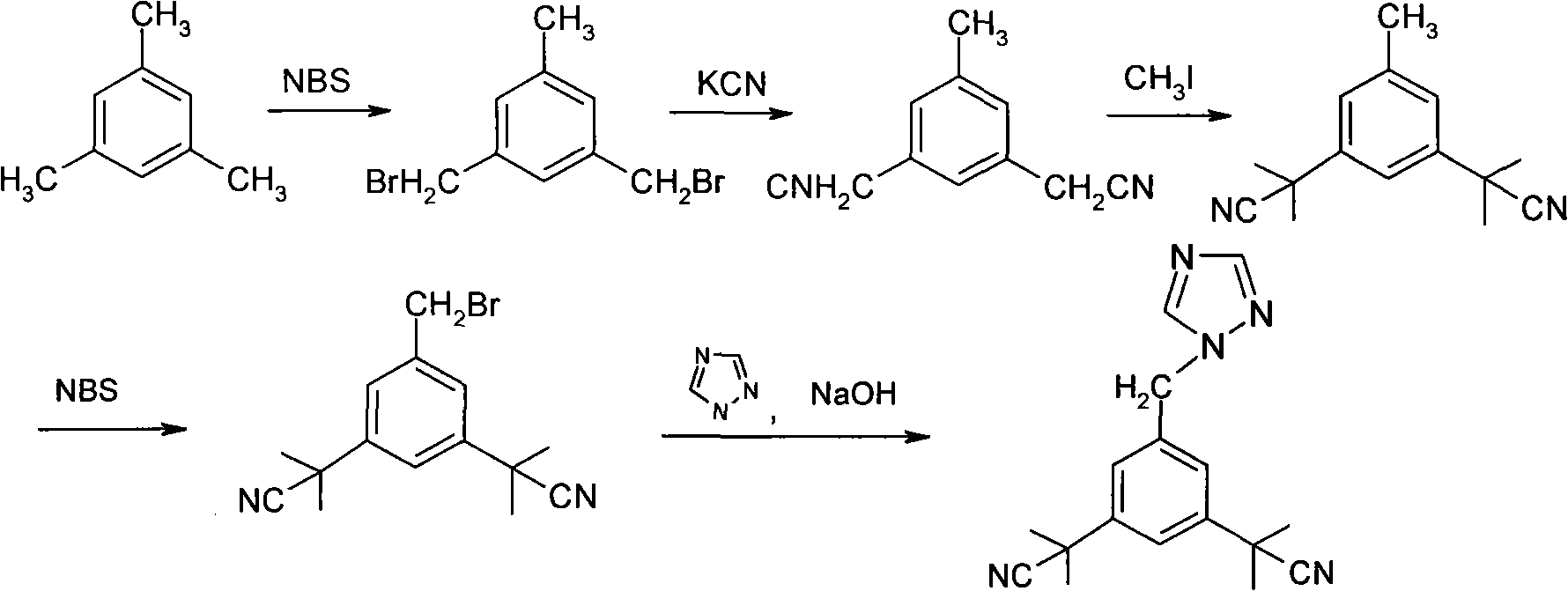
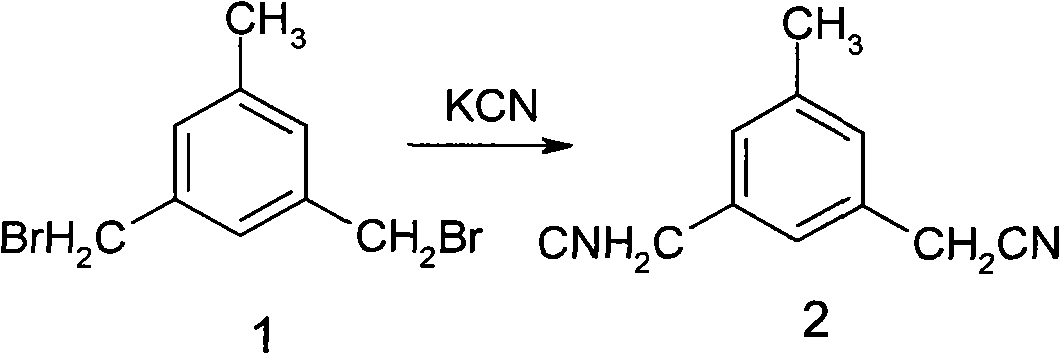
Preparation of 3,5-di(2-cyano-isopropyl)-toluene
ActiveCN101239932AReduce usageHigh purityPreparation by cyanide reactionChemical synthesisP-Toluenesulfonic acid
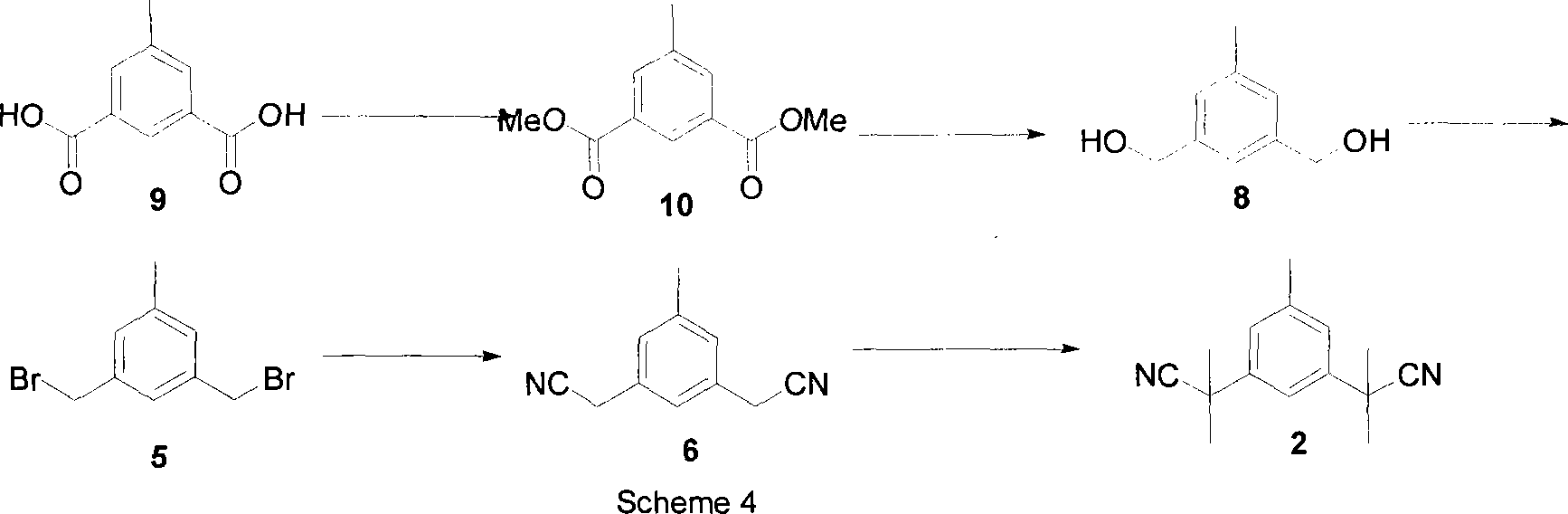
The invention relates to a novel synthesis process of pharmaceutical intermediate 3, 5-bi(2-cyanoindole-isopropyl)-toluene, belonging to the chemical synthesis technology field. The invention uses 5-methyl-1, 3-dimethyl phthalate as the material, synthesizes anastrozole intermediate 3, 5-bi(2-cyanoindole-isopropyl)-toluene by esterification, reduction, brominating, cyaniding and methylation reaction. The reduction method using borohydrides is performed in aether solvent, the method can completely avoid using high-toxicity solvent such as CCl4 or the like; in the methylation reaction, expensive iodomethane is replaced by p-toluenesulfonic acid methyl; the purity of product 3, 5-bi(2-cyanoindole-isopropyl)-toluene (2) is high.
Owner:苏州第壹制药有限公司
Temperature controlled sustained-release injection containing steroids anti-cancer drugs
InactiveCN101273963APharmaceutical delivery mechanismPharmaceutical non-active ingredientsGoserelinTherapeutic effect
The invention relates to a temperature-controlled sustained-release injection containing a hormone anti-cancer drug, which comprises the anti-cancer drug, an amphiphilic block copolymer, a solvent and a certain amount of drug release regulator, wherein, the mixture of the amphiphilic block copolymer and a solvent without organic solvent has the temperature-sensitive gelatinization feature, which is flowable liquid in the environment that is lower than the body temperature and can be automatically converted to the water-insoluble gel that can not flow and be biodegradable for absorption in an endotherm, and the water-insoluble gel can allow the contained hormone anti-cancer drug to have the local sustained release in a tumor and maintain the effective drug concentration for a plurality of weeks to a plurality of months; the viscosity of the temperature-controlled sustained-release injection is 10cp to 3000cp ( at 5 DEG C to 30 DEG C ), and the gelatinization temperature is 35 DEG C to 37 DEG C. The sustained-release injection can be injected in the tumor or the tumor periphery or be arranged in the postoperative tumor cavity, thus significantly reducing the systemic reaction of the drug, selectively strengthening the treatment effects of chemotherapy, radiotherapy and other non-surgical therapies, and being used for the treatment of the tumors in different stages. The anti-cancer drug can be triptorelin, goserelin, leuprorelin, anastrozole, idoxifene, tamoxifen and other hormone anti-cancer drugs.
Owner:SHANDONG LANJIN PHARMA +1
Testosterone Booster Transdermal Compositions
InactiveUS20150065426A1Reduce riskPromote absorptionOrganic active ingredientsPeptide/protein ingredientsPhospholipidBULK ACTIVE INGREDIENT
Formulations and methods for transdermal drug delivery compositions that include synergistic combination of three pharmaceutical active ingredients (APIs), such as testosterone, anastrozole, and HCG are disclosed. TBTC of the present disclosure may be indicated for reducing symptoms of testosterone deficiency. Disclosed TBTC may include permeation enhancers that may improve penetration of testosterone, anastrozole, and HCG in human skin. Permeation enhancer compositions within TBTC may include oils from amazon rainforest such as Pracaxi oil, Plukenetia volubilis seed oil, Inaja oil, and Patauá oil, which includes behenic and oleic fatty acids that may provide penetration power. TBTC may include organic solvents as transdermal penetration enhancers. Additionally, TBTC may include physiological lipids, phospholipids, and one or more butters rich in linoleic acid and linolenic acid that may also provide penetration power with restorative benefits to the skin.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
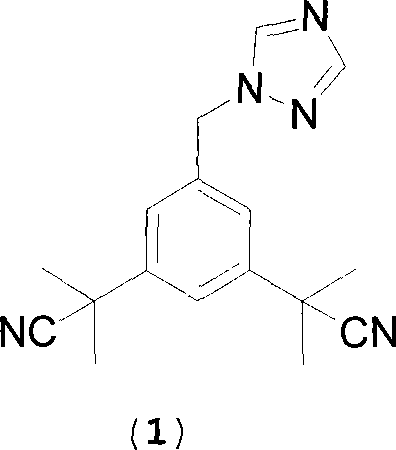
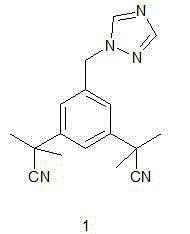
Method for preparing anastrozole
ActiveCN103554041ASimple reaction conditionsQuality improvementOrganic chemistryBenzeneBenzoyl bromide
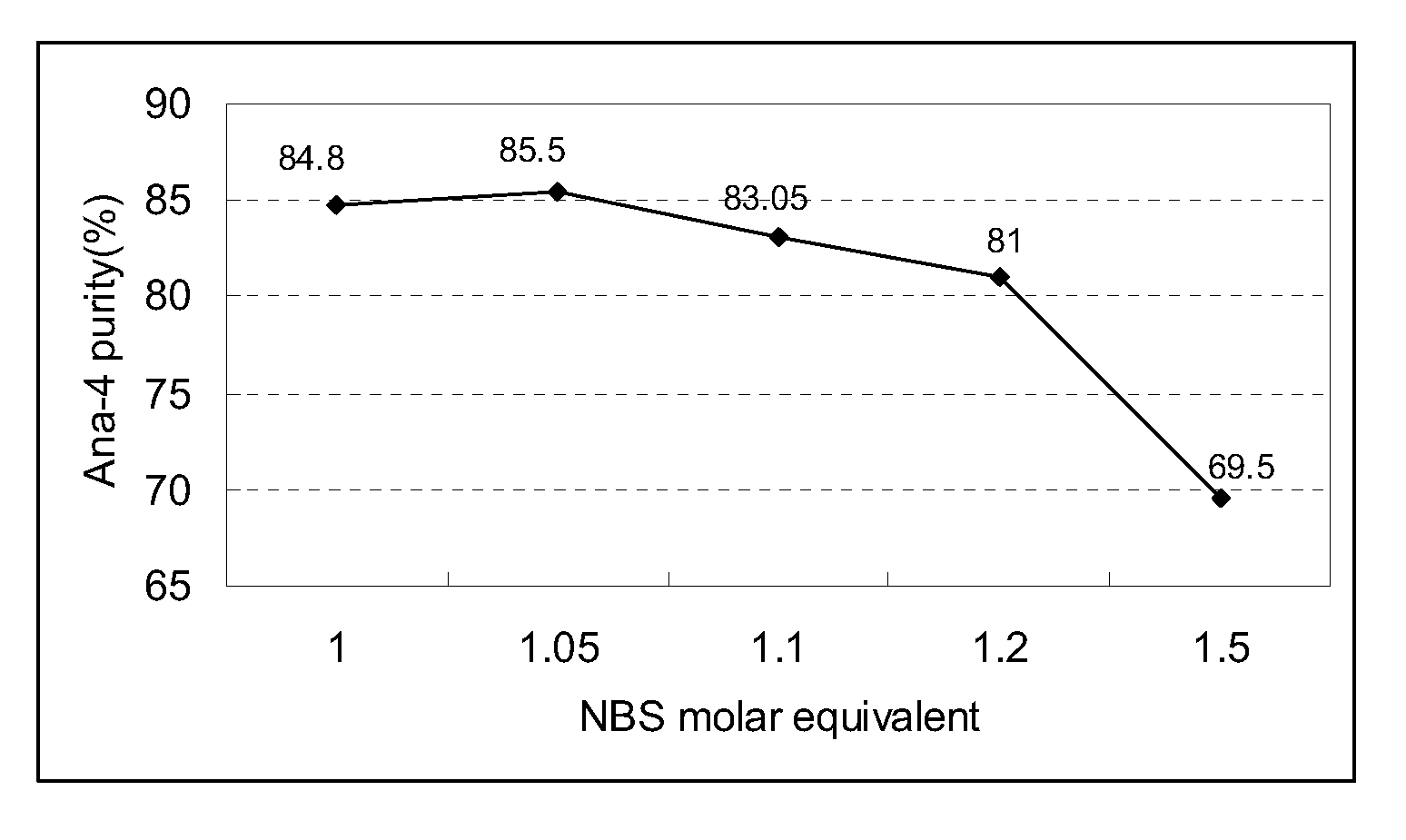
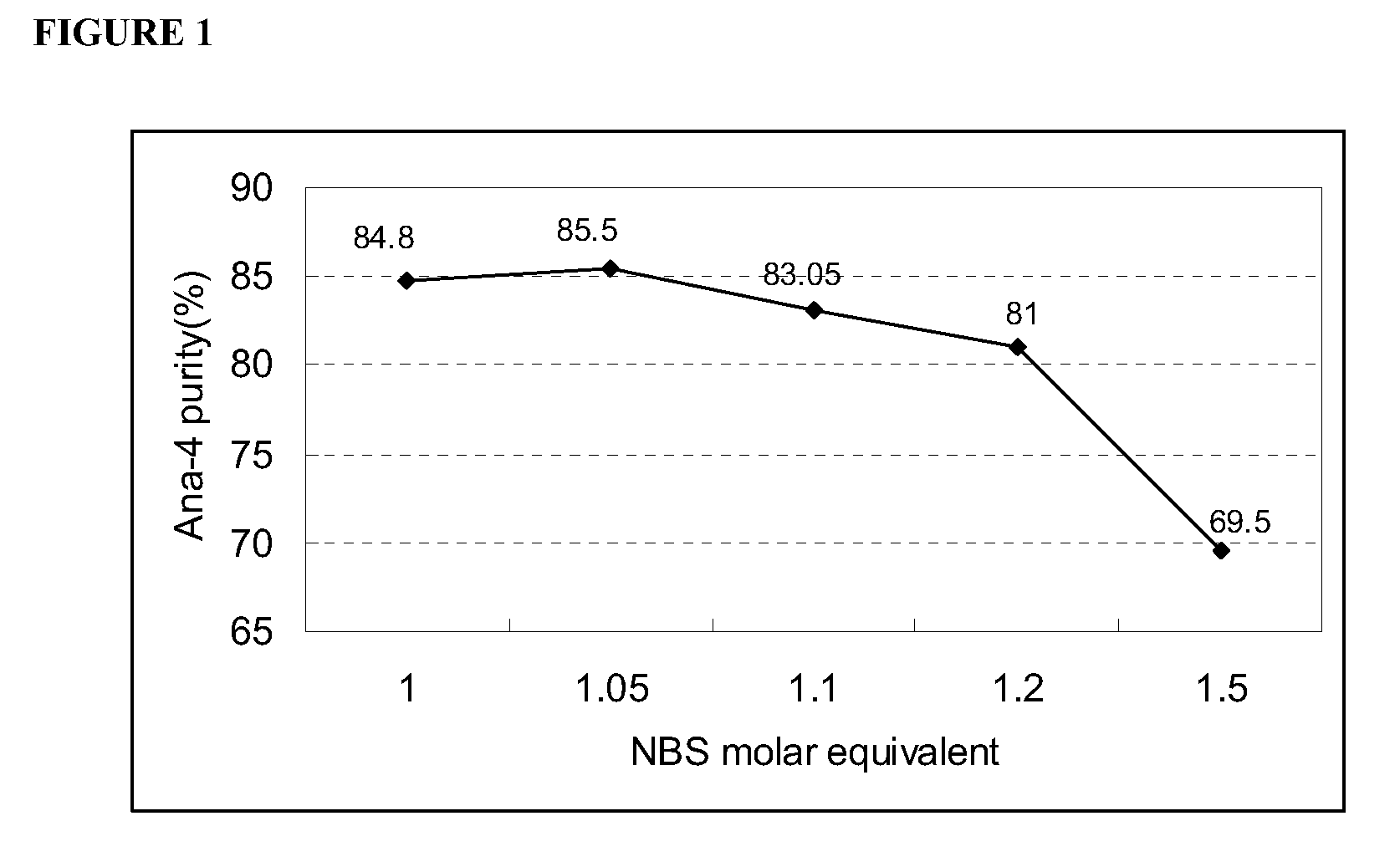
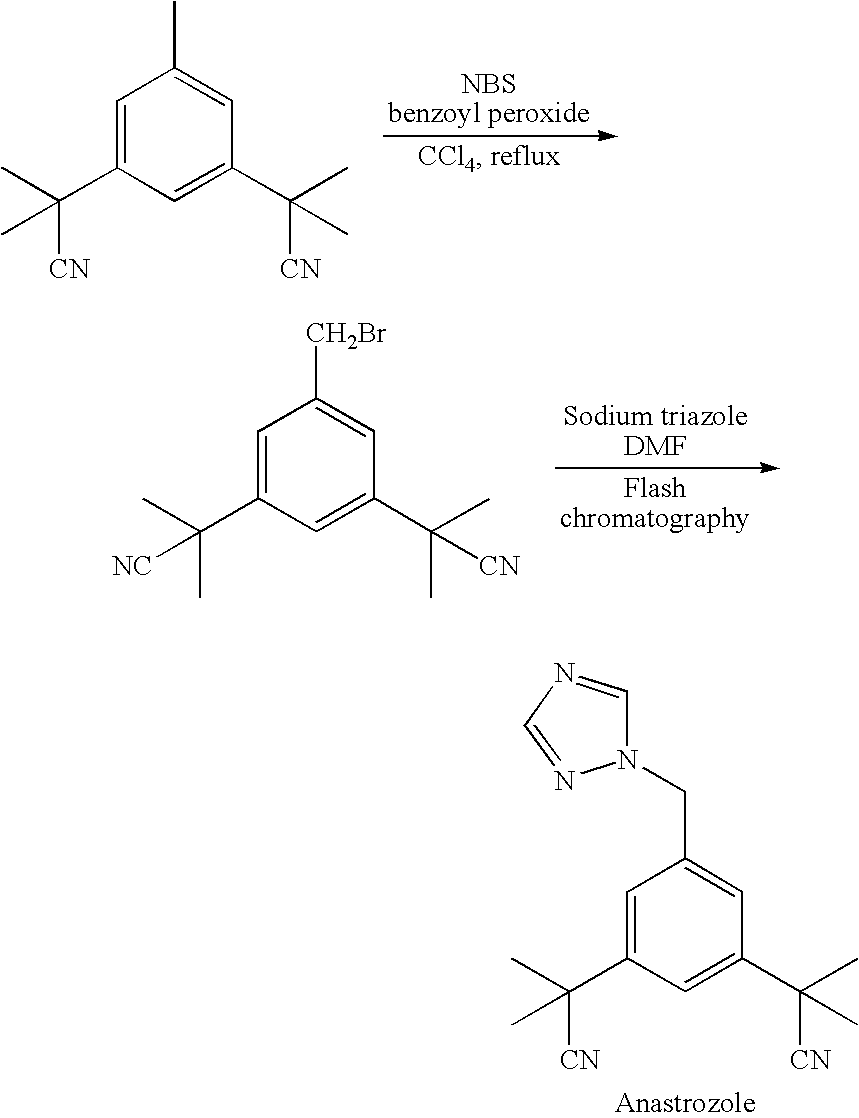
The invention discloses a method for preparing anastrozole. The anastrozole is prepared by using a,a,a',a',5-pentamethyl-1,3-diacetonitrile benzene as a starting material, bromizing under the action of a brominating agent NBS to generate a midbody 3,5-bi[(2,2-dimethyl)cyan methyl]-benzyl bromide, and catalyzing and condensing the midbody with 1,2,4-triazole in water and organic solvent by phase transfer to prepare anastrozole. The method has the advantages of simplicity in operation, mild reaction condition, high yield and high purity of products, and is suitable for industrial production of anastrozole.
Owner:JIANGSU QINGJIANG PHARMA
Transdermal Delivery of Anastrozole for Systemic Effect
Formulations and methods for transdermal drug delivery compositions that include anastrozole are disclosed. Transdermal anastrozole compositions of the present disclosure may be indicated for treating testosterone deficiency. Disclosed transdermal anastrozole compositions may include permeation enhancers that may improve penetration of anastrozole in human skin. Permeation enhancers within transdermal anastrozole compositions may include oils from Amazon rainforest such as Pracaxi oil, Plukenetia volubilis seed oil, Inaja oil, and Patauá oil, which includes behenic and oleic fatty acids that may provide penetration power. Transdermal anastrozole may include organic solvents as transdermal penetration enhancers. Additionally, transdermal anastrozole compositions may include physiological lipids, phospholipids, and one or more butters rich in linoleic acid and linolenic acid that may also provide penetration power with restorative benefits to the skin.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
External patch containing anastrozole and application thereof
InactiveCN105055379ASmall toxicityAvoid first pass effectOrganic active ingredientsAntineoplastic agentsContinuous useAnastrozole
The invention relates to an external patch containing anastrozole and a preparation method thereof. The external preparation uses anastrozole as a main drug, and is used for treating breast cancer. The external patch containing anastrozole is absorbed in a percutaneous manner, is uniform in drug release and mild and durable in action. One patch can be continuously used for 7 days. The patch directly acts on skin without passing through gastrointestinal tracts, so that the first pass effect of liver can be avoided, adverse response is reduced, and the occurrence rate of adverse responses is reduced. The external patch has good patient compliance, and is convenient to use and carry.
Owner:徐静 +1
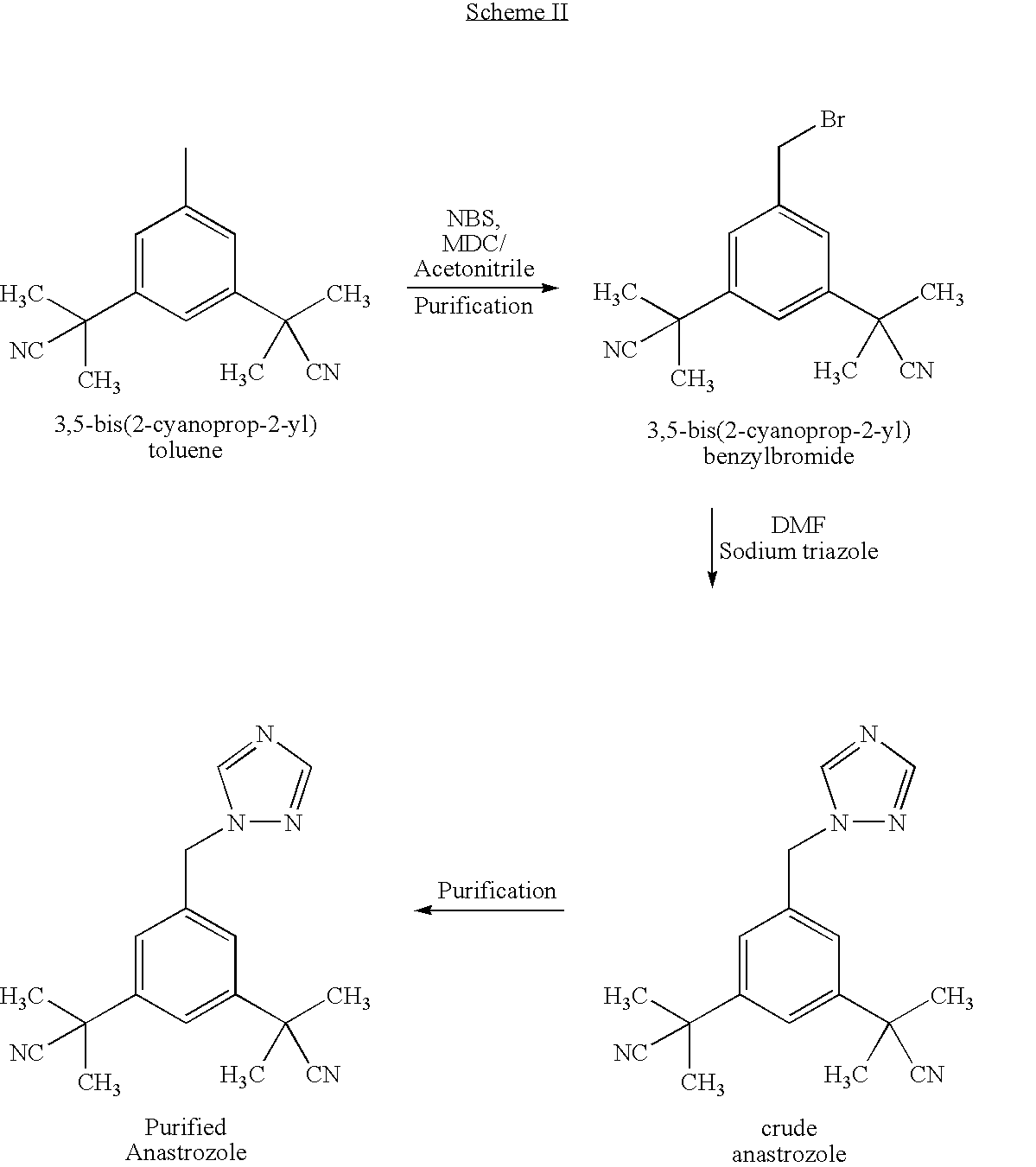
Process for the Preparation of Pure Anastrozole
A process for the preparation of anastrozole which comprises: a) brominating 3,5-bis(2-cyanoprop-2-yl)toluene (II) in an organic solvent using a brominating agent to obtain 3,5-bis(2-cyanoprop-2-yl)benzylbromide (III); b) heating the reaction mass of step a) to the reflux temperature of the organic solvent for a period of time no longer than 3 hours; c) isolating and purifying the bromo intermediate (III) using an organic solvent; d) alkylating the bromo intermediate in the presence of a base, optionally a phase transfer catalyst, a 1,2,4-triazole and an organic solvent to obtain anastrozole; and e) isolating and purifying the anastrozole from an organic solvent.
Owner:CIPLA LTD
Preparation method of anastrozole
The invention provides a preparation method of anastrozole. The preparation method comprises the following steps that 1, 5-bromomethyl-a,a,a',a'-tetramethyl-1,3-xylylene cyanide and 4-amino-1,2,4-triazole are taken as raw materials to react in an organic solvent, and crystallization is conducted to obtain an intermediate I, that is, a,a,a',a'-tetramethyl-5-[(4-amino-1,2,4-triazol)-methylene]-1,3-xylylene cyanide bromide; 2, the intermediate I is dissolved into a solvent, a little amount of water, acid and a metal catalyst are added, sodium azide is added in batches in a reflux state, immediate filtration is conducted after a complete reaction, a mother solution is concentrated until the volume of the solution is 0.25-0.75 times of the original volume of the mother solution, cooling crystallization is conducted, solids are filtered and subjected to PH regulation and extraction, and concentration is conducted to obtain a crude anastrozole product; 3, the crude anastrozole product is dissolved into an alcohol solvent, decoloring and cooling crystallization are conducted, and the fine anastrozole product is obtained. According to the preparation method, iso-anastrozole impurities influencing the medicinal efficacy are prevented from being generated from the source, no iso-anastrozole is detected out, the remaining single impurity content is less than 0.05%, and the remaining total impurity content is less than 0.2%.
Owner:JIANGSU HAICI BIOLOGICAL PHARMA CO LTD OF YANGTZE RIVER PHARMA GRP +1
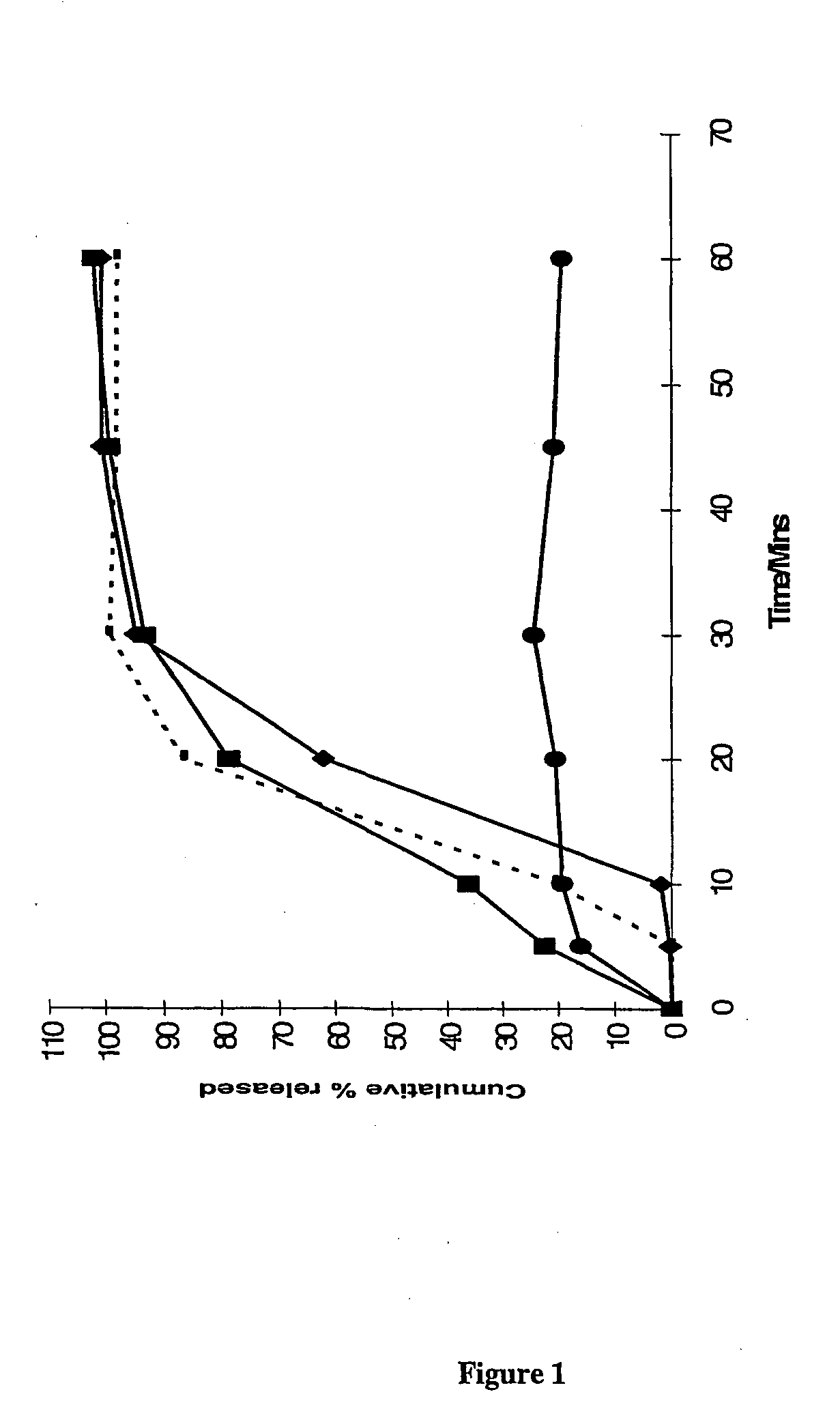
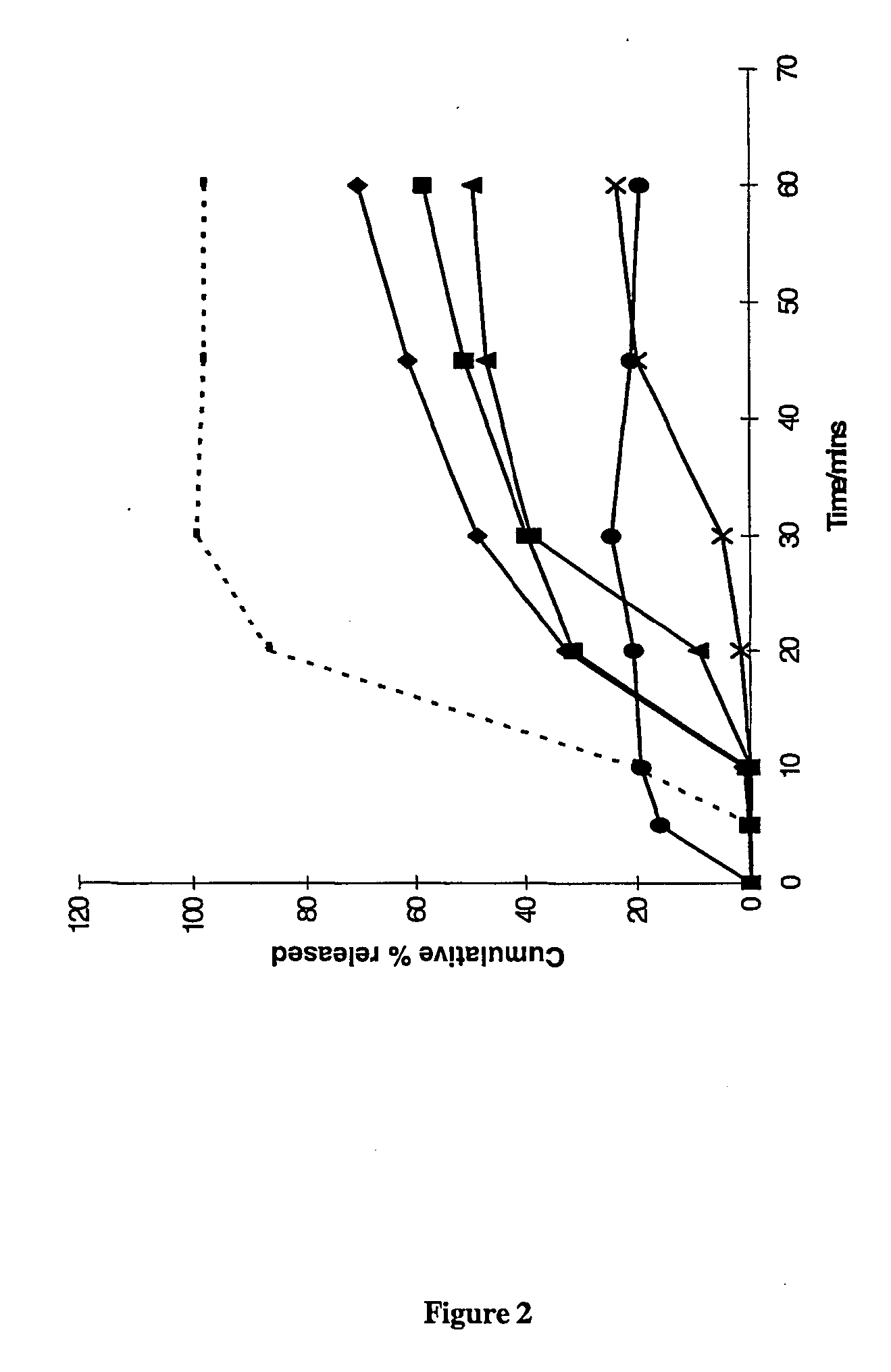
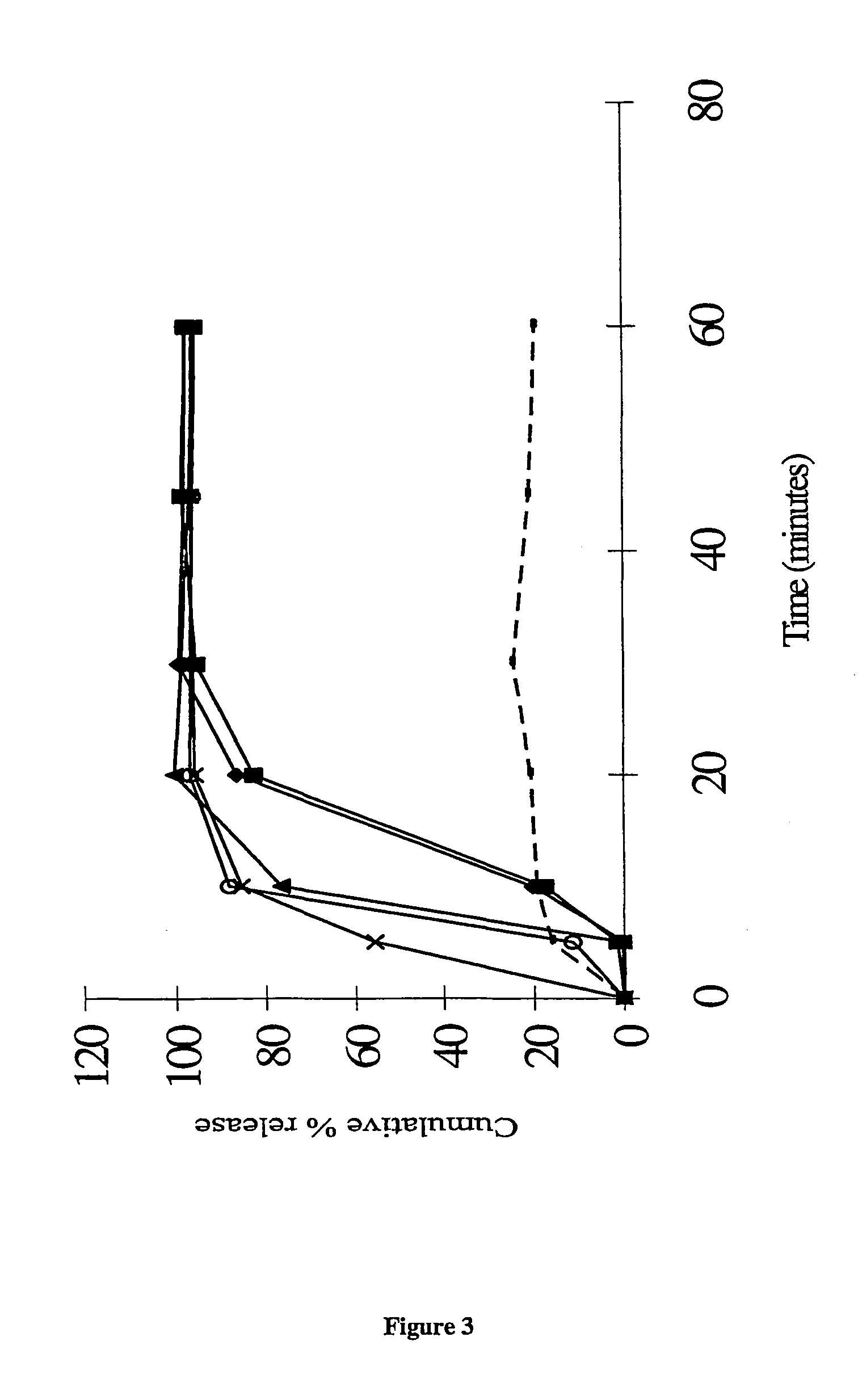
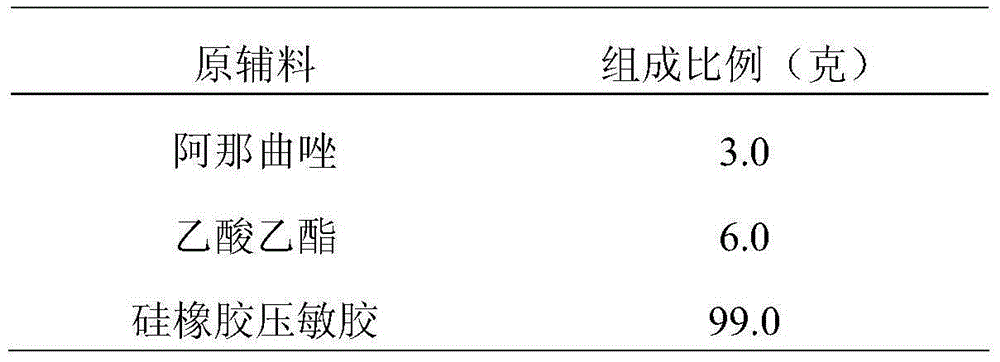
Anastrozole controlled release patch and preparation method thereof
InactiveCN101513397AImprove adhesionImprove flexibilityOrganic active ingredientsPharmaceutical non-active ingredientsSide effectTackifier
The invention belongs to the field of pharmaceutical technology, disclosing an anastrozole controlled release patch and a preparation method thereof. The invention comprises a back lining layer, a medicine reservoir and an anti-sticking layer, wherein, the medicine reservoir comprises remedium cardinale anastrozole, pressure sensitive adhesive and sorbefacient by skin, wherein, the anastrozole accounts for 0.5-30wt%, the pressure sensitive adhesive accounts for 70-90wt% and the sorbefacient by skin accounts for 0.5-50wt%; inert filler, plasticizer, tackifier, chemical inhibitor and the like can also be added to the list. The anastrozole, the pressure sensitive adhesive and the sorbefacient by skin are fully mixed and transferred to be coated on the anti-sticking layer for being dried at the temperature of 40-80 DEG C, and then the mixture are compounded by using PVC or lining materials without woven fabrics and made into the patch of different sizes and specifications by die cutting, thus obtaining the finished product. The patch can not only avoid stimulus to the gastrointestinal tract by oral medication but also reduce the toxic side effect of the medicine by slow release; the release lasts for at least 7 days, thus ensuring lasting and stable therapeutical efficiency; if medicine administration is desired to be halted, removing the patch can achieve the purpose; the patch features convenient use; in addition, the patch is flexible and appropriately adhesive.
Owner:SHENYANG PHARMA UNIVERSITY
Medicinal composition of nimustine and its progression agent
InactiveCN1919171AEasy injectionIncreased sensitivityOrganic active ingredientsSolution deliveryPolymerDrug concentration
Disclosed is a pharmaceutical composition of slow release injection carrying both Nimustine and its synergistic agent, which comprises slow release auxiliary materials and active anticancer constituents, wherein the active anticancer constituents include Nimustine and its synergistic agents (such as Triptorelin, Anastrozole or Decitabine). The viscosity of the slow release injection is 10-650cp (at 20-30 deg C), and the active anticancer constituents can also be made into slow release implanting agent. The slow release subsidiary materials mainly comprise bio-compactable and degradable macromolecular polymers, when locally dispensed on the tumor, the composition not only can lower down the whole body toxicity reaction of the anti-cancer medicament, but also can selectively increase the tumor local medicinal concentration, the treatment effect of the non-operative treatment methods such as chemotherapy, medicament and radiation can also be improved. The solid tumors include hepatic carcinoma, lung carcinoma, breast cancer, cancer of pancreas, intestinal cancer, renal carcinoma, and gastric carcinoma.
Owner:JINAN KANGQUAN PHARMA TECH
Testosterone Combined with Anastrozole Injection Solutions
ActiveUS20140371186A1Improve the level ofOrganic active ingredientsBiocideAnastrozoleIntramuscular injection
Compositions and methods for testosterone booster injection solutions (TBIS) that includes testosterone cypionate in synergistic combination with anastrozole are disclosed. Disclosed TBIS may be administered for treating testosterone deficiency. Disclosed TBIS is an intramuscular injection. The therapeutic dosage and protocol varies, according to the individual person. Different formulations may be designed to provide higher or lower testosterone doses.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
Anastrozole dispersed tablet formulation
InactiveCN100337625CPromote absorptionImprove Medication AdherenceOrganic active ingredientsPill deliveryClinical efficacyAnastrozole
The present invention belongs to the medicine technology, especially an anastrozole dispersed tablet dosage form. Through plentiful clinical investigations, we conquer the technology prejudice in prior art that it is not necessary to exploit anastrozole into dispersed tablets and obtains the present invention. The clinical experiments approve that the adverse reaction in accordance with the present invention is remarkably reduced,the compliableness of said drug is remarkably improved, and unexpected clinical curative effects are acquired. Said medicine is characterized by its low fabricating cost, formulation stabilization, fast absorption, high bioavailability and the like. The present invention is suitable for treating advanced breast carcinoma of females after menopause and provides favorable therapeutic agents dosage form to breast carcinoma patients.
Owner:LUNAN PHARMA GROUP CORPORATION
Process for the preparation of pure anastrozole
A process for the preparation of anastrozole which comprises: a) brominating 3,5-bis(2-cyanoprop-2-yl)toluene (II) in an organic solvent using a brominating agent to obtain 3,5-bis(2-cyanoprop-2-yl)benzylbromide (III); b) heating the reaction mass of step a) to the reflux temperature of the organic solvent for a period of time no longer than 3 hours; c) isolating and purifying the bromo intermediate (III) using an organic solvent; d) alkylating the bromo intermediate in the presence of a base, optionally a phase transfer catalyst, a 1,2,4-triazole and an organic solvent to obtain anastrozole; and e) isolating and purifying the anastrozole from an organic solvent.
Owner:CIPLA LTD
Purification process for Anastrozole intermediate
InactiveUS20070032660A1Antineoplastic agentsCarboxylic acid nitrile purification/separationTolueneAnastrozole
The invention is directed to processes for purifying the Anastrozole intermediate, 3,5-bis(2-cyanoisopropyl)toluene, processes for producing Anastrozole, processes for preparing Anastrozole pharmaceutical compositions, and Anastrozole and Anastrozole pharmaceutical compositions prepared with the processes of the invention.
Owner:SICOR SOC ITAL CORTICOSTEROIDI SPA
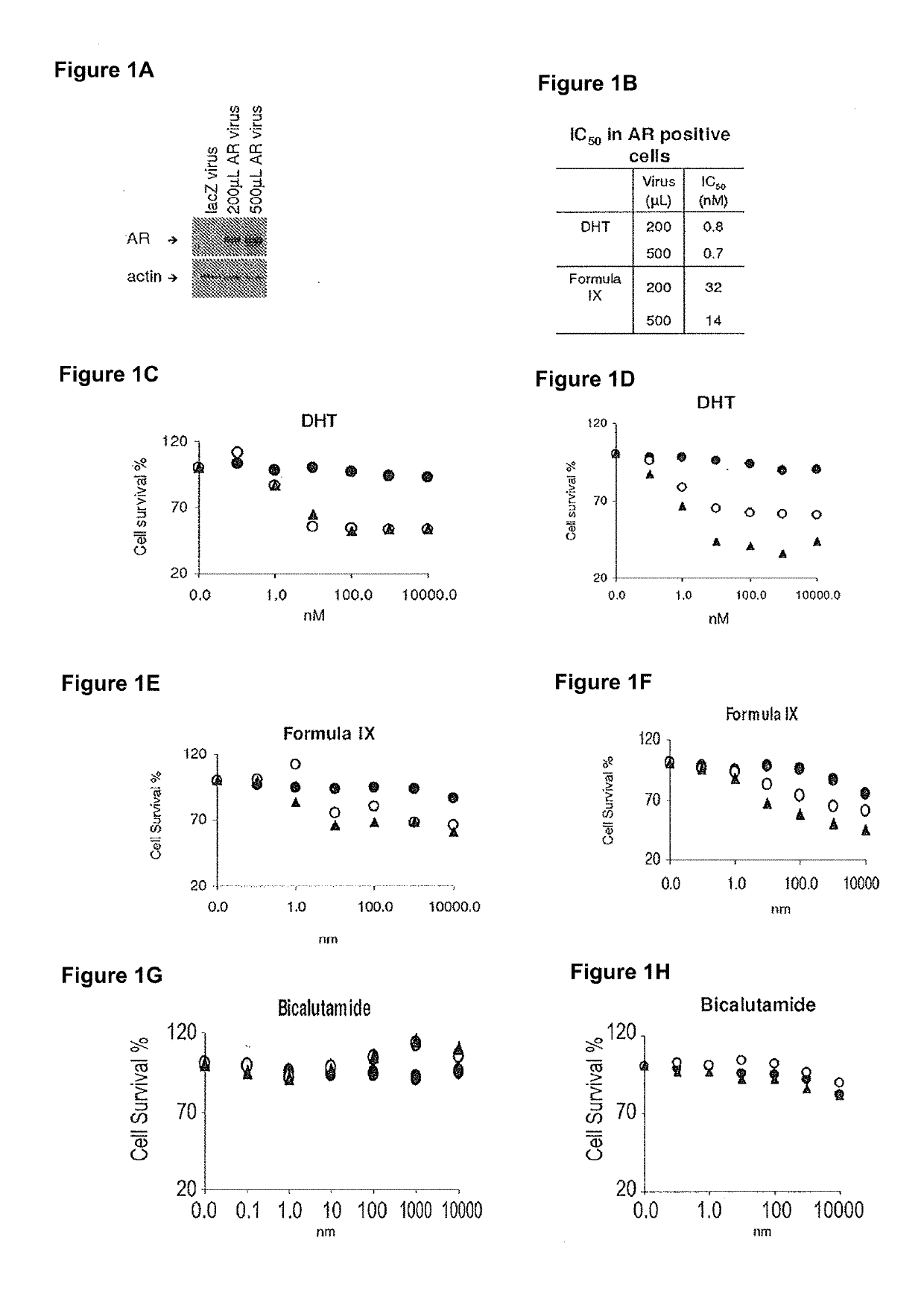
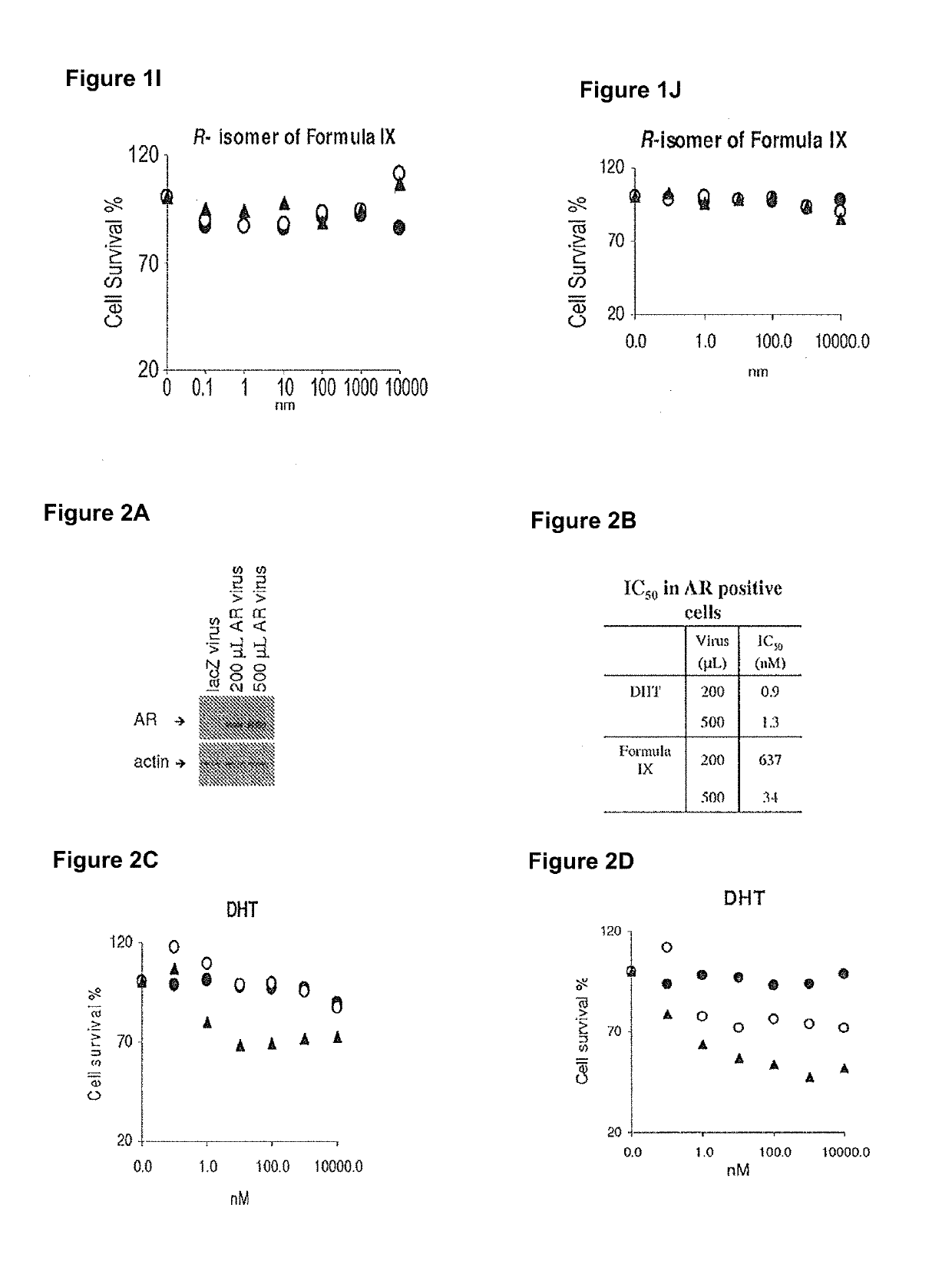
Method of treating er mutant expressing breast cancers with selective androgen receptor modulators (SARMS)
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed selective estrogen receptor modulator (SERM) (tamoxifen, toremifene, raloxifene), gonadotropin-releasing hormone (GnRH) agonist (goserelin), aromatase inhibitor (AI) (letrozole, anastrozole, exemestane), cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitor (palbociclib (Ibrance), ribociclib (Kisqali), abemaciclib (Vorzenio)), mTOR inhibitor (everolimus), trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, neratinib (Nerlynx), olaparib (Lynparza) (an inhibitor of the enzyme poly ADP ribose polymerase (PARP)), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; HER2-positive; and / or treating a subject suffering from ER mutant expressing breast cancer, comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
Docetaxel-containing anti-cancer sustained-release injection
InactiveCN101234085AEasy to operateGood repeatabilityOrganic active ingredientsPharmaceutical delivery mechanismPolyethylene glycolSuspending Agents
The invention relates to anticancer sustained release injection which comprises sustained release microspheres and menstruum, wherein, the sustained release microspheres comprise anticancer active components and sustained release auxiliary material; the menstruum is special menstruum that contains suspending agent. The anticancer active components are fotemustine, nimustine, carmustine or combination of bendamustine and mitozolomide, docetaxel, etoposide, teniposide, vinblastine, anastrozole, tamoxifen, fluorouracil or mitomycin C; the sustained release auxiliary material is polylactic acid and polylactic acid copolymer, polyethylene glycol and polylactic acid copolymer of polyethylene glycol, terminal carboxyl group polylactic acid copolymer, EVAc, fatty acid and decanedioic acid copolymer, etc.; viscosity of the suspending agent is 100cp-3,000cp (at 25 DEG C-30 DEG C), and the suspending agent is selected from sodium carboxymethylcellulose, etc. The sustained release microspheres can also be made into sustained release implant; the injection or implant is injected or placed in or around tumor so as to reduce general reaction of the drug and selectively improve and keep local concentration for about 30-50 days. The anticancer sustained release injection can be used solely and can also promote anti-tumor effects of non-operative treatments, such as chemotherapy and / or radiotherapy, etc.
Owner:JINAN SHUAIHUA PHARMA TECH
Anastrozole dispersible tablet and preparation method thereof
InactiveCN102988313AOrganic active ingredientsPharmaceutical non-active ingredientsAnastrozolePatient compliance
The invention provides an anastrozole dispersible tablet and a preparation method thereof. The anastrozole dispersible tablet comprises a primary component which is anastrozole, and auxiliary components comprising a disintegrating agent, a filler, a flavoring agent and the like. The anastrozole dispersible tablet rapidly forms a uniform suspension after meeting water, has the characteristics of convenient administration, fast absorption, high biological availability and the like, can improve the compliances of patients, and can increase the clinical medication selection of doctors and the patients.
Owner:BEIJING YILING BIOENG
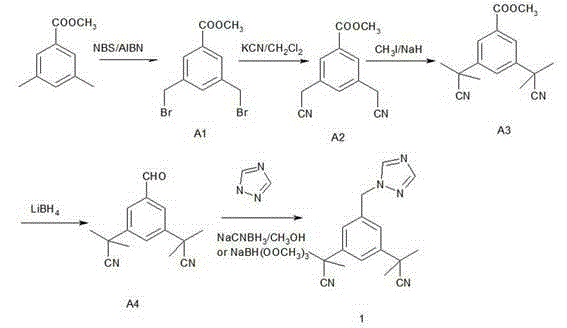
Method for synthesizing aromatase inhibitor
The invention discloses a method for synthesizing an aromatase inhibitor anastrozole. A synthesis route comprises the steps: with 3, 5-dimethylbenzoate as a starting material, carrying out bromination, cyanation and methylation to obtain 3, 5-bis (2-cyano-2-yl) benzoate, reducing the intermediate to obtain 3,5-bis (2-cyano-2-yl) benzaldehyde, and carrying out a reductive amination reaction on the generated intermediate and 1.24-triazole to obtain anastrozole. The method has the advantages of easiness in operation, mild reaction conditions, high yield, high product purity and the like, and is suitable for industrial production of anastrozole.
Owner:JIANGSU QINGJIANG PHARMA
Method for synthesizing anastrozole
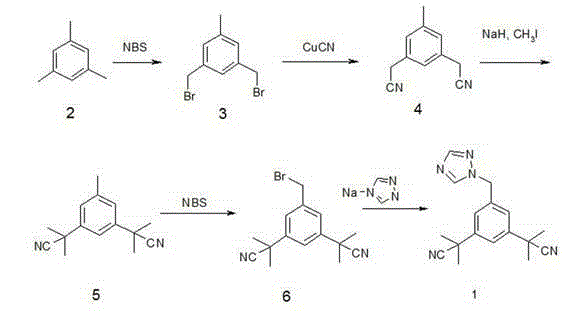
InactiveCN102108066AReduce dosagePromote application developmentPreparation by cyanide reactionN-BromosuccinimideMethyl benzene
The invention discloses a novel method for synthesizing anastrozole, and belongs to the technical field of medicinal chemistry. The method comprises the following steps: 3,5-dibromo toluene used as a starting material reacts to produce 3,5-dibromo methyl toluene under the effect of a brominating agent NBS (N-bromosuccinimide), and reacts to produce an intermediate 3,5-bis[(2,2-dimethyl) cyano methyl]- bromo methyl benzene through a cyanidation reaction, a methylation reaction and a bromination reaction; and then the intermediate reacts with 1,2,4-triple nitrogen oxazole sodium to produce the anastrozole. The novel method is characterized in that the second step of cyano substitution reaction is improved. The method has the advantages that the process is simple, the efficiency is greatly improved, the yield is improved to 83 percent to 88.20 percent, the cost is reduced, the solvent use amount is reduced, the environment is further protected, and little side reaction is caused; and as the cost is reduced, the method is very beneficial to and suitable for industrialized production.
Owner:LUOHE NANJIECUN QUANWEI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com