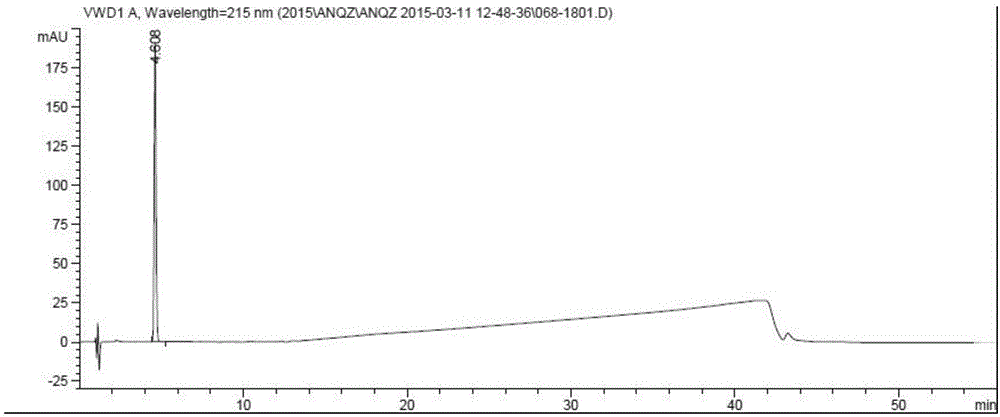
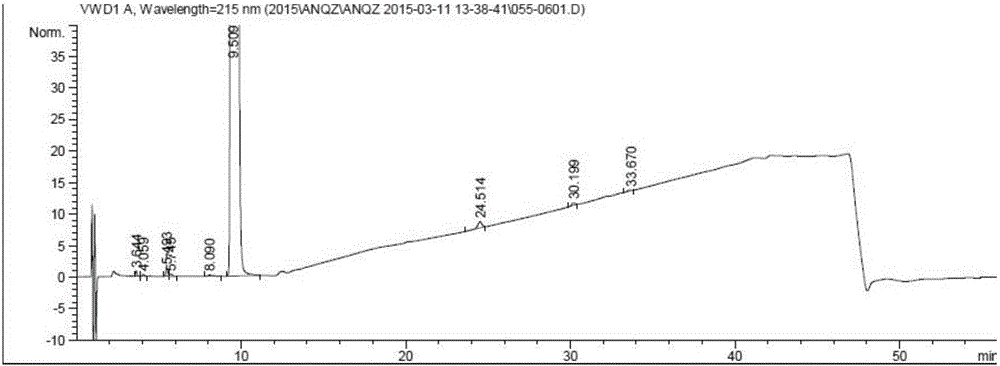
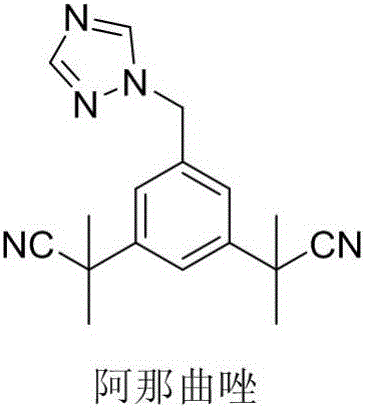
Preparation method of anastrozole
A technology for anastrozole and crude products, which is applied in the field of preparation of anastrozole, can solve the problems of low product content, cumbersome process, unsafety, etc., and achieve the effect of high yield and low content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] In the present embodiment, the preparation method of Anastrozole comprises the following steps:
[0034] 1) Add 30 g of 5-bromomethyl-α, α, α′, α′-tetramethyl-1,3-benzenediacetonitrile, 4-amino-1,2,4-triazol in sequence in a glass reactor 30 g of oxazole and 80 ml of acetonitrile were heated to reflux under stirring, and after 12 hours of reaction, the temperature was lowered and crystallized at 0°C for 8 hours. After suction filtration and drying, 35.58 g of intermediate I was obtained, with a yield of 93.0%;
[0035] 2) Add 30g of the obtained intermediate I, 225ml of anhydrous methanol, 2ml of purified water, and 2g of copper sulfate into the glass reactor in sequence, add 24.0g of concentrated sulfuric acid dropwise under stirring, and heat to reflux under stirring after dropping. Slowly add 12.0 g of sodium azide, after the addition is complete, continue the reflux reaction for 1 h. Suction filter the reaction liquid while it is hot, discard the filter residue, c...
Embodiment 2
[0040] In the present embodiment, the preparation method of Anastrozole comprises the following steps:
[0041] 1) Add 30 g of 5-bromomethyl-α, α, α′, α′-tetramethyl-1,3-benzenediacetonitrile, 4-amino-1,2,4-triazol in sequence in a glass reactor 15g of oxazole and 60ml of acetonitrile were heated to reflux under stirring. After 12 hours of reaction, the temperature was lowered and crystallized at 5°C for 8 hours. Suction filtration and drying yielded 35 g of intermediate I with a yield of 91.6%;
[0042] 2) Add 20.8g of the obtained intermediate I, 150ml of absolute ethanol, 2.0ml of purified water, and 1.3g of copper sulfate into a glass reactor in sequence, add 18.2g of concentrated sulfuric acid dropwise under stirring, and heat to reflux under stirring after dropping. Slowly add 9.1 g of sodium azide, after the addition is complete, continue the reflux reaction for 1 h. Suction filter the reaction solution while it is hot, discard the filter residue, concentrate the filt...
Embodiment 3
[0046]In the present embodiment, the preparation method of Anastrozole comprises the following steps:
[0047] 1) Add 23.5 g of 5-bromomethyl-α, α, α', α'-tetramethyl-1,3-benzenediacetonitrile, 4-amino-1,2,4-tri 15 g of oxazole and 60 ml of acetonitrile were heated to reflux with stirring, and after 8 hours of reaction, the temperature was lowered and crystallized at 5°C. Suction filtration and drying yielded 21 g of Intermediate I with a yield of 92%;
[0048] 2) Add 20 g of intermediate I, 150 ml of absolute ethanol, 1.7 ml of purified water, and 1.3 g of copper sulfate into a glass reactor in sequence, add 17 g of concentrated sulfuric acid dropwise under stirring, and heat to reflux under stirring after dropping. Slowly add 8.5 g of sodium azide, after the addition is complete, continue the reflux reaction for 1 h. Suction filter the reaction solution while it is hot, discard the filter residue, concentrate the filtrate under reduced pressure to 0.5 times the original vo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com