Method for preparing polycyclic carbamoyl pyridone compound
A technology for polycyclic carbamoyl pyridone and compound, which is applied in the field of preparation of polycyclic carbamoyl pyridone compound, can solve the problems of difficulty in removing isomers, long preparation period, many isomers in cyclization steps and the like , to avoid the risk of impurities, save production time, and shorten the process cycle.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
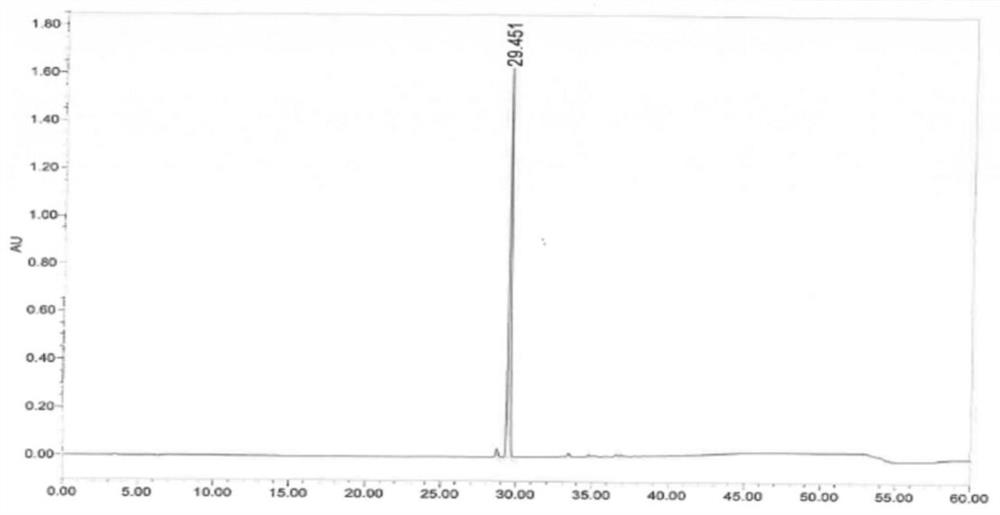
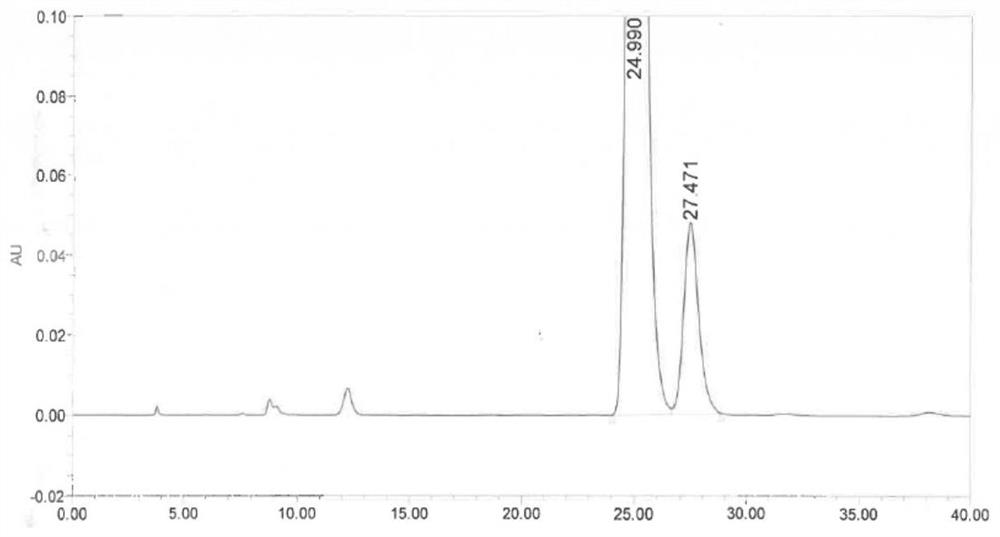
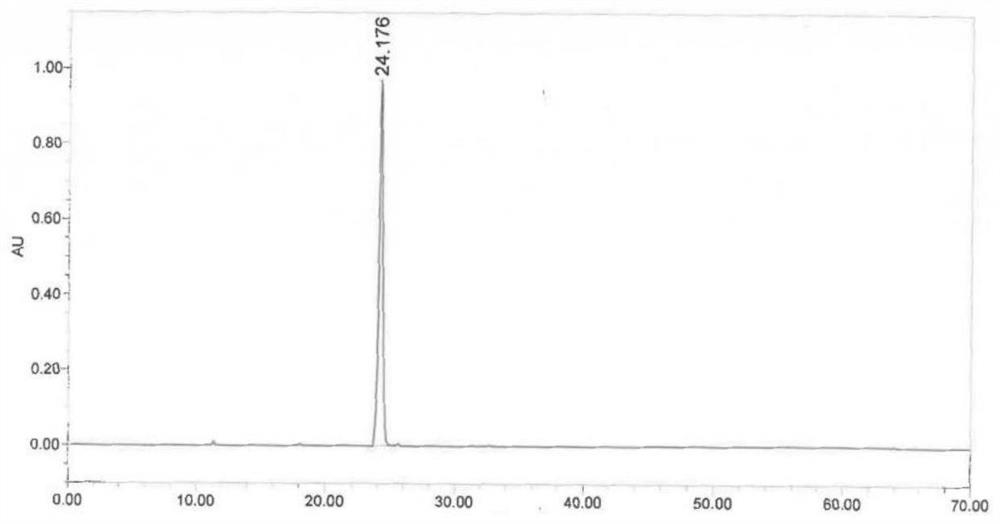
Image
Examples
Embodiment 1
[0108] Example 1: 1-(2,2-Dimethoxyethyl)-1,4-dihydro-3-ethoxy-4-oxo-5-(2,4-difluorobenzylaminoyl ) ethyl pyridine-2-carboxylate
[0109]
[0110] 30.0 g of 1-(2,2-dimethoxyethyl)-5-ethoxy-6-(ethoxycarbonyl)-4-oxo-1,4-dihydropyridine-3-carboxylic acid ( 87.38 mmol, 1.0 eq) was added to 300 ml of tetrahydrofuran, and 15.6 g of CDI (96.21 mmol, 1.1 eq) was added with stirring. The mixture was refluxed for 3 hours, cooled, cooled to -10~0°C, 13.8 g of 2,4-difluorobenzylamine (96.41mmol, 1.1eq) was added, the addition was completed, and stirred at -10~0°C for 0.5 hours, washed with dilute hydrochloric acid and sodium bicarbonate solution, and concentrated under reduced pressure to obtain crude compound 1-(2,2-dimethoxyethyl)-1,4-dihydro-3-ethoxy-4-oxo as an oil. Substituted-5-(2,4-difluorobenzyl)pyridine-2-carboxylic acid ethyl ester. The oil was used directly in the next step without further purification.
Embodiment 2
[0111] Example 2: 1-(2,2-Dihydroxyethyl)-1,4-dihydro-3-ethoxy-4-oxo-5-(2,4-difluorobenzyl)pyridine -2-ethyl formate
[0112]
[0113] 164.0 g of formic acid (4.0 M, 134 ml) was added to the oil obtained in Example 1 (theoretical amount 40.9 g, 1.0 M), and the mixture was stirred at 75-85° C. for 2 hours. Add 269ml of purified water at ℃, stir, filter, wash with purified water, and dry to obtain 37.6g of compound 1-(2,2-dihydroxyethyl)-1,4-dihydro-3-ethoxy-4-oxo -5-(2,4-Difluorobenzyl)pyridine-2-carboxylic acid ethyl ester (97.8% yield).
Embodiment 3
[0114] Example 3: 1-(2,2-Dimethoxyethyl)-1,4-dihydro-3-ethoxy-4-oxo-5-(2,4-difluorobenzylaminoyl ) ethyl pyridine-2-carboxylate
[0115]
[0116] 30.0 g of 1-(2,2-dimethoxyethyl)-5-ethoxy-6-(ethoxycarbonyl)-4-oxo-1,4-dihydropyridine-3-carboxylic acid ( 87.38 mmol, 1.0 eq) was added to 300 ml of tetrahydrofuran, and 18.4 g of CDI (113.48 mmol, 1.3 eq) was added with stirring. The mixture was refluxed for 3 hours, cooled, cooled to 5-15°C, 16.3 g of 2,4-difluorobenzylamine (113.87mmol, 1.3eq) was added, the addition was completed, and the mixture was stirred at 5-15°C for 0.5 hour, Wash with diluted hydrochloric acid and sodium bicarbonate solution, and concentrate under reduced pressure to give crude compound 1-(2,2-dimethoxyethyl)-1,4-dihydro-3-ethoxy-4-oxo- 5-(2,4-Difluorobenzyl)pyridine-2-carboxylic acid ethyl ester. The oil was used directly in the next step without further purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com