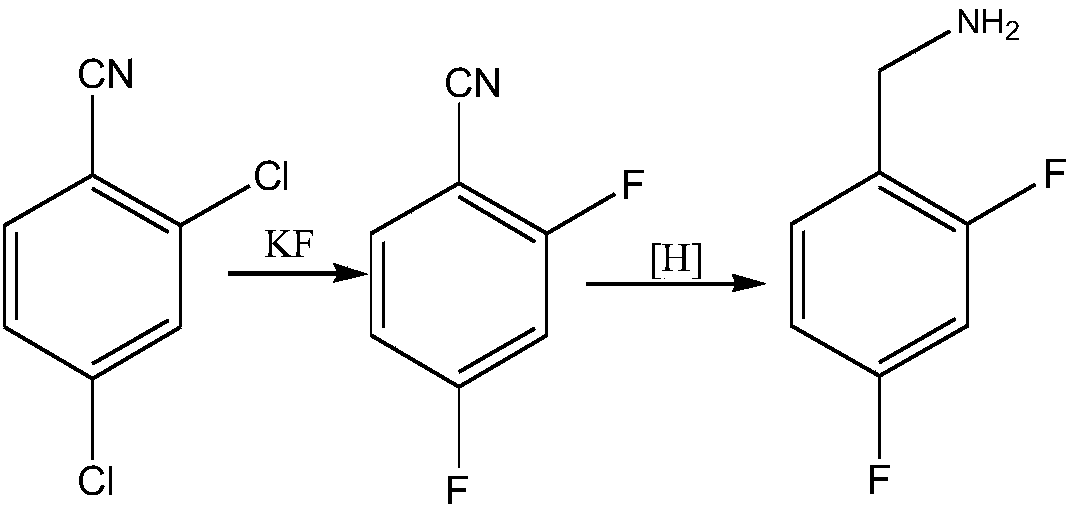
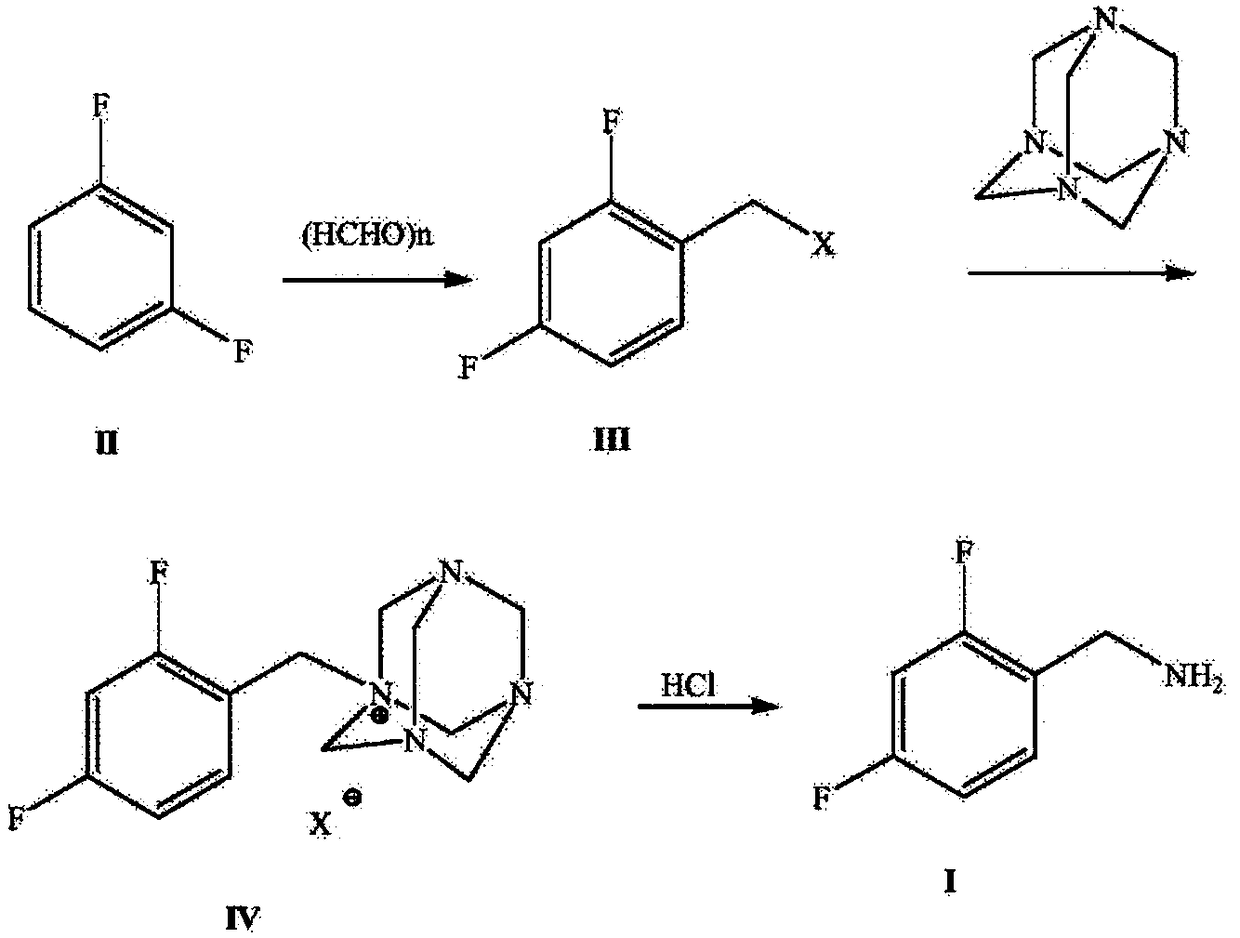
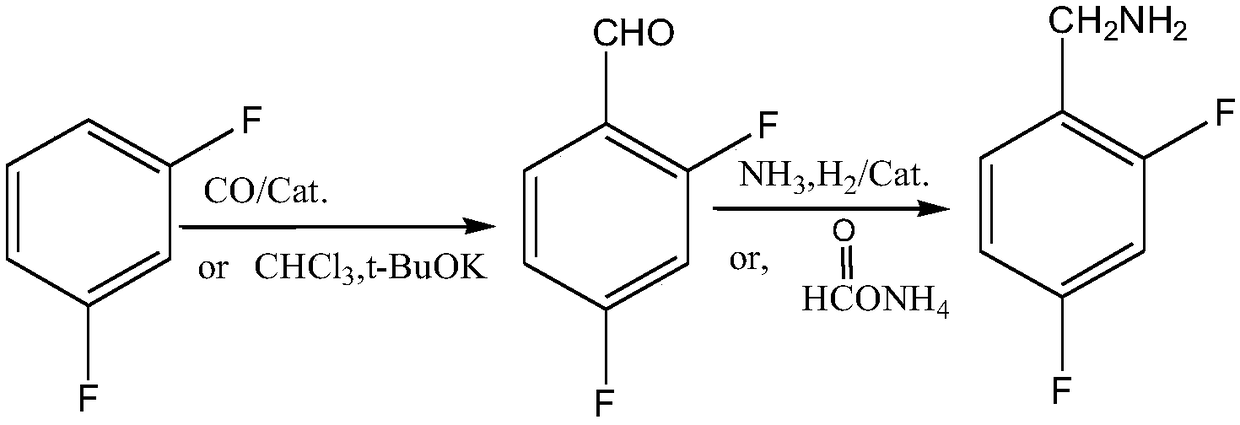
Novel method for synthesizing dolutegravir key intermediate 2,4-difluorobenzylamine
A technology of difluorobenzylamine and dolutegravir, applied in the field of synthesis of 2,4-difluorobenzylamine, the key intermediate of dolutegravir, can solve the problems of harsh operating conditions, low yield and high cost, and achieve The effect of simple raw materials, easy access to raw materials, and simple routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Take m-difluorobenzene 114g (1.0mol), AlCl 3 133g (1.0mol) was added to 300ml containing 4.6g Na(Co(CO) 4 ) (0.024mol) in methanol solution (self-made), vacuumize, replace with nitrogen 3 times, fill with CO under stirring until the system pressure reaches 3.5MPa, heat to 60°C, the heat release is severe at the initial stage of the reaction, and condensed water is introduced to control the temperature Not more than 80°C. After 3 hours the reaction was complete. After the reaction, the residual gas was discharged, and 80ml of 10% hydrochloric acid was slowly dropped into the system, filtered, and most of the solvent was evaporated from the filtrate under reduced pressure, and the obtained pale yellow transparent liquid was directly subjected to the next step of reaction.
[0030] Add the light yellow transparent liquid in the previous step to 400ml of methanol, add 5.7g of Raney nickel while stirring, vacuumize the system for nitrogen replacement 3 times, slowly inject...
Embodiment 2
[0032] Take m-difluorobenzene 114g (1mol) and chloroform 125g (1.05mol) into 300ml tetrahydrofuran, stir to dissolve, keep the temperature between 30-40°C, slowly add potassium tert-butoxide 118g (1.05mol) in batches under stirring, The whole feeding process is about 1.5 hours. After the addition, continue to stir and react for 1 hour, filter, and evaporate most of the tetrahydrofuran. Slowly add 100 g (2.1 mol) of formic acid to the system, raise the temperature to 65 ° C and stir for 3 hours. After the reaction, evaporate under reduced pressure. To remove most of the excess formic acid, add 150ml of dichloromethane to the system, wash with 200ml of sodium bicarbonate once, wash once with 200ml of saturated saline, separate the organic layer, distill off the dichloromethane, and the obtained pale yellow liquid is directly used in the next step reaction.
[0033] Add the light yellow liquid oil in the previous step to 400ml of methanol, add 10.5g of 5% Pd / C while stirring, vac...
Embodiment 3
[0035] Take m-difluorobenzene 114g (1mol) and chloroform 125g (1.05mol) into 300ml tetrahydrofuran, stir to dissolve, keep the temperature between 30-40°C, slowly add potassium tert-butoxide 118g (1.05mol) in batches under stirring, The whole feeding process is about 1.5 hours. After the addition, continue to stir and react for 1 hour, filter, and evaporate most of the tetrahydrofuran. Slowly add 100 g (2.1 mol) of formic acid to the system, raise the temperature to 65 ° C and stir for 3 hours. After the reaction, evaporate under reduced pressure. To remove most of the excess formic acid, add 150ml of dichloromethane to the system, wash with 200ml of sodium bicarbonate once, wash once with 200ml of saturated saline, separate the organic layer, distill off the dichloromethane, and the obtained pale yellow liquid is directly used in the next step reaction.
[0036] Add 126g of ammonium formate (2mol) to the light yellow liquid oil in the previous step, slowly raise the temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com