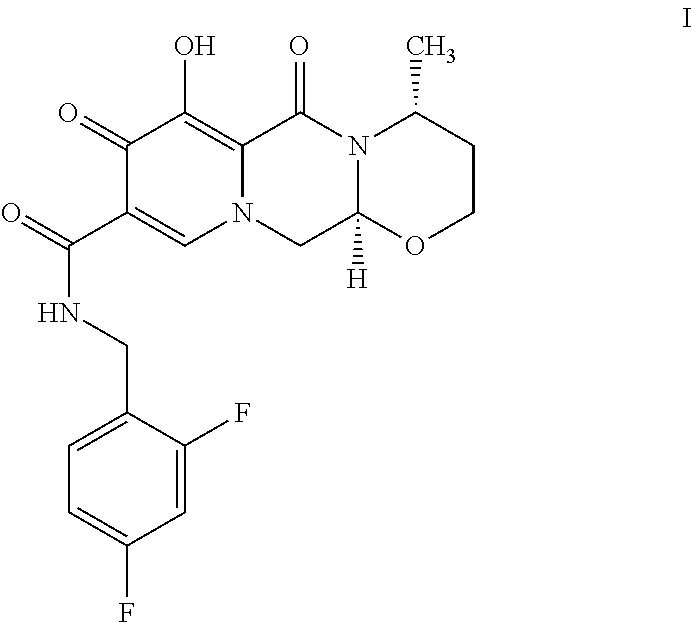
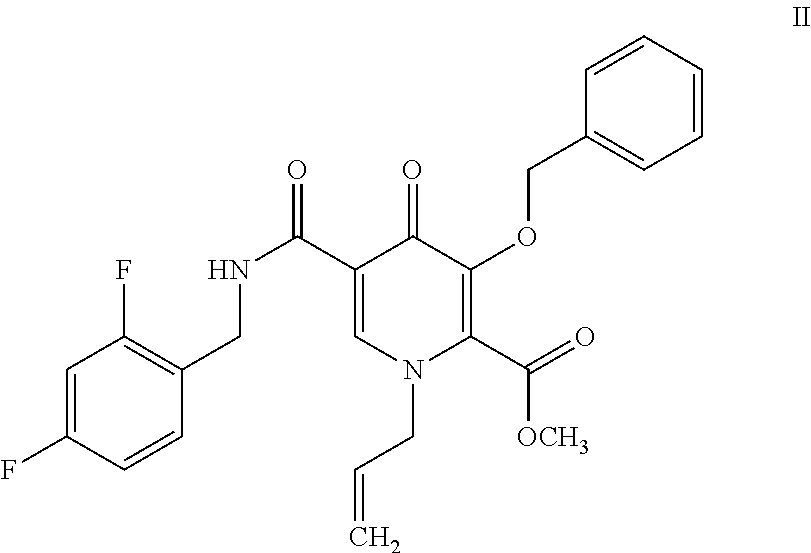
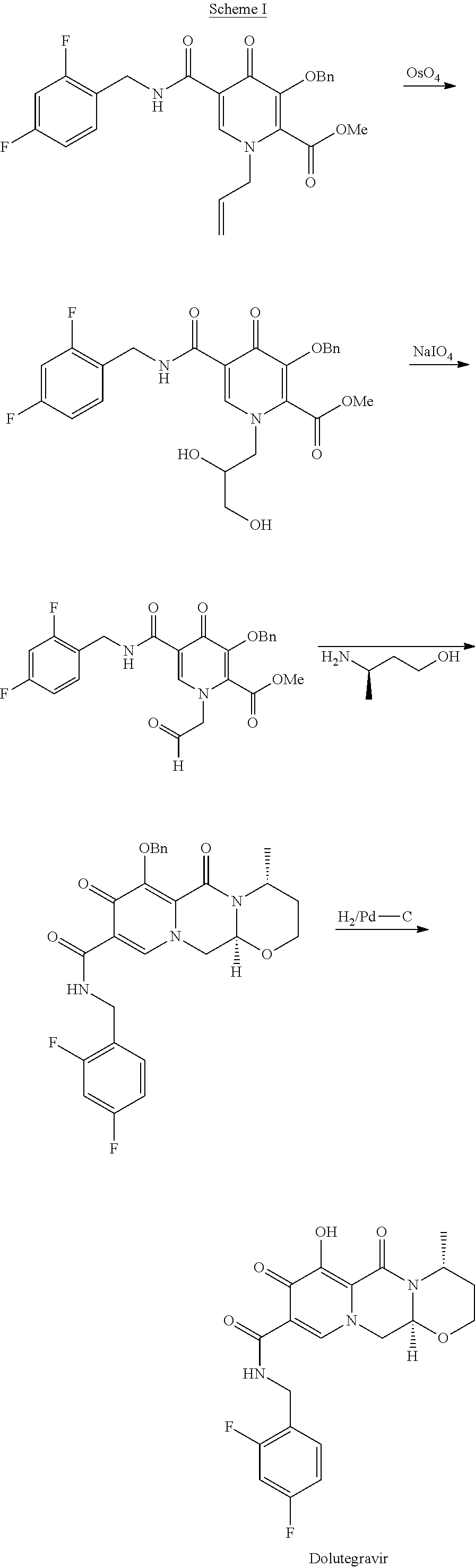
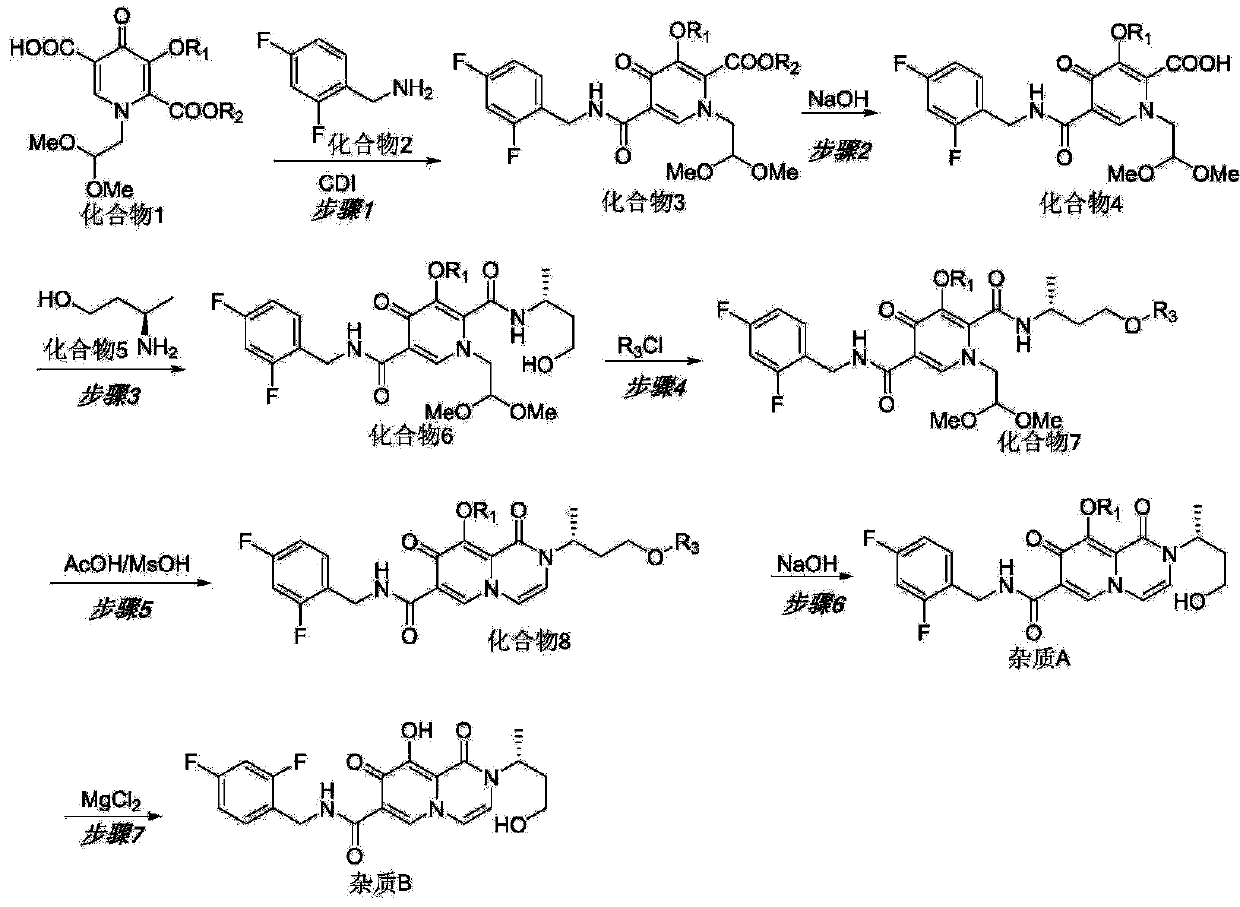
Patents
Literature
45 results about "Dolutegravir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
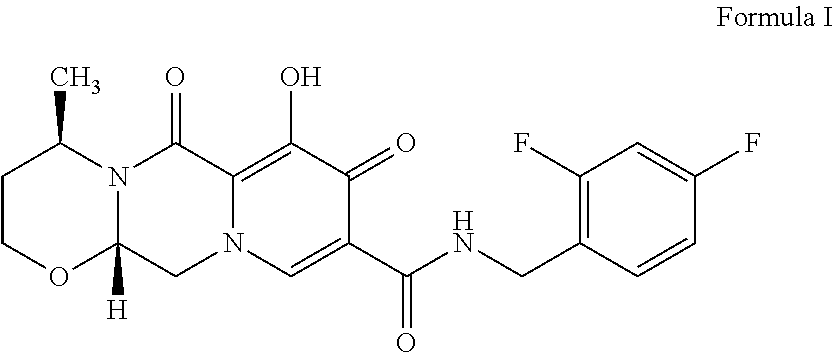
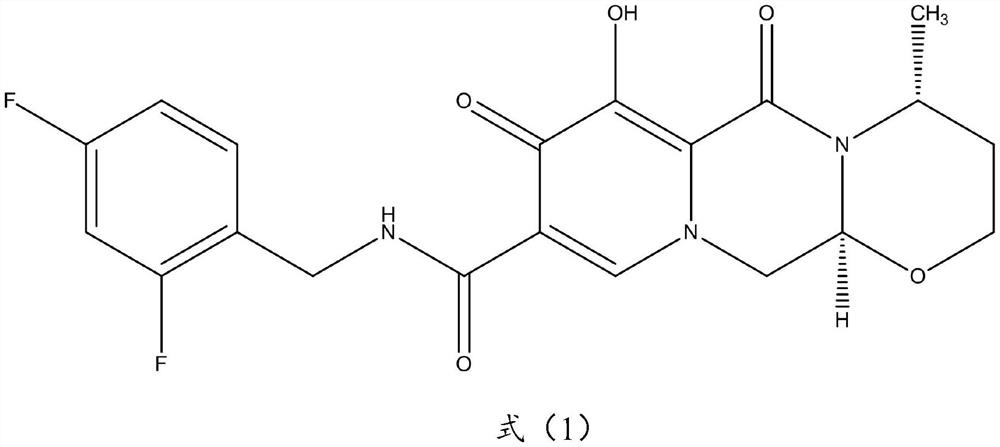
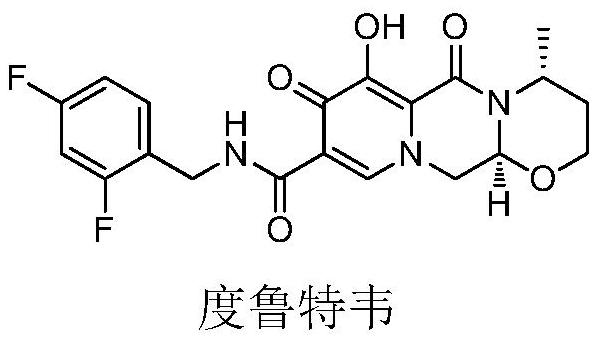
Dolutegravir is used with other HIV medications to help control HIV infection.
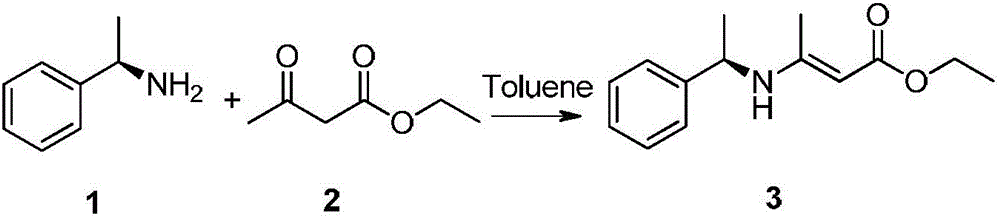
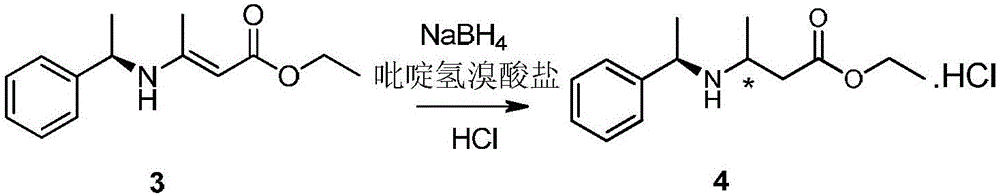
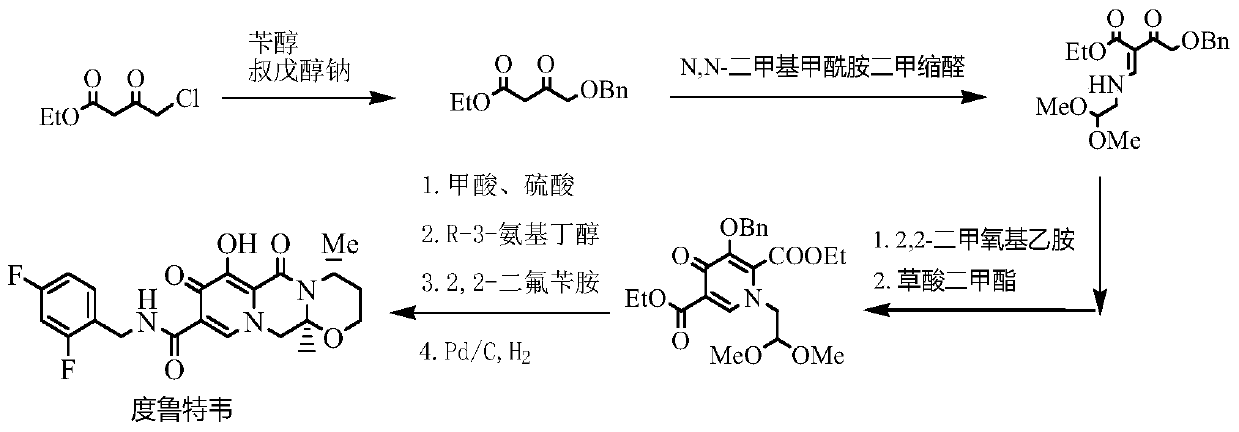
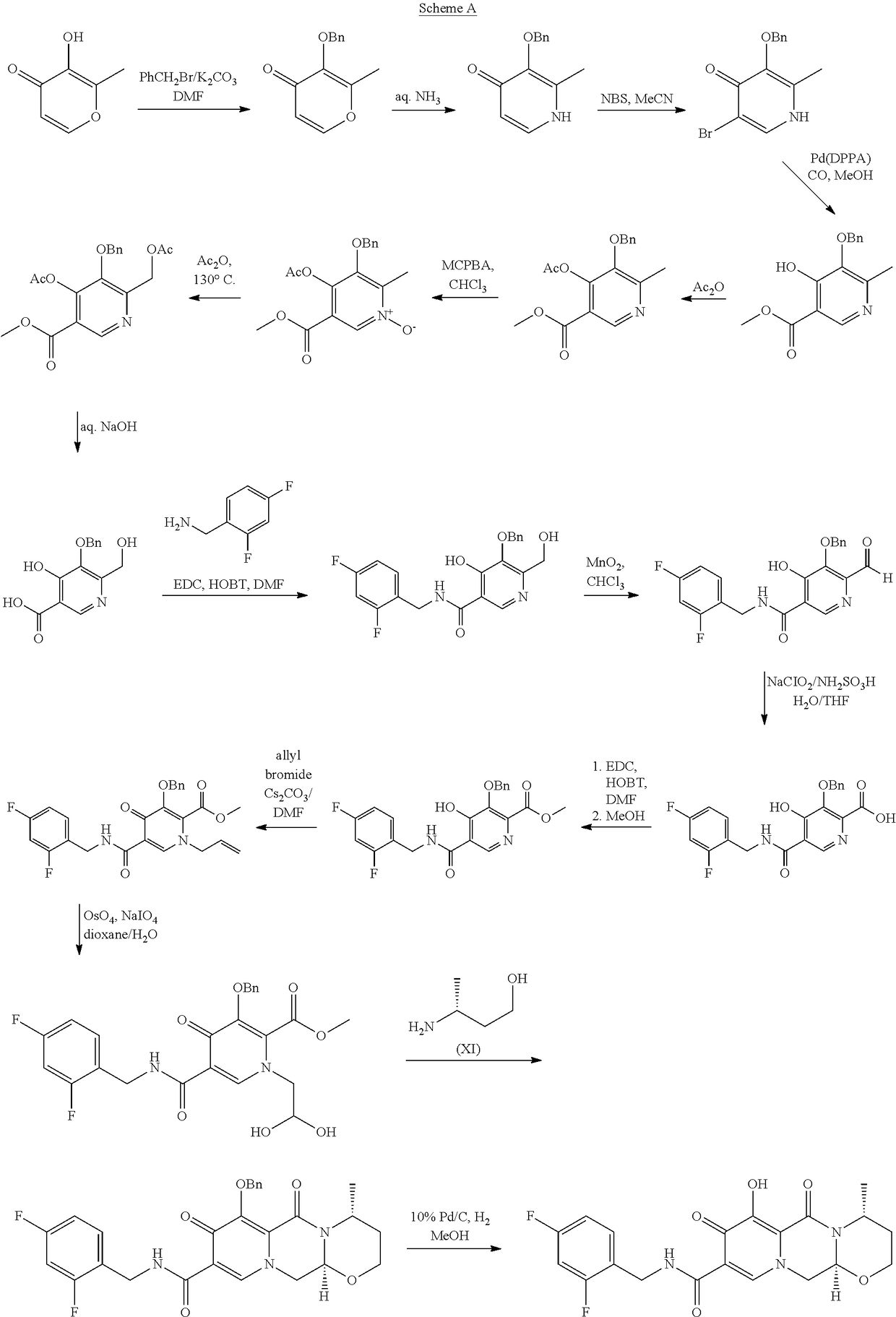
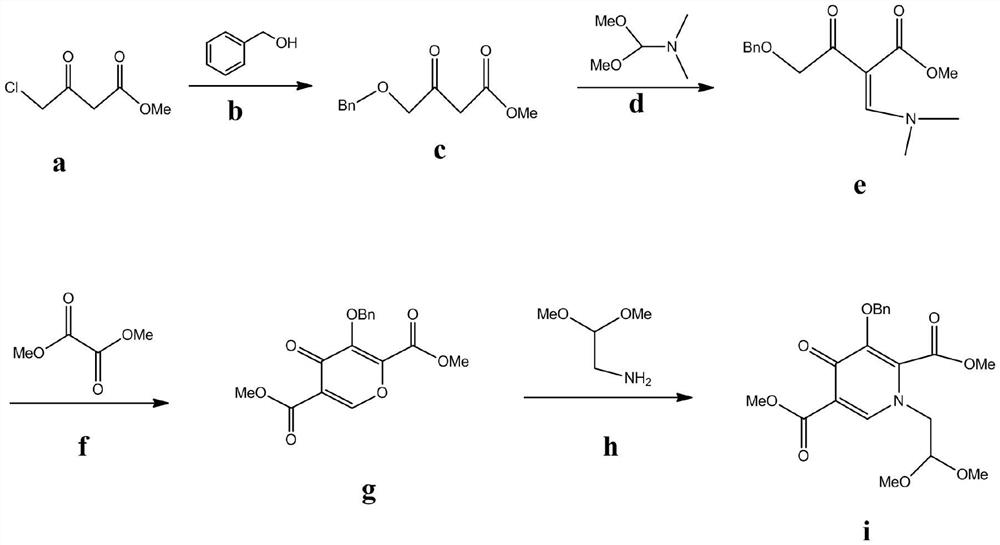
Improved method for synthesizing dolutegravir
ActiveCN108299466ALow priceReduce pollutionOrganic chemistryBulk chemical productionSolventImproved method
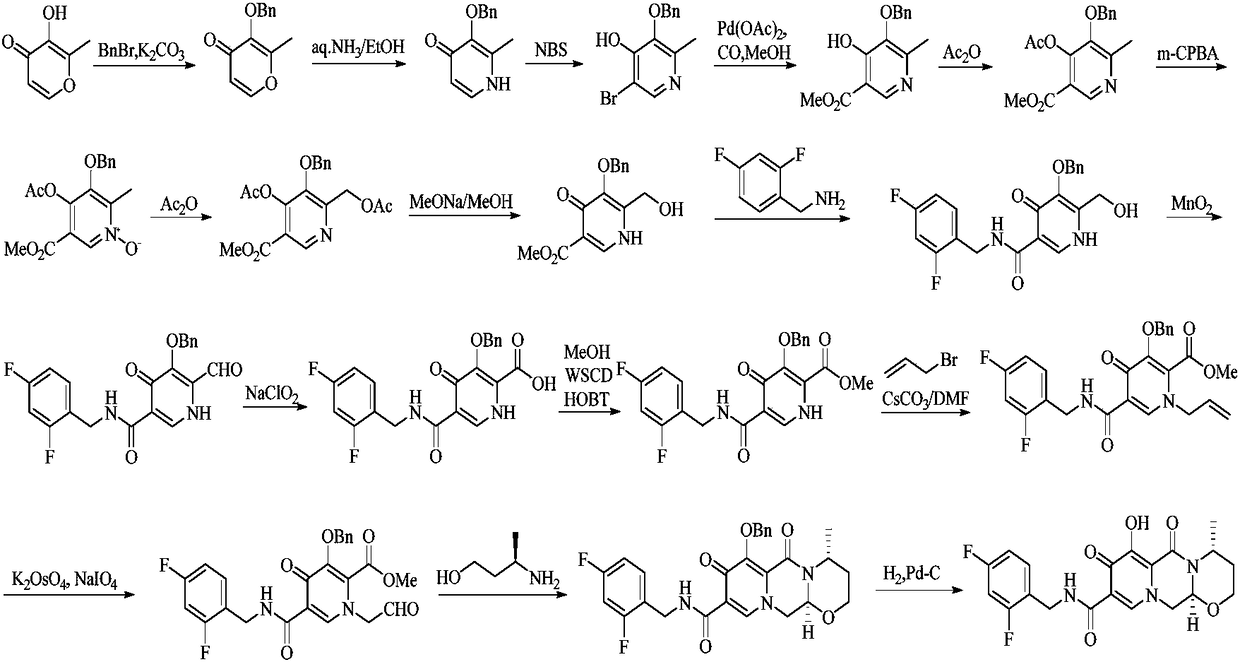
The invention relates to an improved method for synthesizing dolutegravir and belongs to the field of medicinal chemistry. The method takes maltol (compound 1) as a raw material, and a target is synthesized by the following route. The process raw material is cheap and easy to obtain, a reaction solvent can be recycled, the post-treatment operation is simple, the yield and the purity are high, especially the carbamoylation and debenzylation reaction are carried out in one step, the synthesis route is simplified, the cost is reduced, and the large-scale industrial production is facilitated.
Owner:仁合熙德隆药业有限公司 +2
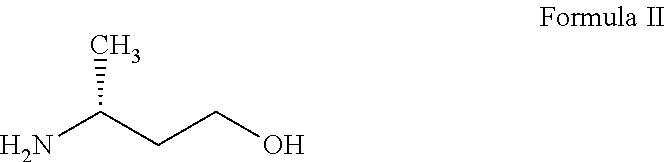
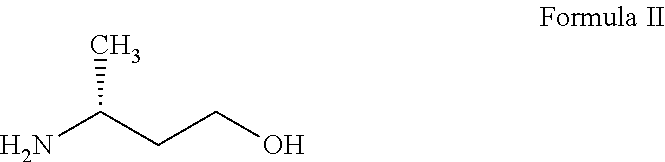
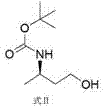
Synthetic method of dolutegravir key intermediate (R)-3-aminobutanol
InactiveCN106748816AReduce usageHigh optical purityOrganic compound preparationAmino-carboxyl compound preparationPhotochemistryDolutegravir
The invention discloses a synthetic method of dolutegravir key intermediate (R)-3-aminobutanol. The synthesis route is disclosed in the invention, and is suitable for industrialized large-scale production of (R)-3-aminobutanol; product optical purity is increased effectively; using of raw materials dangerous in operation is avoided; product yield is extremely high; and the synthetic method is suitable for industrialized popularization and application.
Owner:HENAN NORMAL UNIV
An improved process for the preparation of Dolutegravir
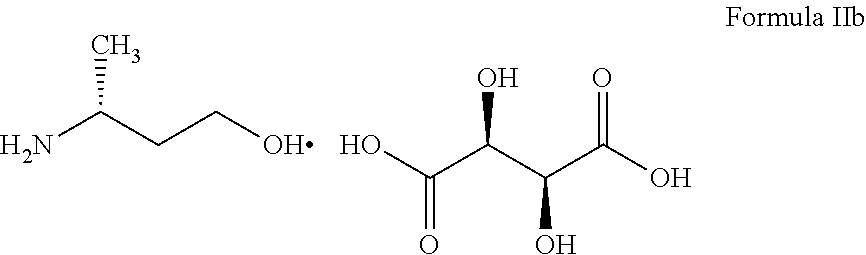
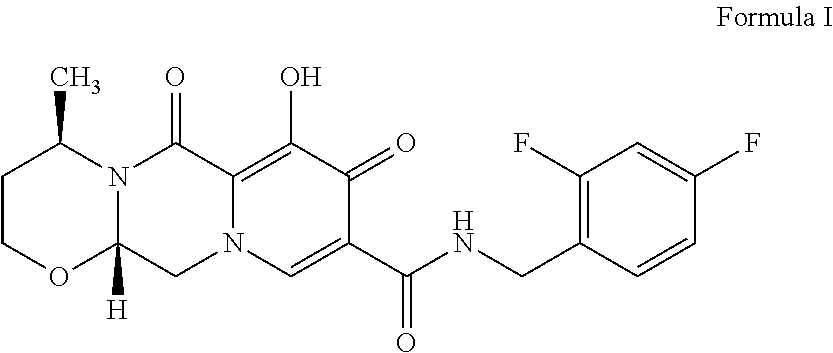
ActiveUS20160108058A1Simple and cost-effective processHigh purityOrganic compound preparationAmino-hyroxy compound preparationTartrateMedicinal chemistry
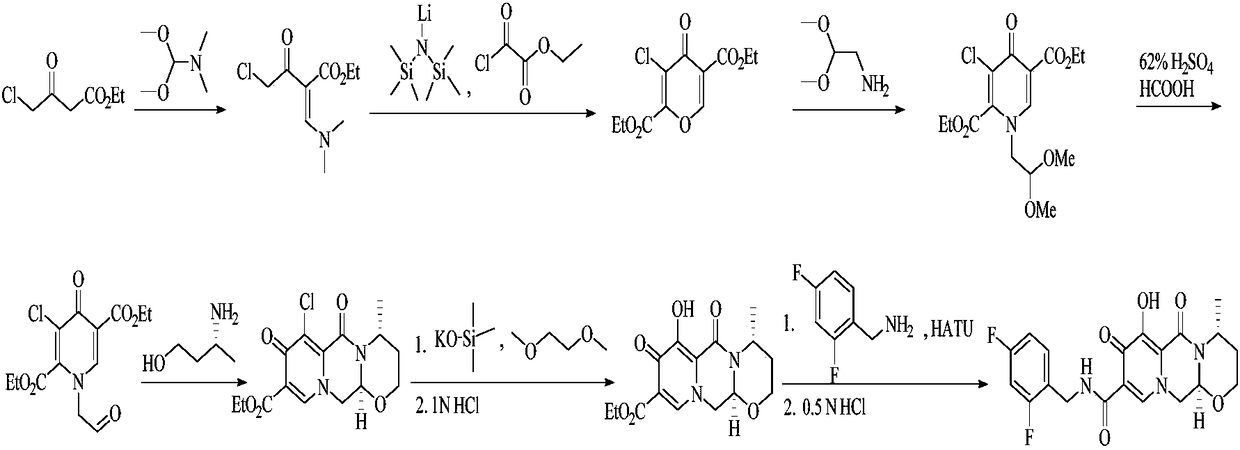
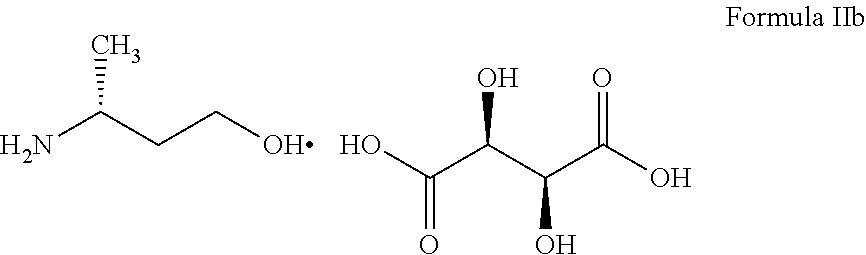
The present invention provides (R)-3-Amino-1-butanol (D)-tartarate (IIb); process for its preparation and its conversion to Dolutegravir. The present invention also provides an improved process for the preparation of Dolutegravir (I) or pharmaceutically acceptable salts wherein compound (XVI) is reacted with an optically active acid addition salt of (R)-3-amino-1-butanol (IIa).
Owner:AUROBINDO PHARMA LTD
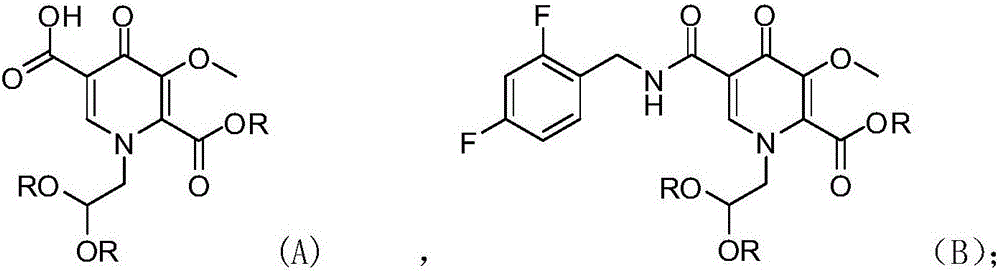
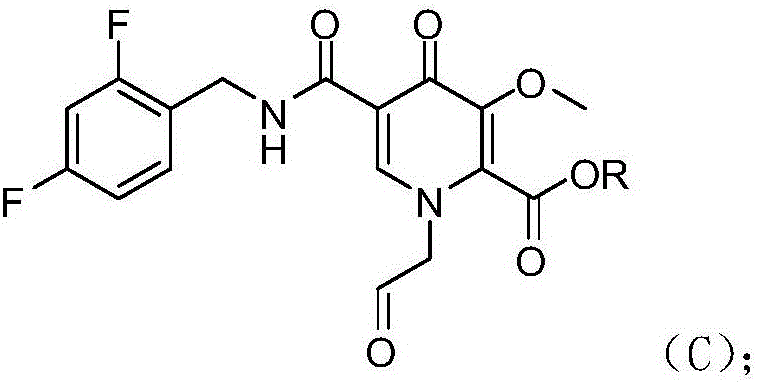
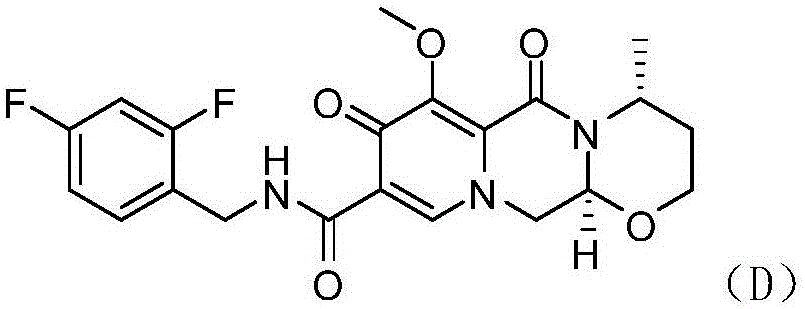
Novel method for preparing dolutegravir
InactiveCN106565747AHigh yieldReduce usageOrganic chemistryBulk chemical productionProtecting groupMedicinal chemistry
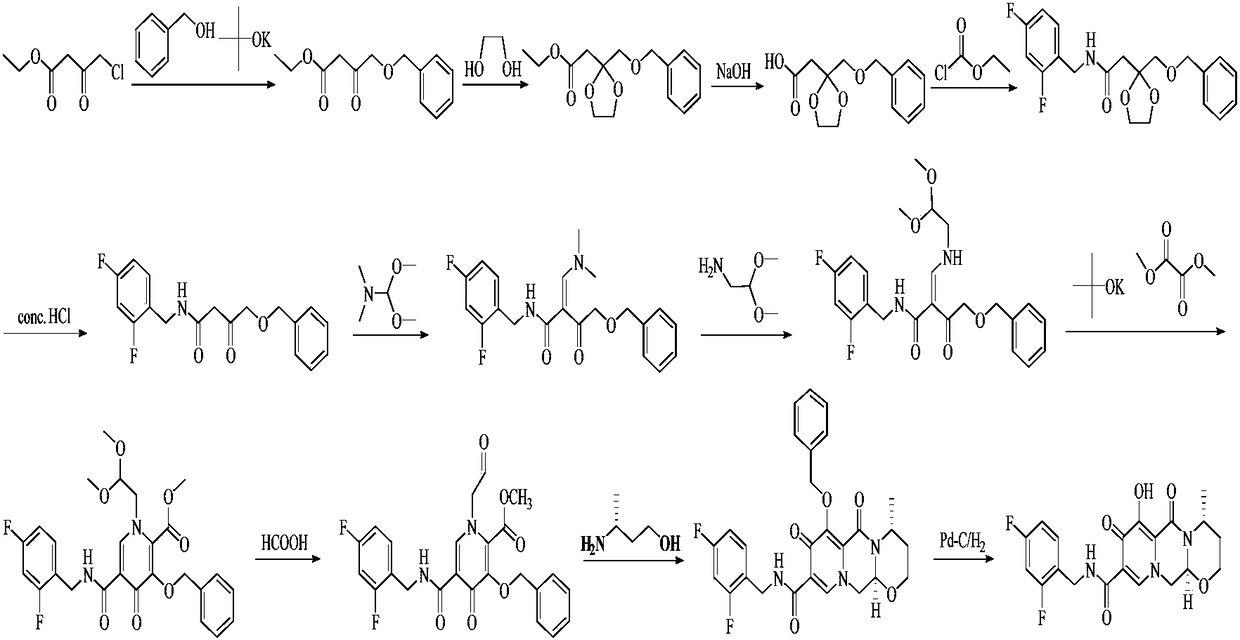
The invention discloses a novel method for preparing dolutegravir (E) defined in the description, and relates to the field of medicinal chemistry. The method comprises the following steps of 1) performing a condensation reaction of a compound (A) and 2,4-difluorobenzylamine to prepare a compound (B) defined in the description; 2) performing aldehyde group protecting group removal on the compound (B) to obtain a compound (C) defined in the description; 3) performing a cyclization reaction of the compound (C) and R-3-amino-1-butanol to prepare a compound (D) defined in the description; and 4) performing a demethylation reaction of the compound (D) to obtain the dolutegravir (E). According to the method, a novel route is adopted, and reaction conditions are continuously optimized, so that the total yield is greatly increased; and the total yield calculated by taking the compound (A) as a starting material is 75% or more, and the yields of single reactions are all 90% or more.
Owner:厦门蔚扬药业有限公司
Process for the preparation of intermediate of dolutegravir
ActiveUS9963430B2Organic compound preparationCarboxylic acid amides preparationDihydropyridineCarboxylate
Owner:HETERO RES FOUND
Preparation method of dolutegravir mother nucleus intermediate
The invention discloses a preparation method of a dolutegravir intermediate, and relates to the field of organic synthetic medicinal chemistry. The preparation method comprises the following steps: (1) carrying out condensation reaction on a compound P1 and a compound P2 to obtain a compound P3; (2) carrying out substitution reaction on the compound P3 and aminoacetaldehyde dimethyl acetal to obtain a compound P4; (3) reacting the compound P4 with halogenated acetate to obtain a compound P5, and further performing intramolecular cyclization on the compound P5 to obtain a compound P6; and (4) carrying out hydrolysis reaction on the compound P6 to obtain the dolutegravir mother nucleus intermediate P7. The method is simple to operate, high in chemical reaction yield, less in three wastes, low in raw material cost, high in finished product purity and suitable for industrial production.
Owner:内蒙古永太化学有限公司
Process for the preparation of intermediate of dolutegravir
ActiveUS20170334858A1Organic compound preparationCarboxylic acid amides preparationDihydropyridineCarboxylic salt
The present invention provides a novel processes for preparation of methyl 3-(benzyloxy)-5-(2,4-difluorobenzylcarbamoyl)-4-oxo-1-(2-oxoethyl)-1,4-dihydropyiridine-2-carboxylate using novel intermediates.
Owner:HETERO RES FOUND
Preparation method of dolutegravir ring-opening impurities, and impurities thereof
InactiveCN110655517ASimple reaction conditionsMild reaction conditionsOrganic chemistryBulk chemical productionChemical compoundCombinatorial chemistry
The invention discloses a preparation method of dolutegravir ring-opening impurities. The preparation method comprises steps of preparation of an impurity A and an impurity B, specifically comprises following steps: taking a compound 1 as an initial raw material, condensing with a compound 2 to generate a compound 3, hydrolyzing into a compound 4 under an alkaline condition, then reacting with a compound 5 to generate a compound 6, obtaining the impurity A from the compound 6 by adopting a protection-ring closing-deprotection method, and further reacting to obtain the impurity B. The inventionalso discloses the impurities. Compared with the prior art, the preparation method disclosed by the invention has the advantages that the preparation of the impurity A and the impurity B of the dolutegravir bulk drug is completed by using a new synthetic route disclosed for the first time; the designed impurity synthesis route is simple and mild in reaction condition, good in yield and safe in reaction operation, and the problems that the dolutegravir degradation impurities A and B are small in amount in an acid-base damage test and cannot be separated are solved; characterization work of theimpurity A and the impurity B is completed, and subsequent further research on the impurity A and the impurity B is facilitated.
Owner:ANHUI BIOCHEM BIO PHARMA
Method for synthesizing diastereomer impurity in dolutegravir raw material
InactiveCN110396099ARaise quality standardsSimple processOrganic chemistry methodsDiastereomerSolvent
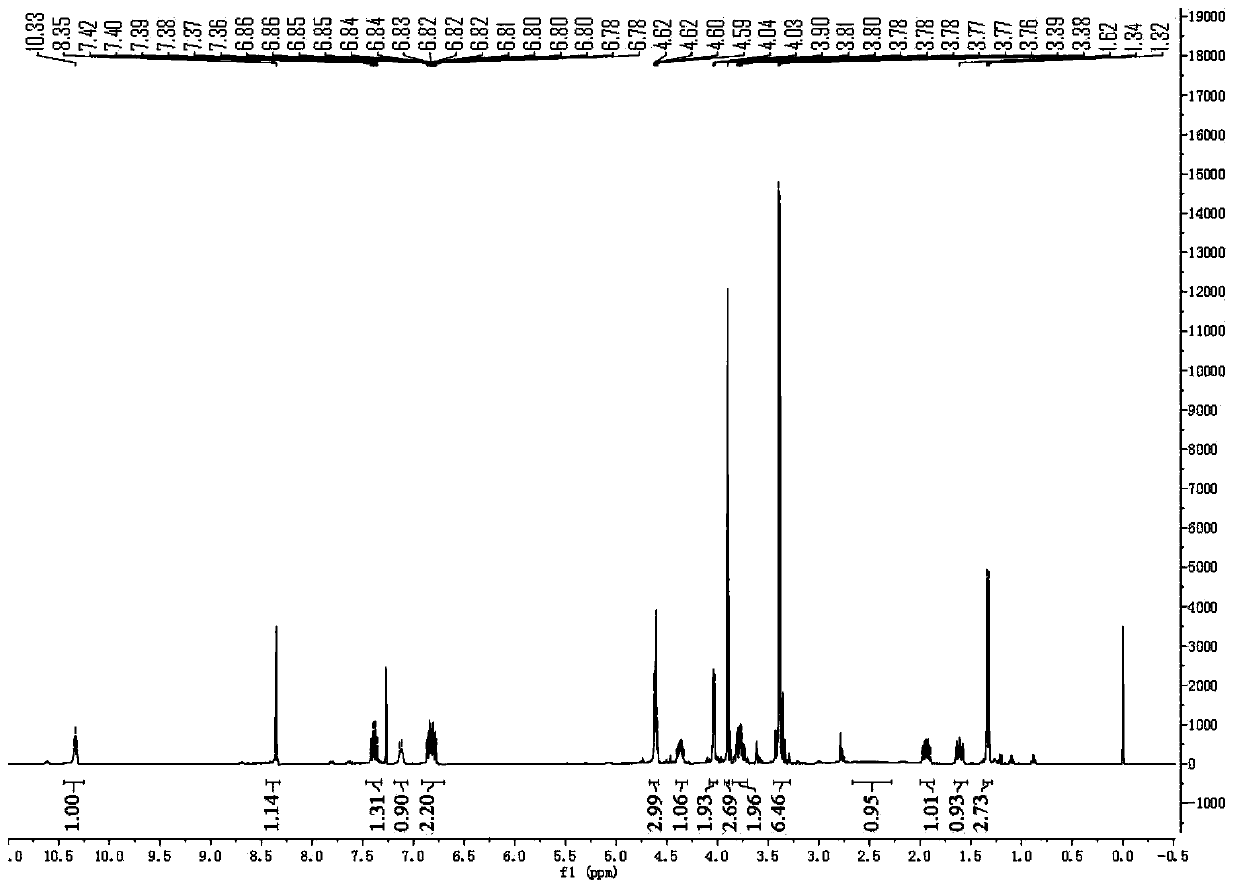
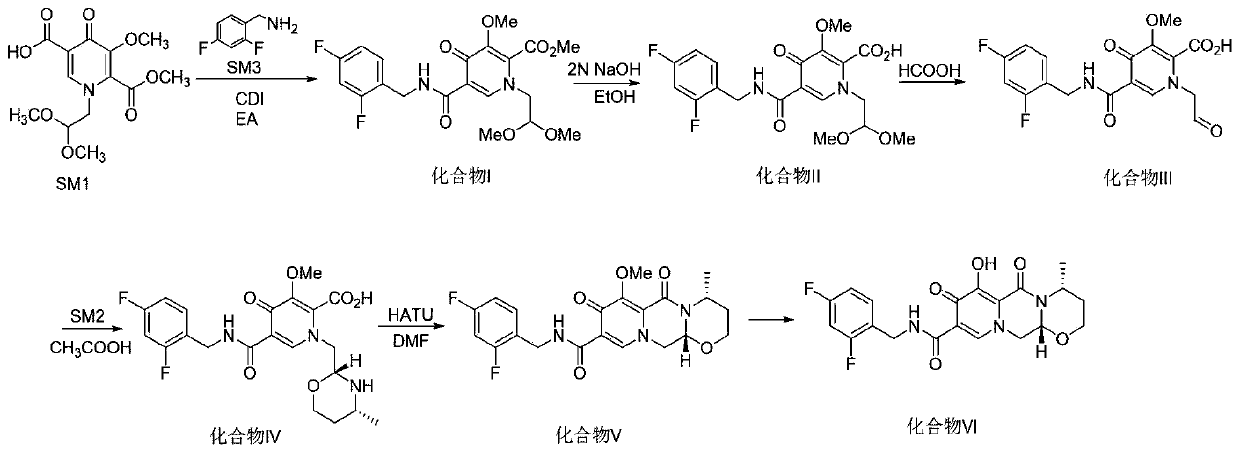
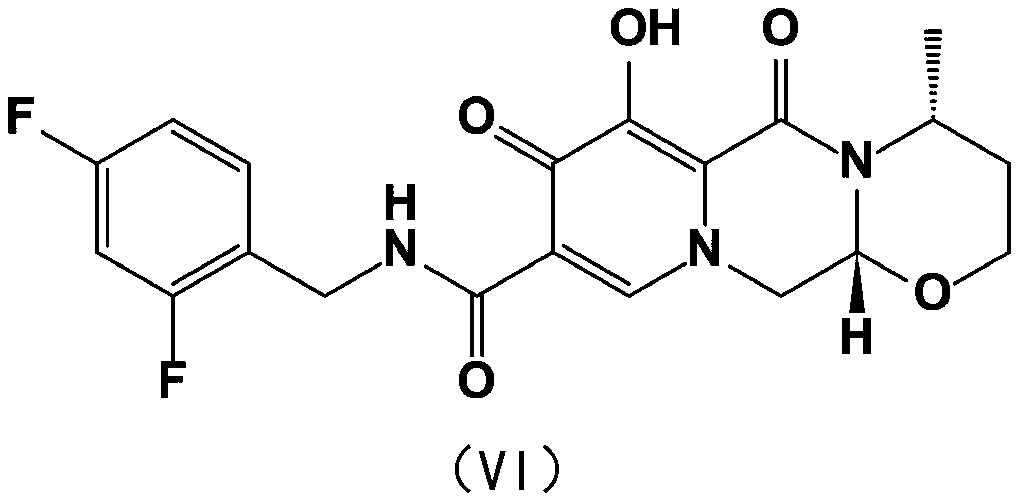
The invention provides a method for synthesizing a diastereomer impurity in a dolutegravir raw material, which comprises the following steps: 1, carrying out condensation reaction on SM1 serving as araw material and 2,4-difluorobenzylamine to generate a compound I; 2, reacting the compound I with an alkali in a reaction solvent to generate a compound II; 3, hydrolyzing the compound II under an acidic condition to generate a compound III; 4, carrying out acid catalytic reaction on the compound III and R-aminobutanol in a reaction solvent to generate a compound IV; 5, generating a compound V from the compound IV in a reaction solvent under the catalysis of a condensing agent; and 6, demethylating the compound V under the action of an alkali metal salt to generate an impurity VI. The synthetic method of the diastereoisomer impurity in the dolutegravir raw material is simple in process and available in raw material, and the prepared new impurity can provide a reference substance for quality analysis of dolutegravir, so that the quality standard of dolutegravir is improved.
Owner:BIONNA (BEIJING) MEDICAL TECH CO LTD
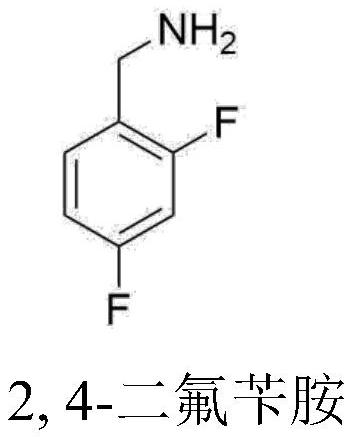
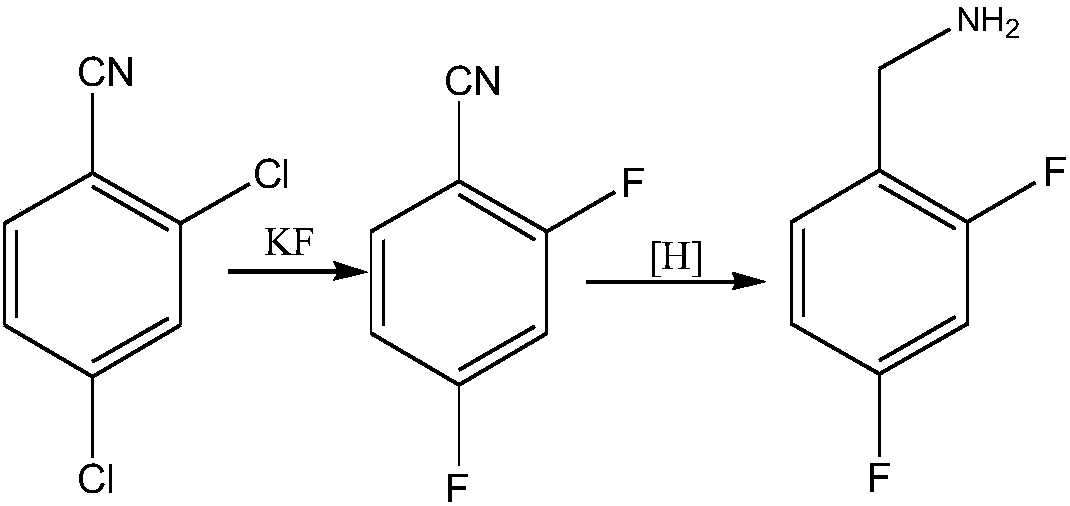
Novel method for synthesizing dolutegravir key intermediate 2,4-difluorobenzylamine
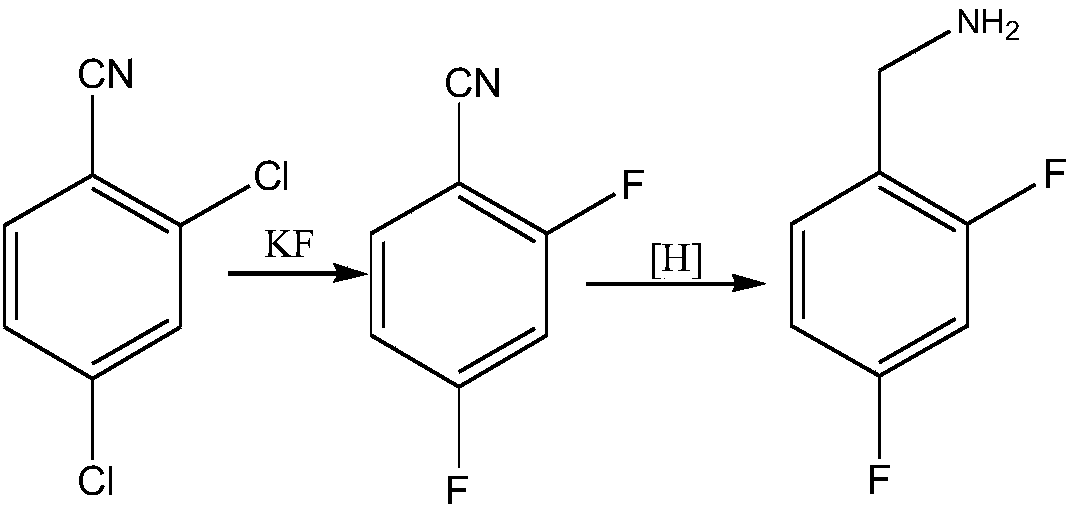
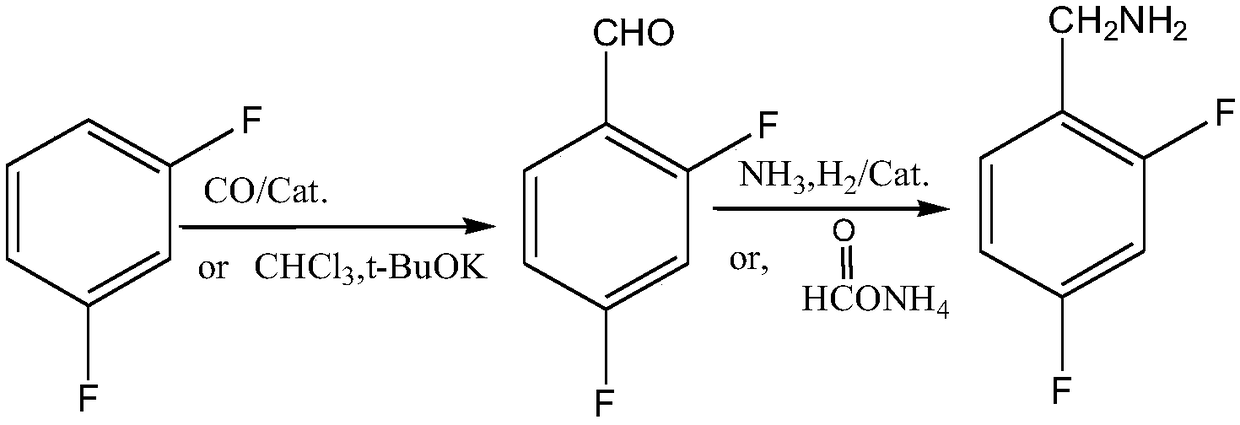
ActiveCN108752217ARaw materials are easy to obtainEasy to routePreparation by reductive alkylationPreparation by carbon monoxide reactionFormylation reactionCarbene
The invention discloses a novel production method which is concise and green in route, low in cost and easy to industrialize to prepare 2,4-difluorobenzylamine. The method comprises the following twosteps: a) at certain pressure, carrying out a carbonylation reaction on m-difluorobenzene and CO in the presence of a catalyst so as to generate 2,4-difluorobenzaldehyde, or carrying out a formylationreaction on m-difluorobenzene, namely carrying out chlorocarbene substitution on m-difluorobenzene in a chloroform strong base system, and further carrying out hydrolysis so as to prepare a product 2,4-difluorobenzaldehyde (Reimer-Tiemann reactions); b) putting the product 2,4-difluorobenzaldehyde of the step a) into an alcohol solvent, at certain pressure, in the presence of the catalyst, carrying out a reduction ammonolysis reaction on the component with an ammonia gas and hydrogen directly in the presence of a catalyst, or enabling the component to react with ammonium formate, thereby obtaining the 2,4-difluorobenzylamine. The preparation method disclosed by the invention is simple and easy in raw material obtaining, concise in route, green and environmentally friendly, low in cost andeasy in industrial production.
Owner:SHAXING CHEM TAIZHOU CITY
Preparation method of 2,4-difluorobenzylamine
PendingCN112410380ARaw material safetyThe production process is environmentally friendlyFermentationRedox enzymesBiochemical engineering
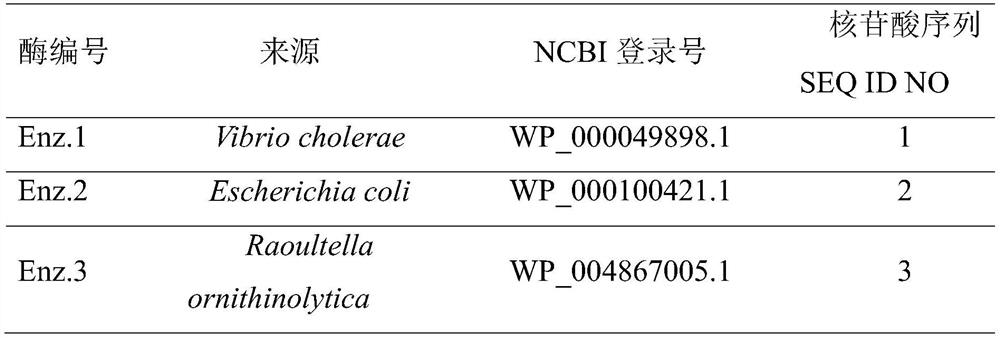
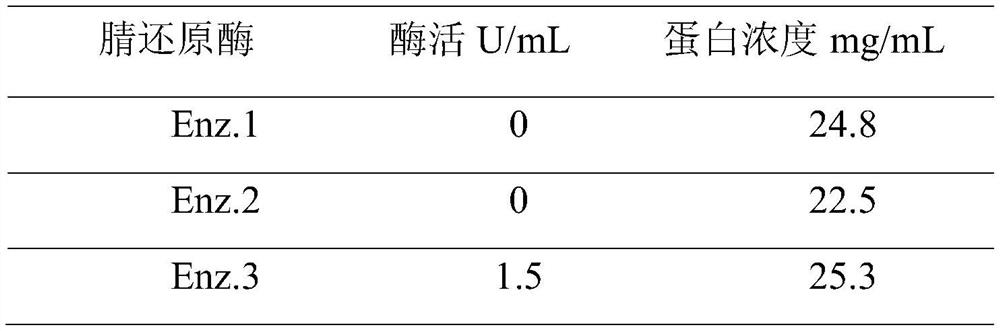
The invention discloses a preparation method of 2,4-difluorobenzylamine, which comprises the following steps: contacting 2,4-difluorobenzonitrile with nitrile oxidoreductase, and reducing 2,4-difluorobenzonitrile into 2,4-difluorobenzylamine. The nitrile oxidoreductase is nitrile oxidoreductase with an NCBI (National Center of Biotechnology Information) login number of WP_004867005.1. The invention also discloses an application of the nitrile oxidoreductase in preparation of dolutegravir or an intermediate of dolutegravir. According to the preparation method provided by the invention, the conversion rate of can reach 70%, the raw materials are safe, the production process is environmentally friendly, and the preparation method is suitable for large-scale industrial production.
Owner:ABIOCHEM BIOTECH CO LTD
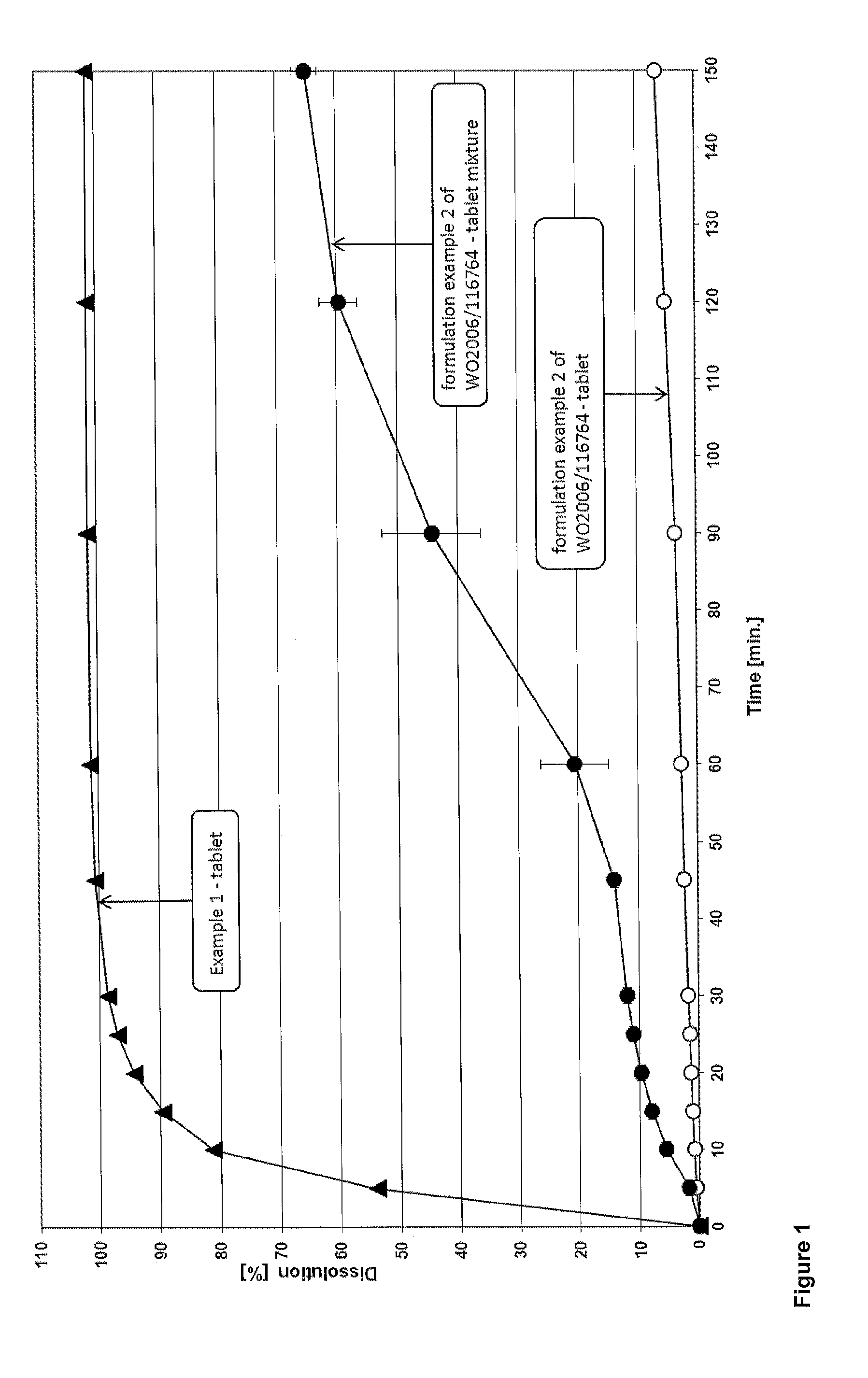
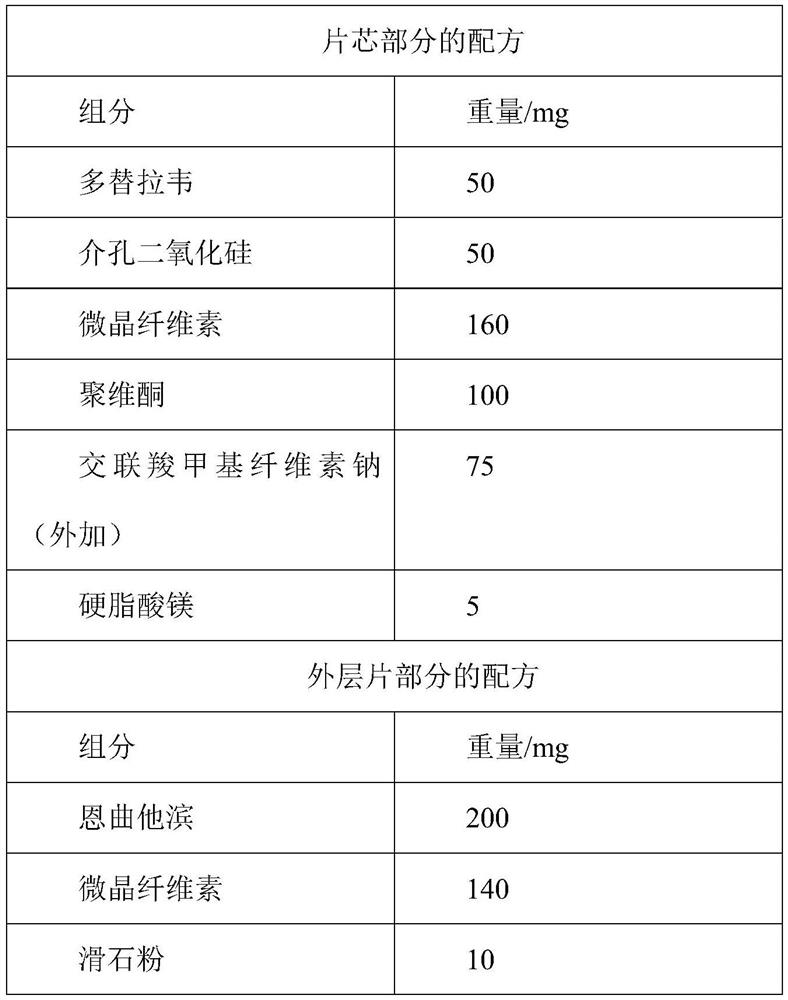
Dolutegravir film coated tablet preparation and preparation method thereof
InactiveCN107998095AStable blood concentrationImprove convenienceOrganic active ingredientsAntiviralsPolyethylene glycolSilica gel
The invention discloses a dolutegravir film coated tablet preparation and a preparation method thereof, wherein the dolutegravir film coated tablet preparation comprises the following active components by mass: 10-20% of dolutegravir, 10-90% of a diluent, 2.0-8.0% of a disintegrant, and 0.5-1.5% of a lubricant, wherein the diluent is one or a plurality of materials selected from mannitol, microcrystalline cellulose, lactose and polyethylene glycol, preferably mannitol and microcrystalline cellulose, the disintegrant is one or a plurality of materials selected from croscarmellose sodium, sodiumcarboxymethyl starch, povidone and hypromellose, preferably sodium carboxymethyl starch and povidone, and the lubricant is one or a plurality of materials selected from magnesium stearate, talc powder, micro-powder silica gel and sodium stearyl fumarate, preferably magnesium stearate and sodium stearyl fumarate. According to the present invention, the preparation method has advantages of simple process, high yield, good product stability, easy mass industrial production and the like.
Owner:顾世海
Process for the preparation of Dolutegravir
ActiveUS9573965B2High puritySimple and cost-effectiveOrganic compound preparationAmino-hyroxy compound preparationTartrateButanol
The present invention provides (R)-3-Amino-1-butanol (D)-tartarate (IIb); process for its preparation and its conversion to Dolutegravir. The present invention also provides an improved process for the preparation of Dolutegravir (I) or pharmaceutically acceptable salts wherein compound (XVI) is reacted with an optically active acid addition salt of (R)-3-amino-1-butanol (IIa).
Owner:AUROBINDO PHARMA LTD
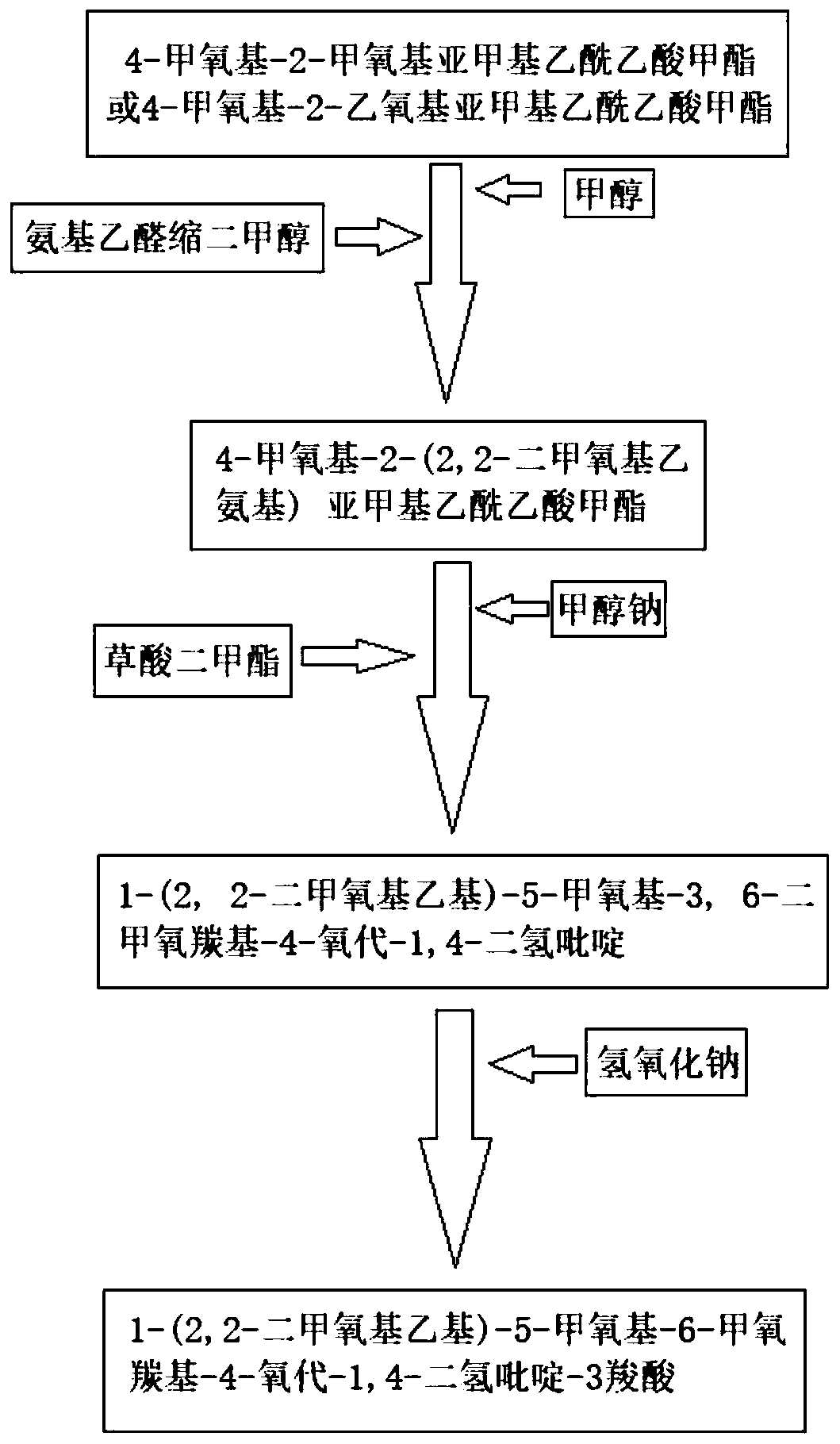
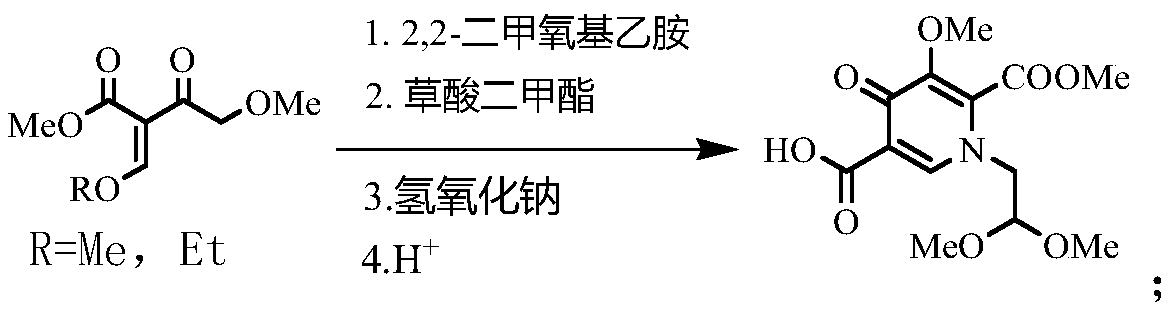
Synthesis method of dolutegravir key intermediate
InactiveCN110294705ASynthetic operation is simpleMild reaction conditionsOrganic chemistryDihydropyridineSynthesis methods
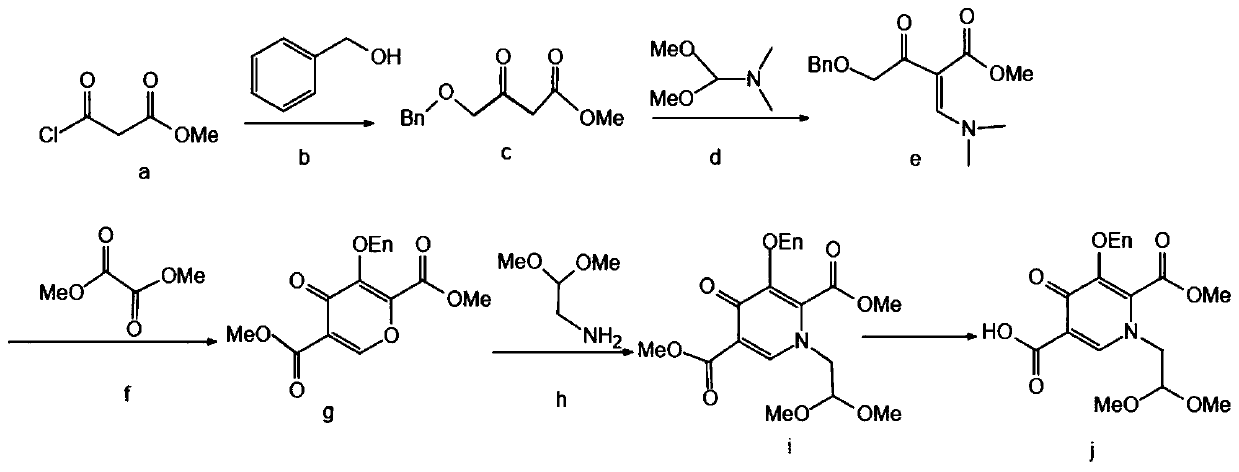
The invention discloses a synthesis method of an dolutegravir key intermediate, and relates to the field of dolutegravir compounds. The method includes the synthesis of 1-(2,2-dimethoxyethyl)-5-methoxyl-6-methoxycarbonyl-4-oxo-1,4-dihydropyridine-3 carboxylic acid, 4-methoxyl-2-methoxymethylene acetoacetate or 4-methoxyl-2-ethoxymethylene acetoacetate is taken as a raw material to synthesize the target product 1-(2,2-dimethoxyethyl)-5-methoxy-6-methoxycarbonyl-4-oxo-1,4-dihydropyridine-3 carboxylic acid through substitution, cyclization and hydrolysis reaction. The operation of synthesis is simple, the reaction conditions are mild, the cost is low, the product quality is good, the yield is high, the total yield is more than 90%, and the dolutegravir key intermediate is suitable for large-scale industrial production.
Owner:SHAXING CHEM TAIZHOU CITY
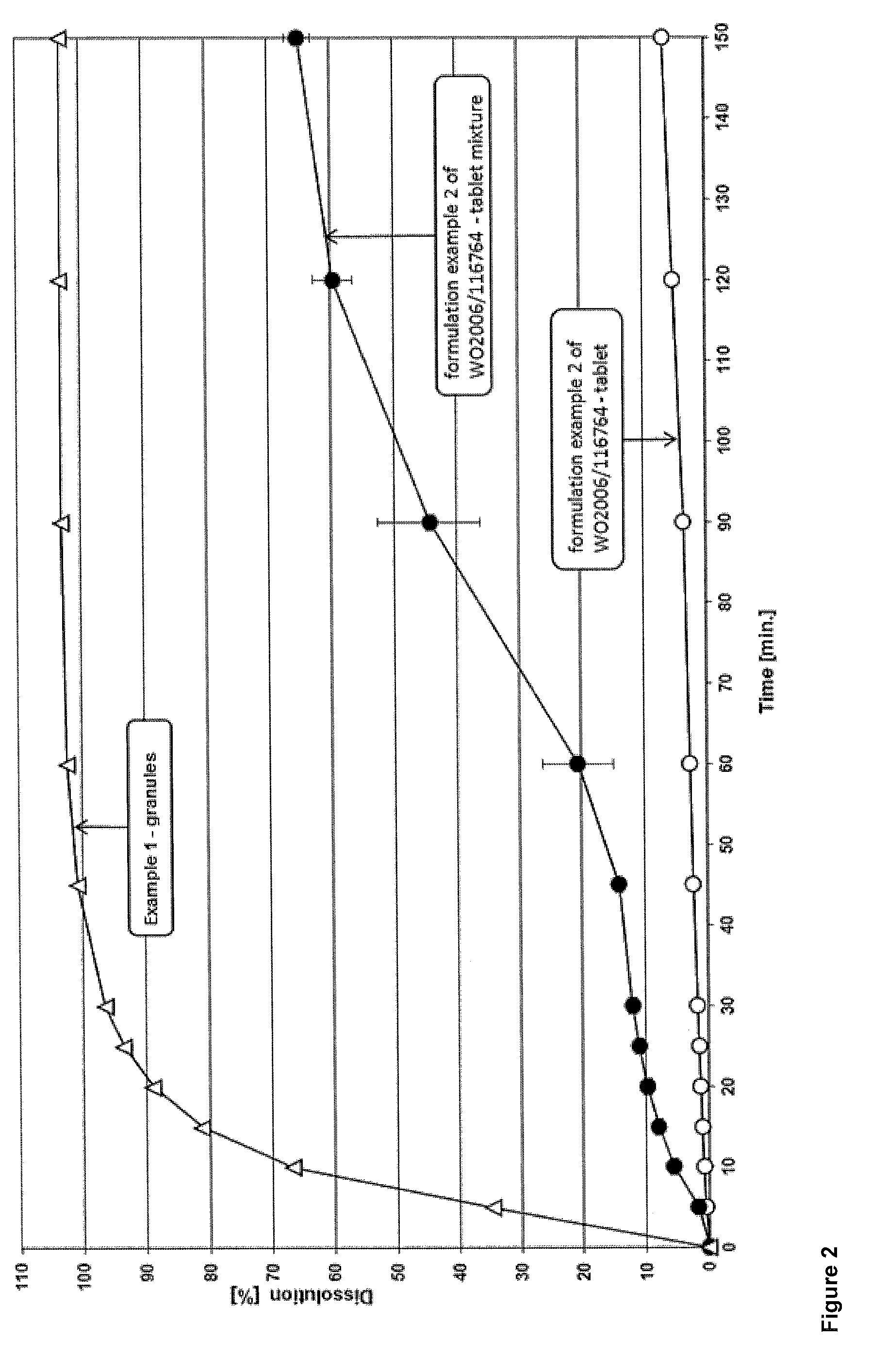
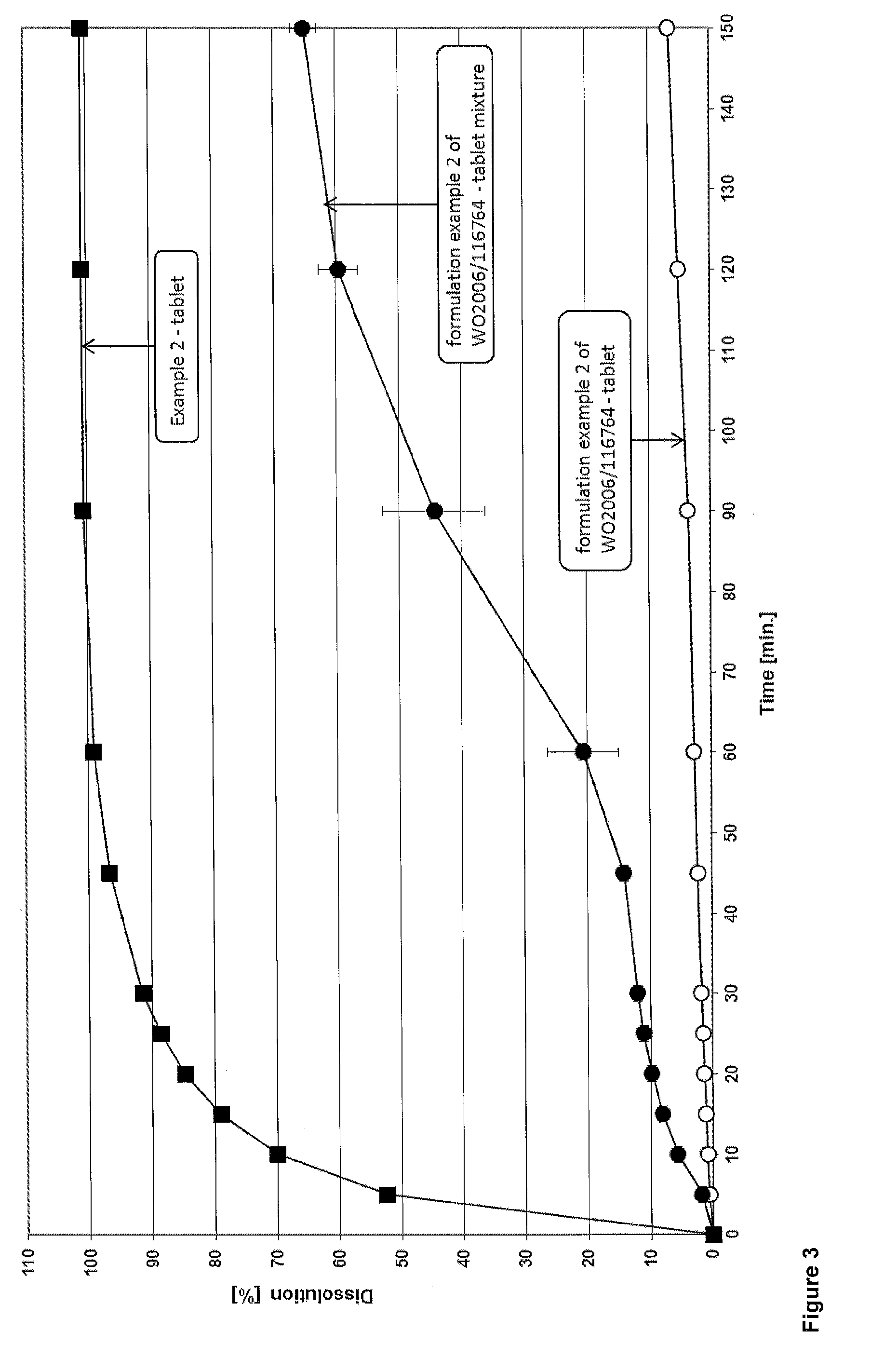
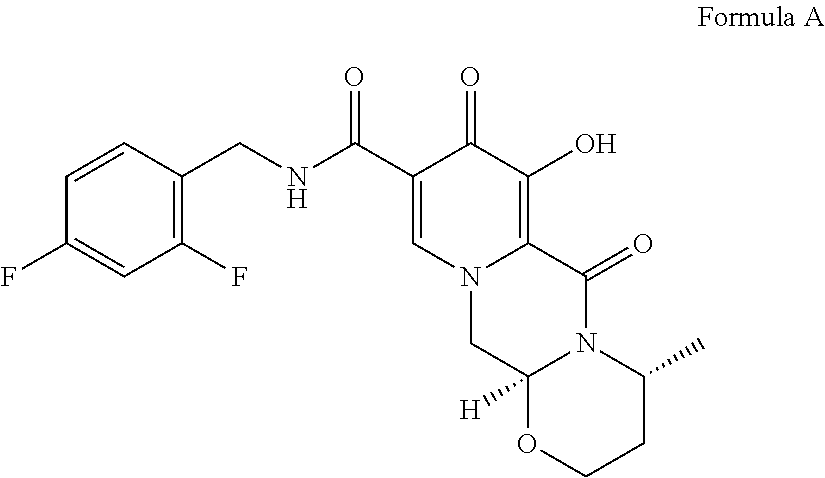
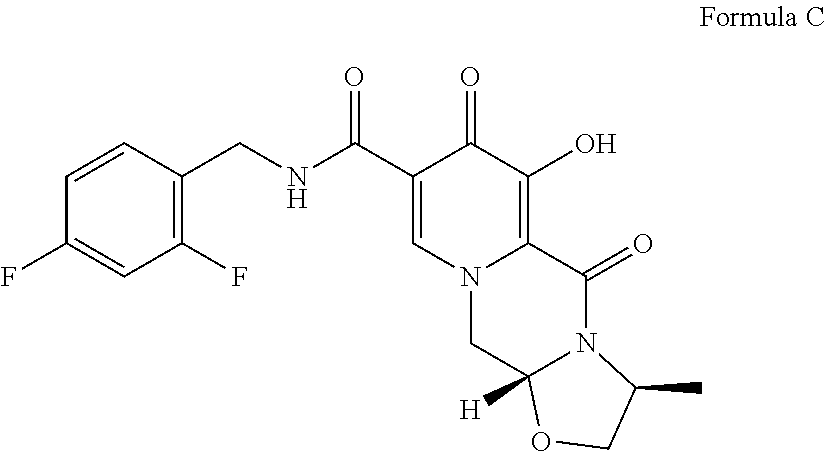
Solid pharmaceutical dosage form of dolutegravir
The present invention relates to a solid pharmaceutical dosage form comprising dolutegravir, a method of its preparation and its use in the treatment of an HIV infection.
Owner:RATIOPHARM GMBH
A Multi-Class Anti-Retroviral Composition
The present invention is related to an anti-retroviral composition. In particular, the present invention relates to a solid oral composition comprising combination of multi-class drugs particularly darunavir, dolutegravir and cobicistat and process for preparing the same.
Owner:HETERO LABS LTD
Processes for Preparing Dolutegravir and Cabotegravir and Analogues Thereof
ActiveUS20170368040A1Process environmental protectionOrganic active ingredientsOrganic chemistryCabotegravirStereochemistry
The present invention relates to processes for preparing substances with antiviral activity, in particular the integrase inhibitors dolutegravir and cabotegravir and analogues thereof, as well as intermediates useful in the processes.
Owner:LEK PHARMA D D
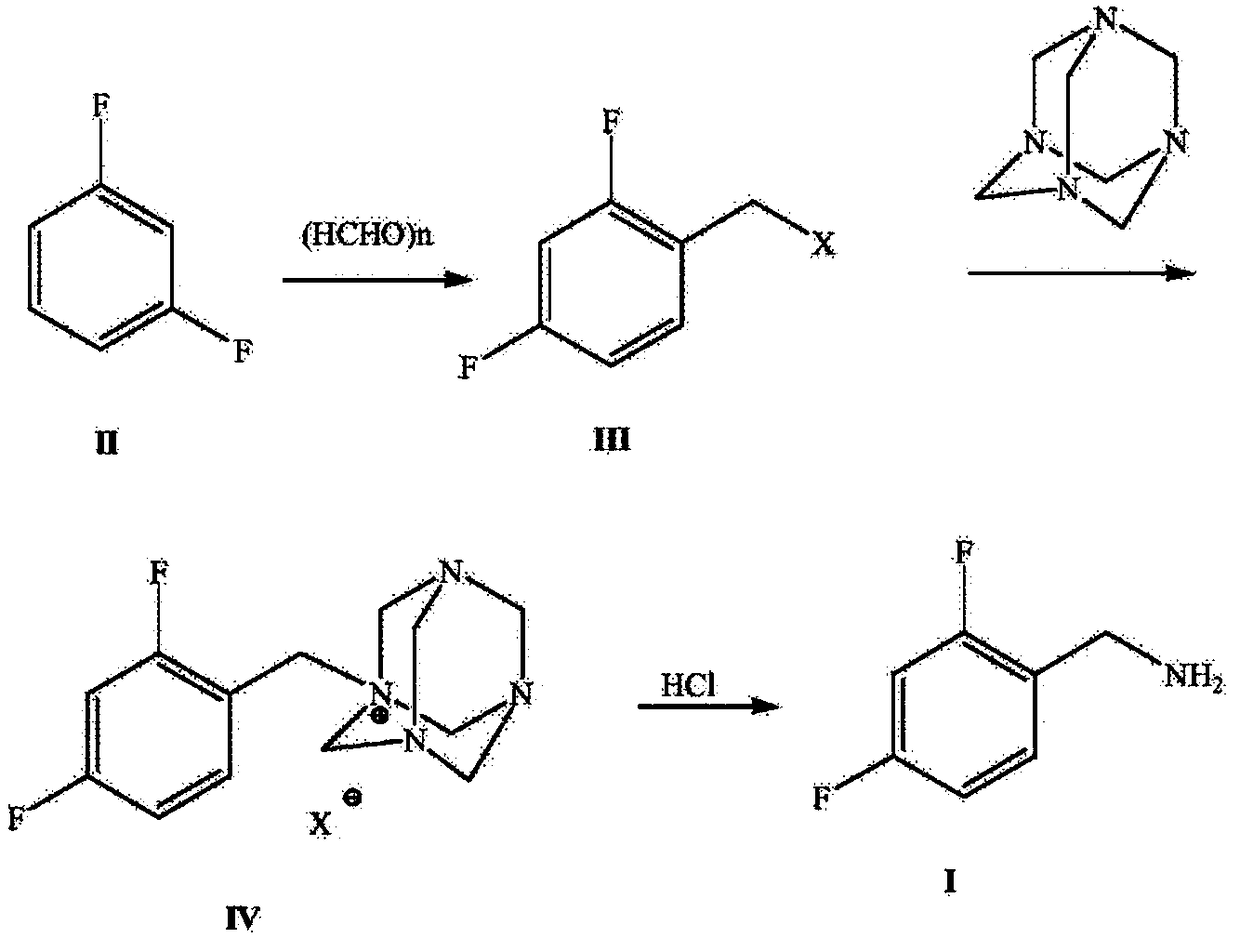
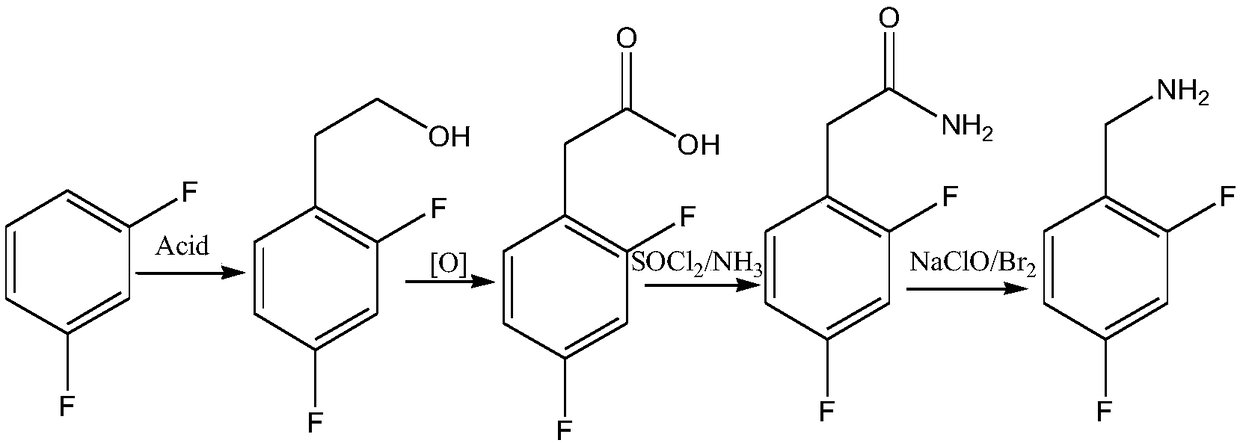
Novel route for preparing dolutegravir key intermediate 2,4-difluorobenzylamine
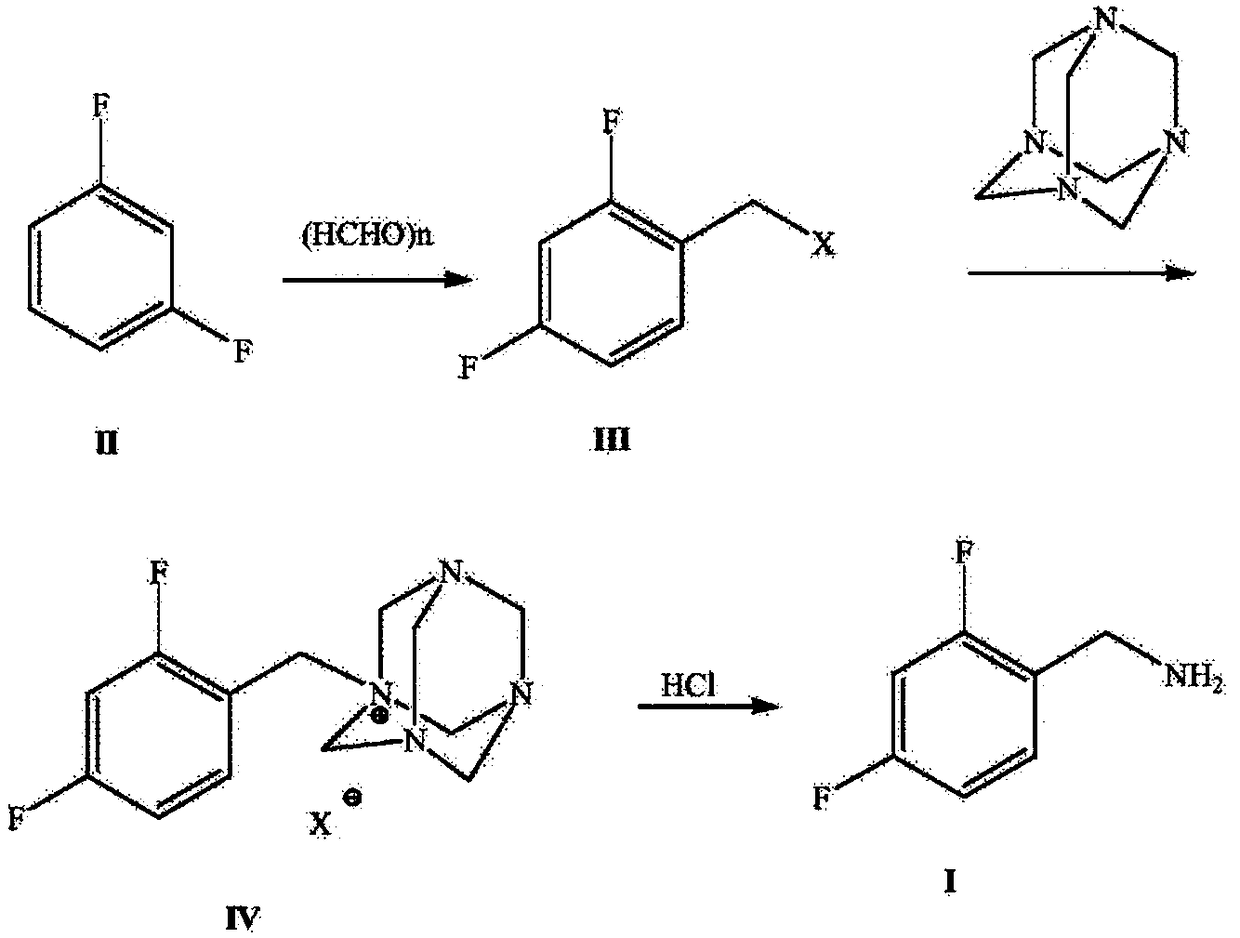
ActiveCN108752218ARaw materials are easy to obtainEasy to routeOrganic compound preparationHydroxy compound preparationHalichondramidePhenylacetic acid
The invention discloses a production route which is concise and green in route, low in cost and easy to industrialize to prepare 2,4-difluorobenzylamine. The route comprises the following four steps:a) by taking m-difluorobenzene as an initial raw material, under catalysis of lewis acid, carrying out a Foucault alkylation reaction with ethylene oxide so as to prepare an intermediate 2,4-difluorobenzene ethanol; b) carrying out a second step of reactions on the product of the step a) without separation or purification, and oxidizing the 2,4-difluorobenzene ethanol to generate 2,4-difluorobenzene phenylacetic acid; c) enabling the product 2,4-difluorobenzene phenylacetic acid of the step b) with sulfoxide chloride and an ammonia gas to react so as to prepare 2,4-difluorophenylacetamide; d)under induction of bromine, enabling the product 2,4-difluorophenylacetamide of the step c) to react with a sodium hypochlorite solution, and carrying out Hofmann degradation, thereby obtaining a target product 2,4-difluorobenzylamine. The preparation method disclosed by the invention is simple and easy in raw material obtaining, concise in route, green and environmentally friendly, low in cost and easy in industrial production.
Owner:浙江沙星科技股份有限公司
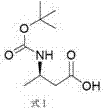
Preparation method of (R)-3-aminobutanol
ActiveCN106966912AHigh optical purityShort reaction pathCarbamic acid derivatives preparationOrganic compound preparationEnvironmental resistanceButyric acid
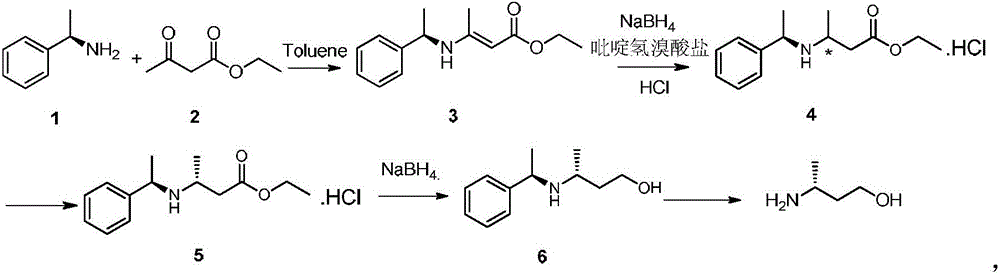
The invention relates to the technical field of synthesis of drug intermediates and particularly relates to a preparation method of dolutegravir intermediate (R)-3-aminobutanol, comprising: using 3-(Boc-amino)butyric acid as a starting material, chirally splitting to obtain a compound of formula I; reducing with sodium borohydride and Lewis acid to obtain a compound of formula II; performing amino deprotection to obtain (R)-3-aminobutanol. The materials used herein are low in price and easy to obtain, the reaction conditions are mild, the safety is reliable, process stability is high, the yield is high, optical purity is high, and the preparation method is green and suitable for industrial production.
Owner:CANGZHOU SENARY CHEM SCI TEC
Anti-retroviral compositions
The present invention relates to pharmaceutical antiretroviral compositions comprising a combination of antiretroviral agents (darunavir, dolutegravir and ritonavir), the manufacturing process thereof and use of the said compositions for the prevention, treatment or prophylaxis of HIV infection.
Owner:HETERO LABS LTD
Antiretroviral pharmaceutical composition and preparation method thereof
ActiveCN111991558AReasonably high bioavailabilityGood dissolution effectOrganic active ingredientsAntiviralsNucleoside Reverse Transcriptase InhibitorEmtricitabine
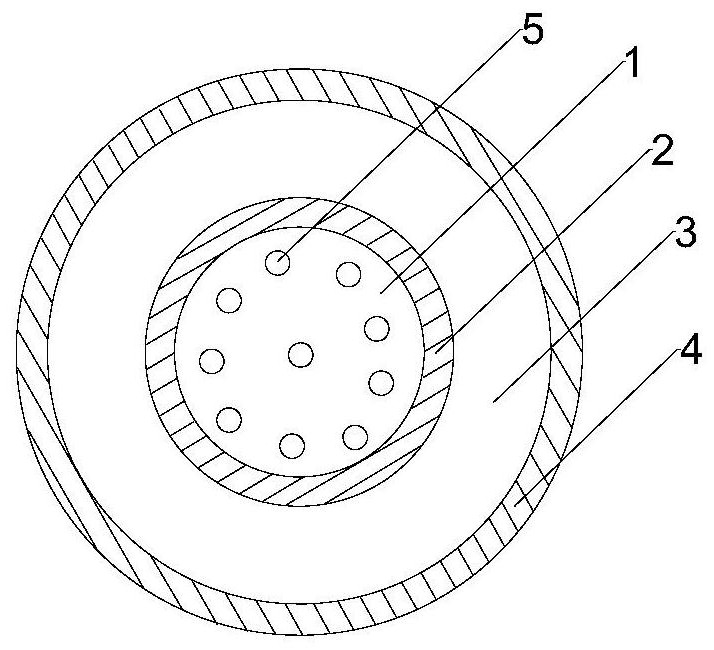
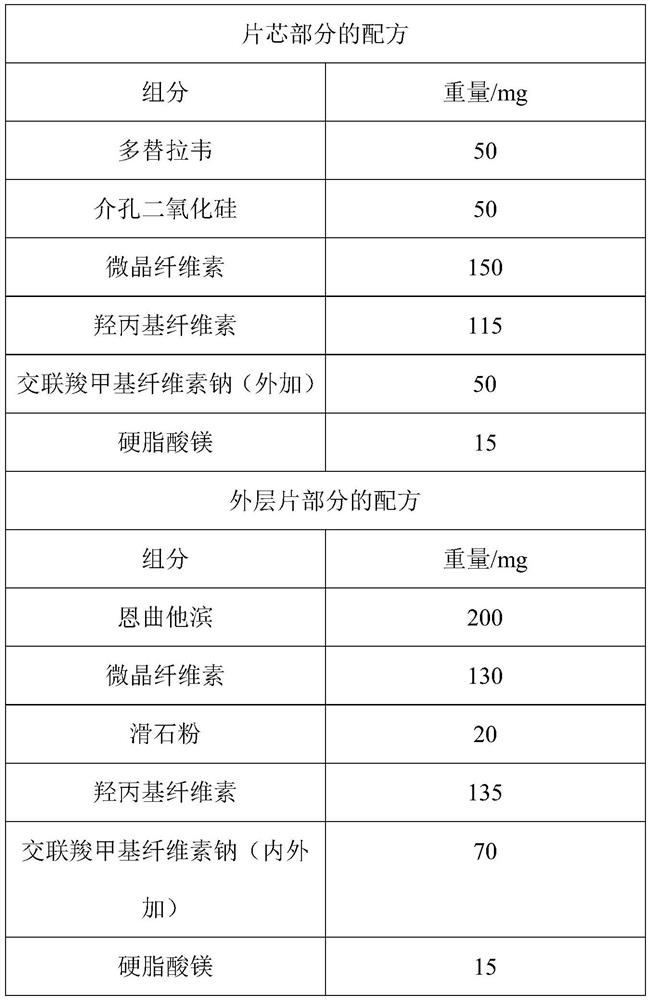
The invention discloses an antiretroviral pharmaceutical composition. The pharmaceutical composition comprises a nucleoside reverse transcriptase inhibitor, an integrase inhibitor and one or more pharmaceutically acceptable excipients, wherein the weight ratio of the nucleoside reverse transcriptase inhibitor to the integrase inhibitor is 4: 1. The invention also discloses a preparation method ofthe composition. In the preparation method, plasma-modified mesoporous silica is used as a carrier to prepare a dolutegravir and modified mesoporous silica solid dispersion, so that the bioavailability of dolutegravir is improved; a tablet is designed into a coated tablet core, and permeation holes are formed in the tablet core, so that the dissolution rate of the main drug dolutegravir in the tablet core is increased, the dissolution rates of the two main drugs dolutegravir and emtricitabine are synchronous, the synergistic effect of the two main drugs is increased, the stability of the tablet is improved, and the curative effect is enhanced; and the prepared compound coated tablet core is high in bioavailability, good in dissolution effect of active ingredients, good in stability and lowin content of related substances.
Owner:ANHUI BIOCHEM BIO PHARMA
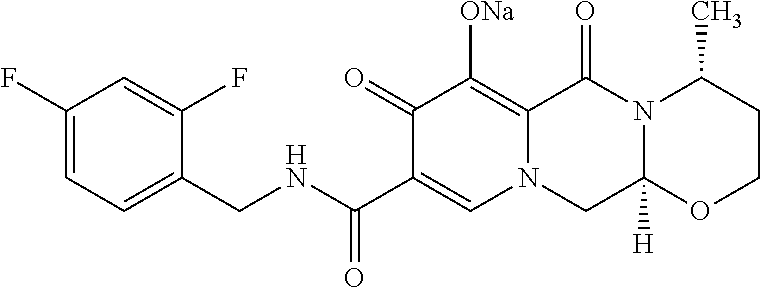
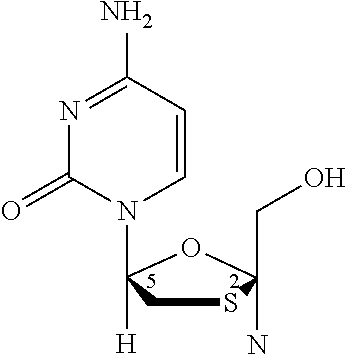
Methods for treating HIV with dolutegravir and lamivudine
Invented are methods for treating HIV in a human in need thereof which comprises the administration of a therapeutically effective amount of a combination of dolutegravir or a pharmaceutically acceptable salt thereof and lamivudine or a pharmaceutically acceptable salt thereof, to such human.
Owner:VIIV HEALTHCARE CO
Anti-retroviral compositions
ActiveUS11045423B2Pharmaceutical product form changeAntiviralsPharmaceutical drugANTIRETROVIRAL AGENTS
The present invention relates to pharmaceutical antiretroviral compositions comprising a combination of antiretroviral agents (darunavir, dolutegravir and ritonavir), the manufacturing process thereof and use of the said compositions for the prevention, treatment or prophylaxis of HIV infection.
Owner:HETERO LABS LTD
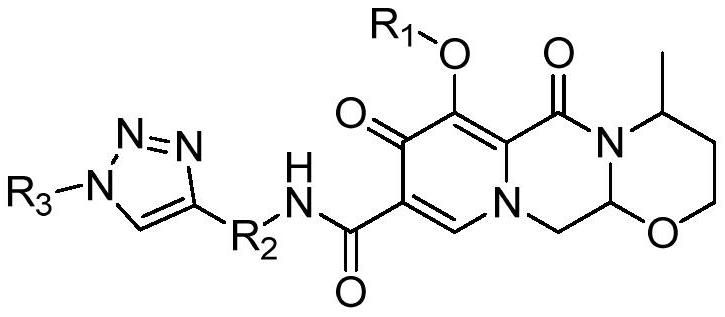
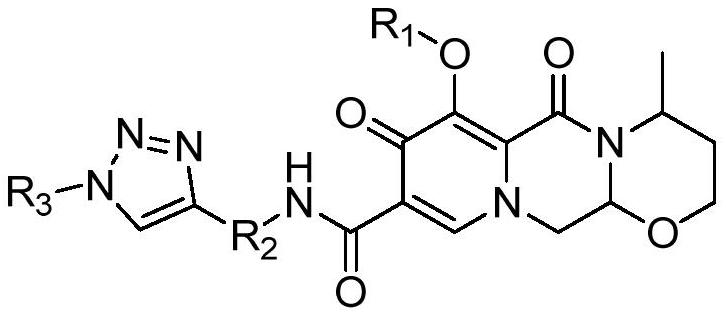
Triazole derivatives capable of affecting calcium ion channels of tumor cells, preparation method and application thereof
ActiveCN113527334BShort reaction pathHigh reaction yieldOrganic chemistry methodsAntineoplastic agentsChemical synthesisCalcium flux
The invention relates to the technical field of pharmaceutical chemical synthesis, in particular to a triazole derivative capable of affecting calcium ion channels of tumor cells, a preparation method and application thereof. The present invention uses dolutegravir as a lead compound, utilizes the principle of drug splicing, and through a click reaction, changes the benzyl group in the dolutegravir structure into a 1,2,3-triazole structure to obtain a new compound, It can inhibit the growth of tumor cells by affecting calcium ion channels in tumor cells, and has significant application value.
Owner:JINAN ASIA PHARMA TECH +1
A kind of synthetic method of dolutegravir intermediate and its related substance detection method
ActiveCN108101838BSimple reaction conditionsAnalysis method is simpleOrganic chemistryComponent separationCombinatorial chemistryMethyl acetate
Owner:汉瑞药业(荆门)有限公司
Dolutegravir derivative with biological activity as well as preparation method and application thereof
ActiveCN113527333AGrowth inhibitionNovel structureOrganic chemistryAntineoplastic agentsChemical synthesisHuman tumor
The invention relates to the technical field of pharmaceutical chemistry synthesis, in particular to a dolutegravir derivative with biological activity and a preparation method thereof. The structural formula of the derivative is shown in the specification, wherein R1 is methyl or hydrogen; R2 is methyl, isopropyl, phenyl, benzyl, a nitrogen-containing heterocyclic ring, a sulfur-containing heterocyclic ring or an oxygen-containing heterocyclic ring; and R3 is hydrogen, methyl, ethyl, phenyl, benzyl, a nitrogen-containing heterocyclic ring, a sulfur-containing heterocyclic ring or an oxygen-containing heterocyclic ring. The preparation method comprises the following steps of: deprotecting 1-(2, 2-dimethoxyethyl)-1, 4-dihydro-3-methoxy-4-oxo-2, 5-pyridinedicarboxylic acid-2-methyl ester to obtain aldehyde, cyclizing the aldehyde with aminobutanol, then carrying out acylation reaction on the cyclized aldehyde and an amino compound, and finally carrying out click reaction with azide to obtain a target compound. The derivative provided by the invention has proliferation inhibition activity on human tumor cells.
Owner:NANKAI UNIV +2
Processes for preparing dolutegravir and cabotegravir and analogues thereof
The present invention relates to processes for preparing substances with antiviral activity, in particular the integrase inhibitors dolutegravir and cabotegravir and analogues thereof, as well as intermediates useful in the processes.
Owner:LEK PHARMA D D
Combination and uses and treatments thereof
PendingUS20200113838A1Improve stabilityStability benefitOrganic active ingredientsAntiviralsPharmaceutical drugPharmaceutical medicine
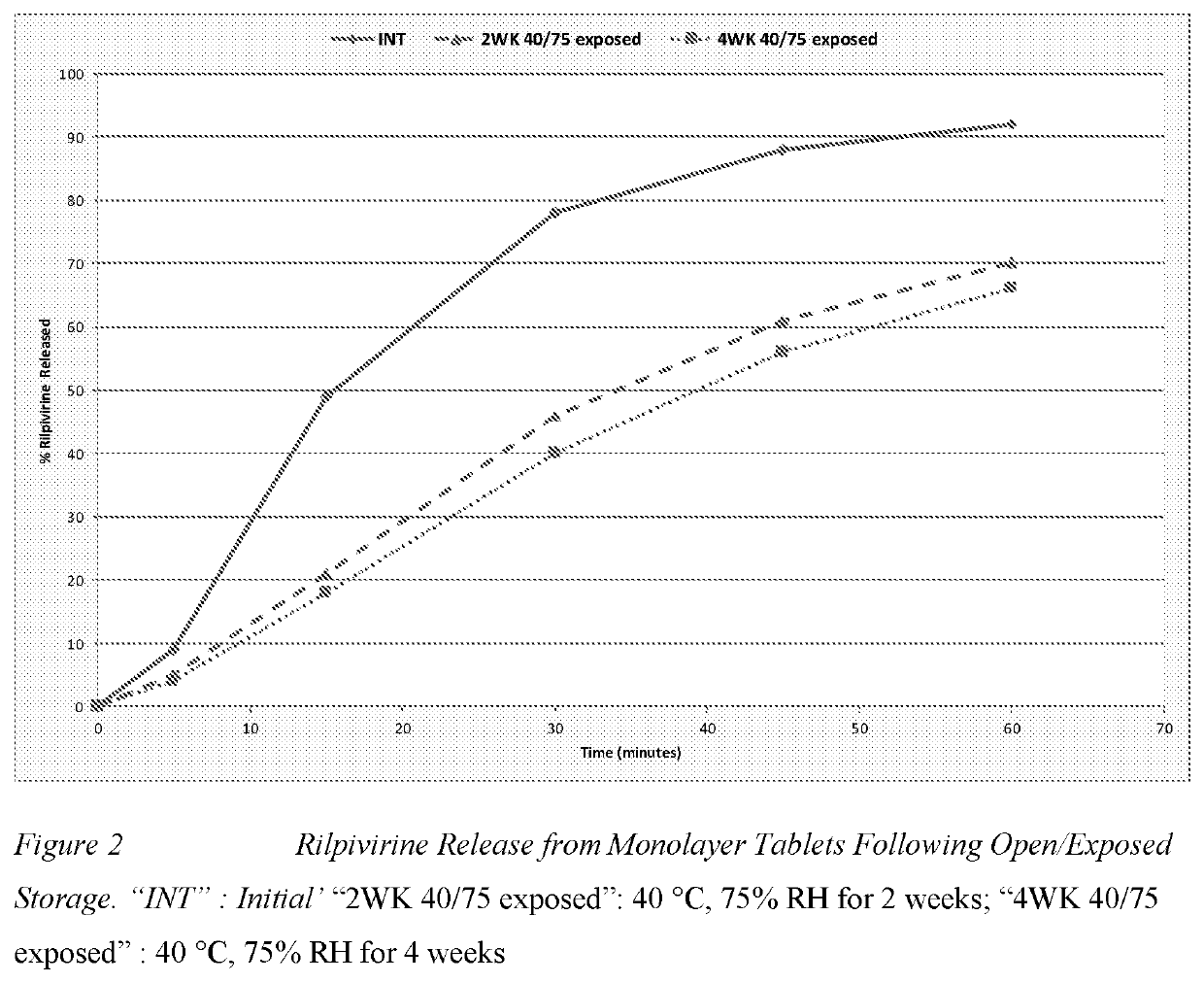
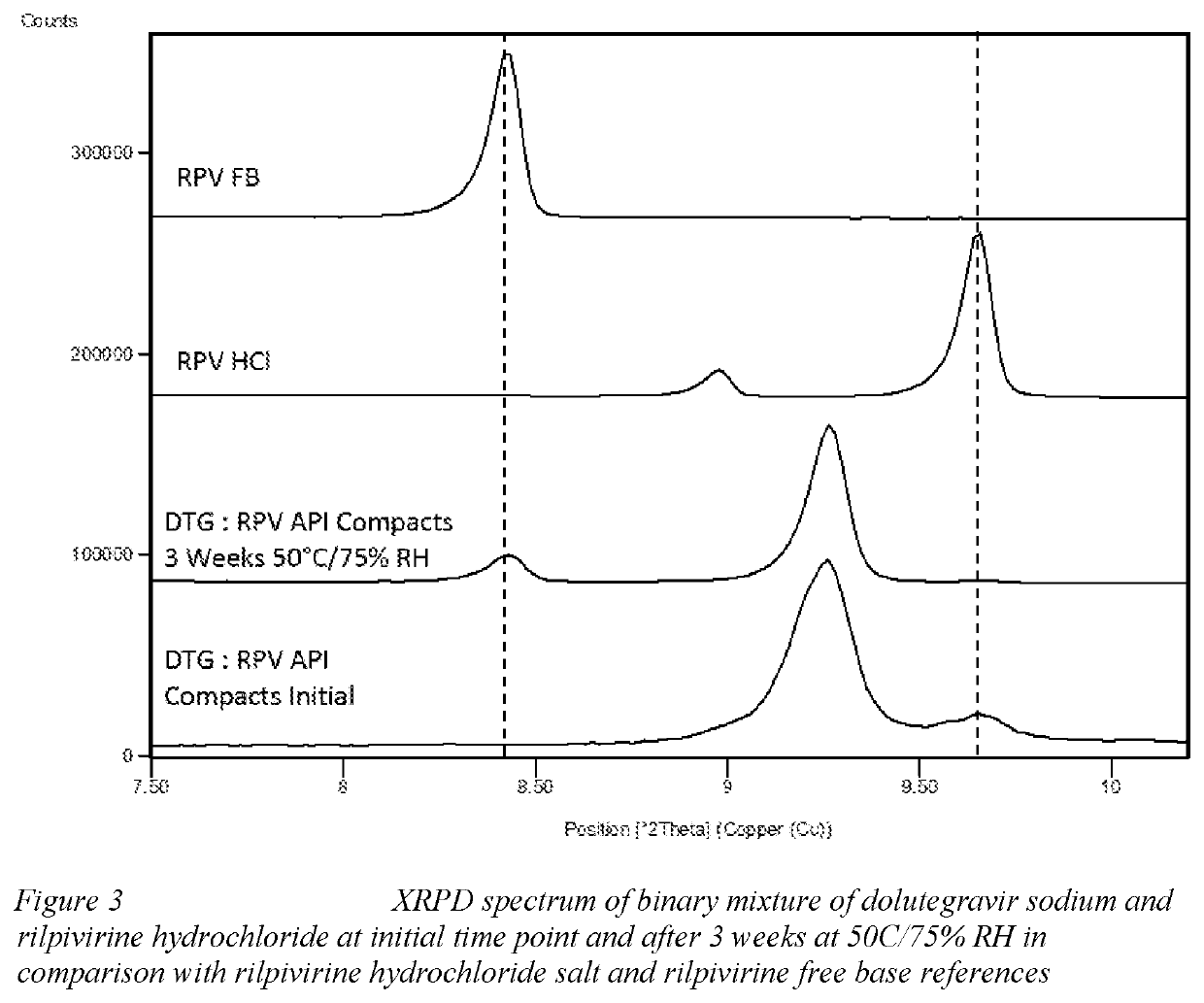
Methods are provided for treating or preventing human immunodeficiency virus-1 (HIV-1) or human immunodeficiency virus-2 (HIV-2) in a virologically suppressed patient in need thereof comprising switching the patient from an antiretroviral treatment regimen comprising at least three antiretroviral agents to a treatment regimen comprising only two antiretroviral agents. In one aspect the two treatment regimen consists of dolutegravir, rilpivirine and at least one pharmaceutically acceptable excipient, diluent or carrier. In another aspect of the invention, there is provided a multilayer tablet comprising dolutegravir or a pharmaceutically acceptable salt thereof and rilpivirine or a pharmaceutically acceptable salt thereof.
Owner:VIIV HEALTHCARE CO +1
Synthesis method of diastereoisomer impurities in dolutegravir raw materials and intermediates
InactiveCN110128448ARaise quality standardsSimple processOrganic chemistryBulk chemical productionAlcoholSynthesis methods
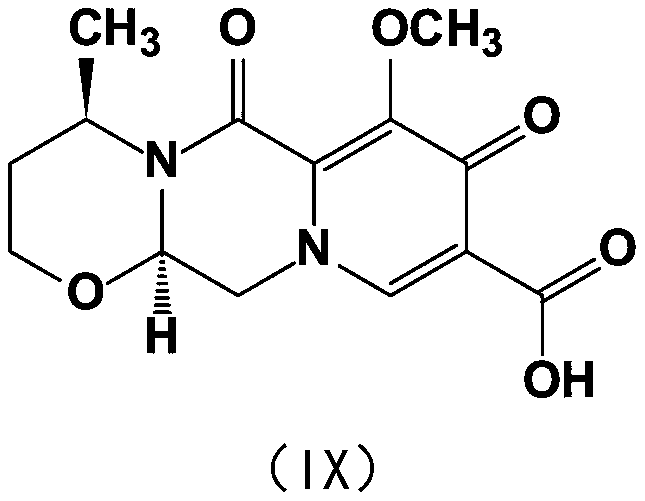
The invention provides a synthesis method of diastereoisomer impurities in dolutegravir raw materials and intermediates. The synthesis method comprises the following steps: 1) with a compound V as a starting raw material, performing a reaction with a large-steric-hindrance protecting group under an alkaline condition to generate a compound VII; 2) carrying out alcoholysis on the compound VII and areaction solvent under an alkaline condition to generate a compound VIII; and 3) carrying out hydrolysis on the compound VIII and an alcohol solvent under an alkaline condition to obtain a compound IX. The invention provides the synthesis method of the diastereoisomer impurities in dolutegravir raw materials and intermediates, which is simple in process and easily available in raw materials. Theprepared new impurity can provide a reference substance for quality analysis of dolutegravir, so that the quality standard of dolutegravir is improved.
Owner:BIONNA (BEIJING) MEDICAL TECH CO LTD
Synthesis method of dolutegravir core intermediate
ActiveCN114349635AAchieve separationGood choiceOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCombinatorial chemistryMethyl acetate
The invention discloses a synthesis method of a dolutegravir core intermediate. Comprising the following steps: firstly adding methanol into a reaction kettle, stirring, then adding strongly basic anion exchange resin, finally adding a raw material methyl 4-chloroacetoacetate, heating to reflux, reacting for 4-8 hours and the like. The reaction post-treatment process is greatly simplified, the process is simple, few three wastes are generated, and the cost is low. According to the method, the yield of the ethyl 4-methoxy acetoacetate is greater than 98%, and the content of the ethyl 4-methoxy acetoacetate is greater than 99%.
Owner:JIANGSU YUXIANG CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com