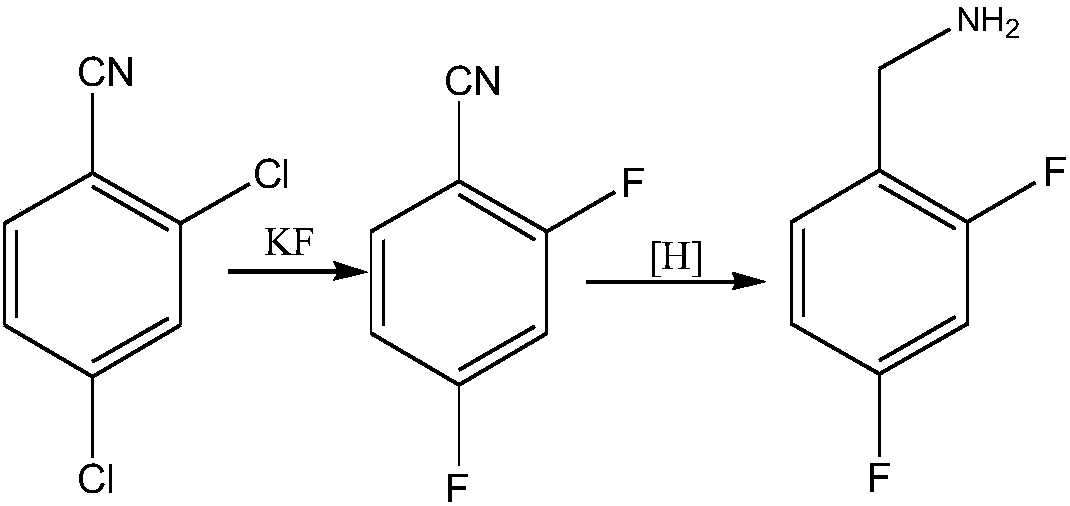
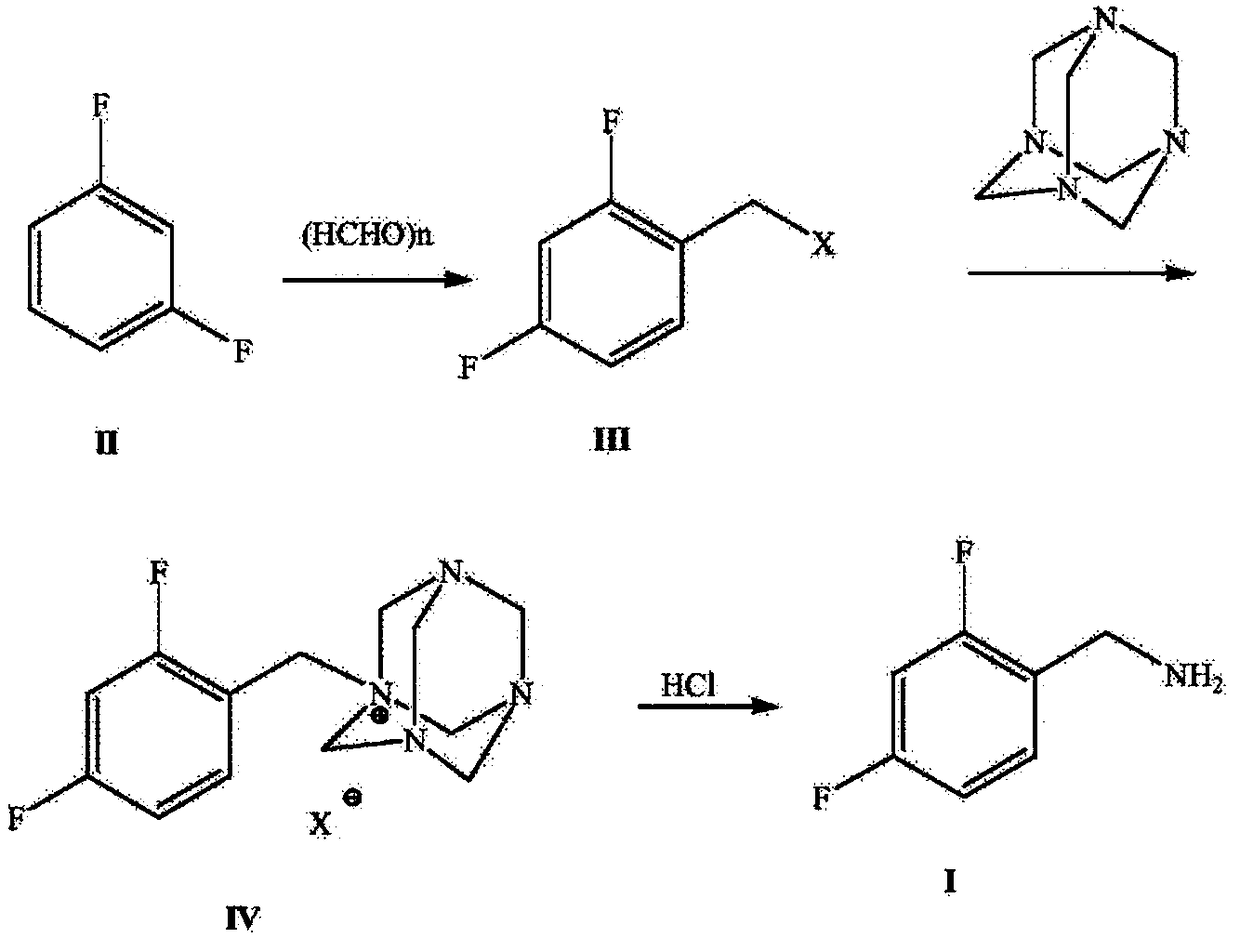
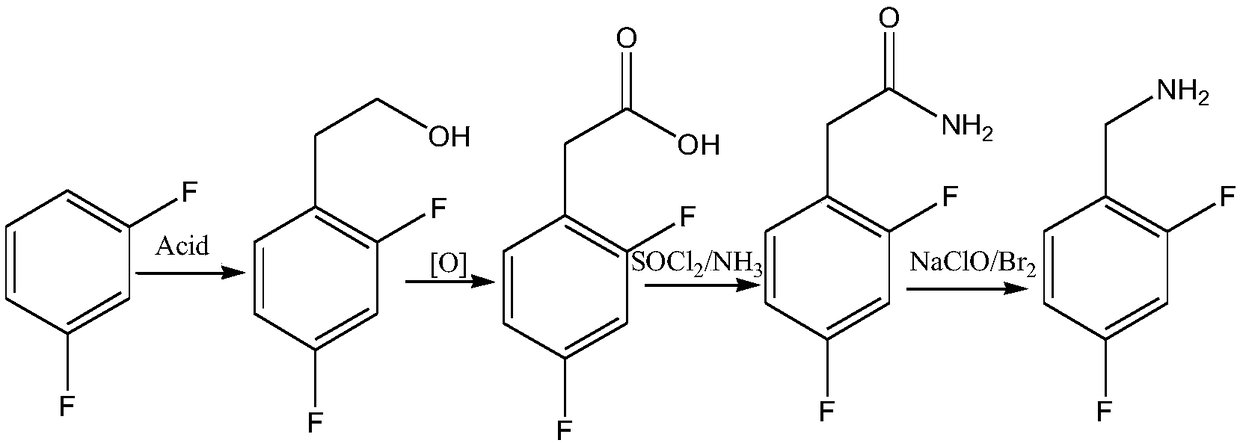
Novel route for preparing dolutegravir key intermediate 2,4-difluorobenzylamine
A technology of difluorobenzylamine and dolutegravir, which is applied in the field of new synthesis routes of 2,4-difluorobenzylamine, a key intermediate of dolutegravir, can solve the problems of high cost, harsh operating conditions, low yield, etc. problem, to achieve the effect of simple route, easy access to raw materials, and simple raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] In order to illustrate the technical solutions in the embodiments of the present invention more clearly, the embodiments of the present invention will be described in detail below in conjunction with the examples, but those skilled in the art will understand that the following examples are only used to illustrate the present invention, and should not considered as limiting the scope of the invention. Those who do not indicate the specific conditions in the examples are carried out according to the conventional conditions or the conditions suggested by the manufacturer. The reagents or instruments used were not indicated by the manufacturer, and they were all conventional products that could be purchased from the market.
[0025] Take m-difluorobenzene 114g (1mol), AlCl 3 173g (1.3mol) was added to 167g nitrobenzene (200ml, 1.36mol), heated to 70°C, and 46.5g (1.05mol) of ethylene oxide was slowly introduced under vigorous stirring, the system had obvious exotherm, cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com