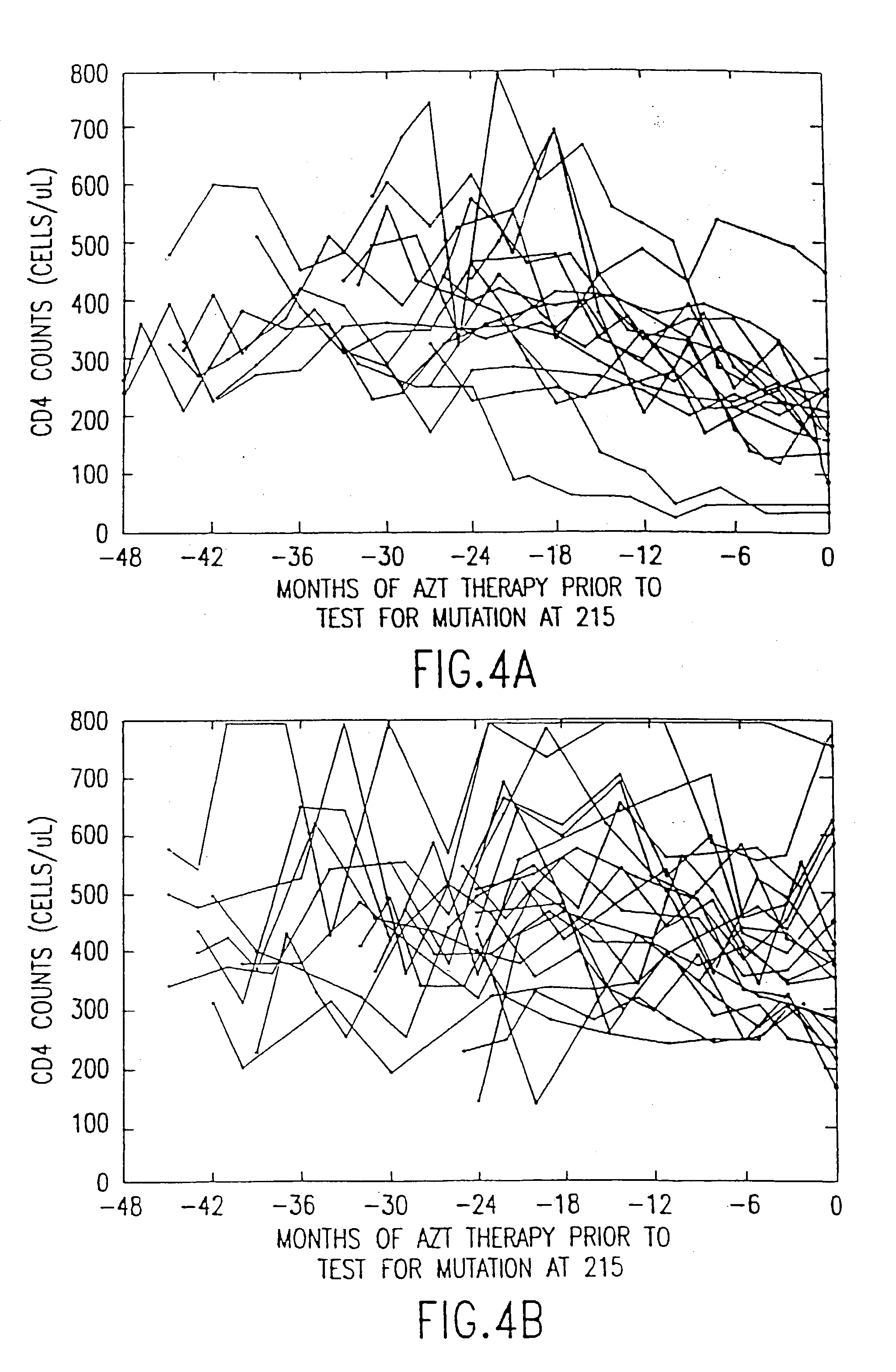
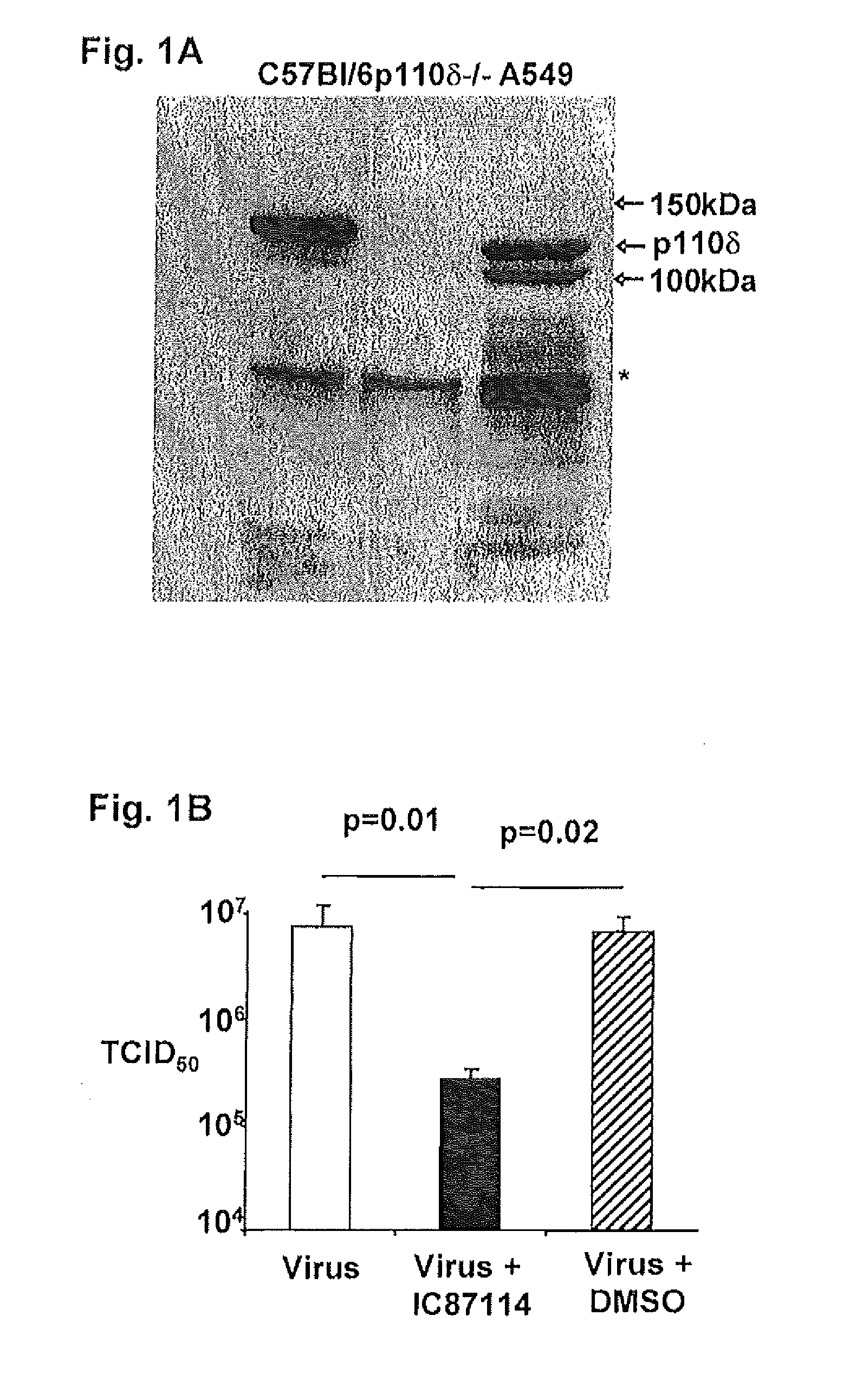
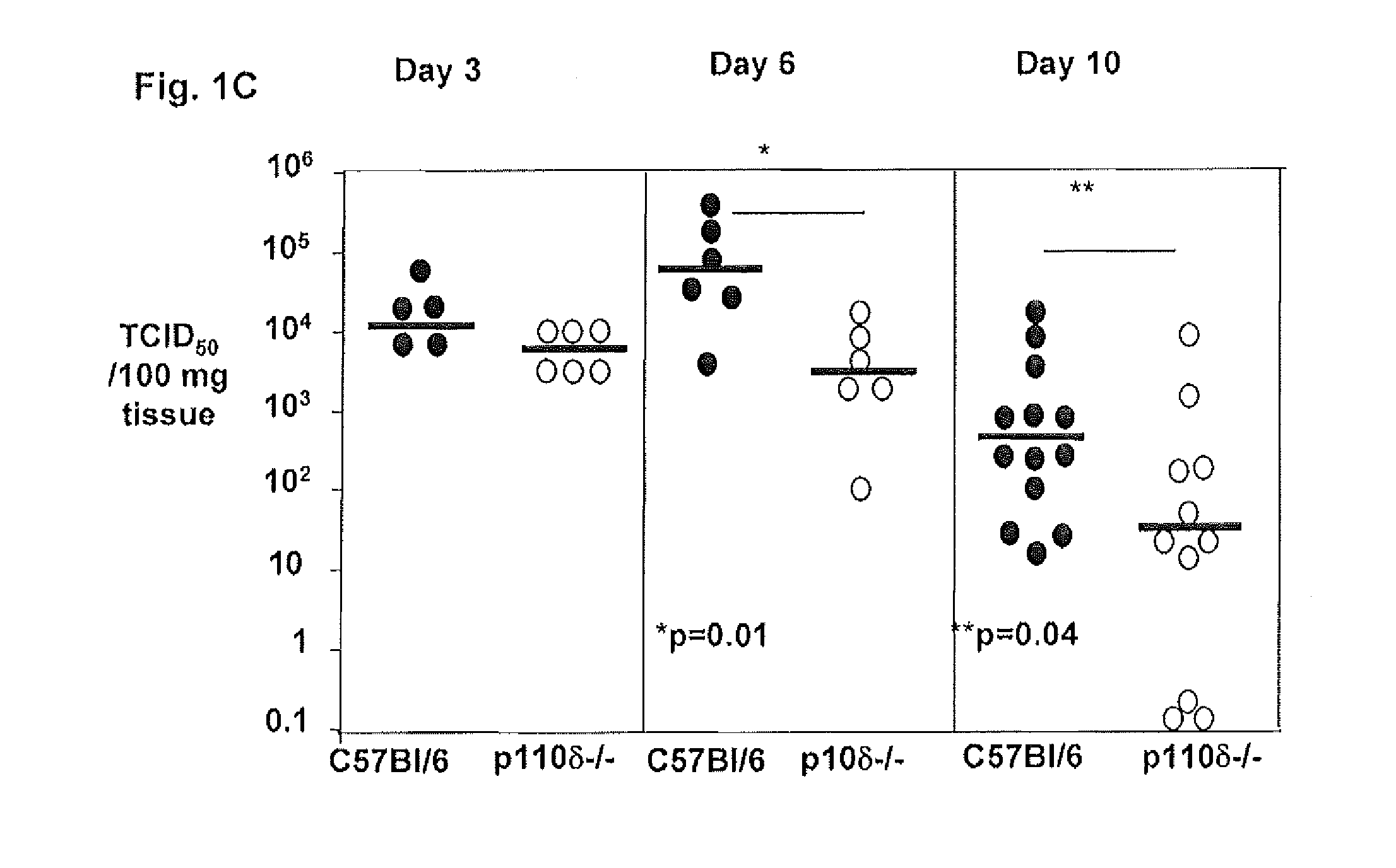
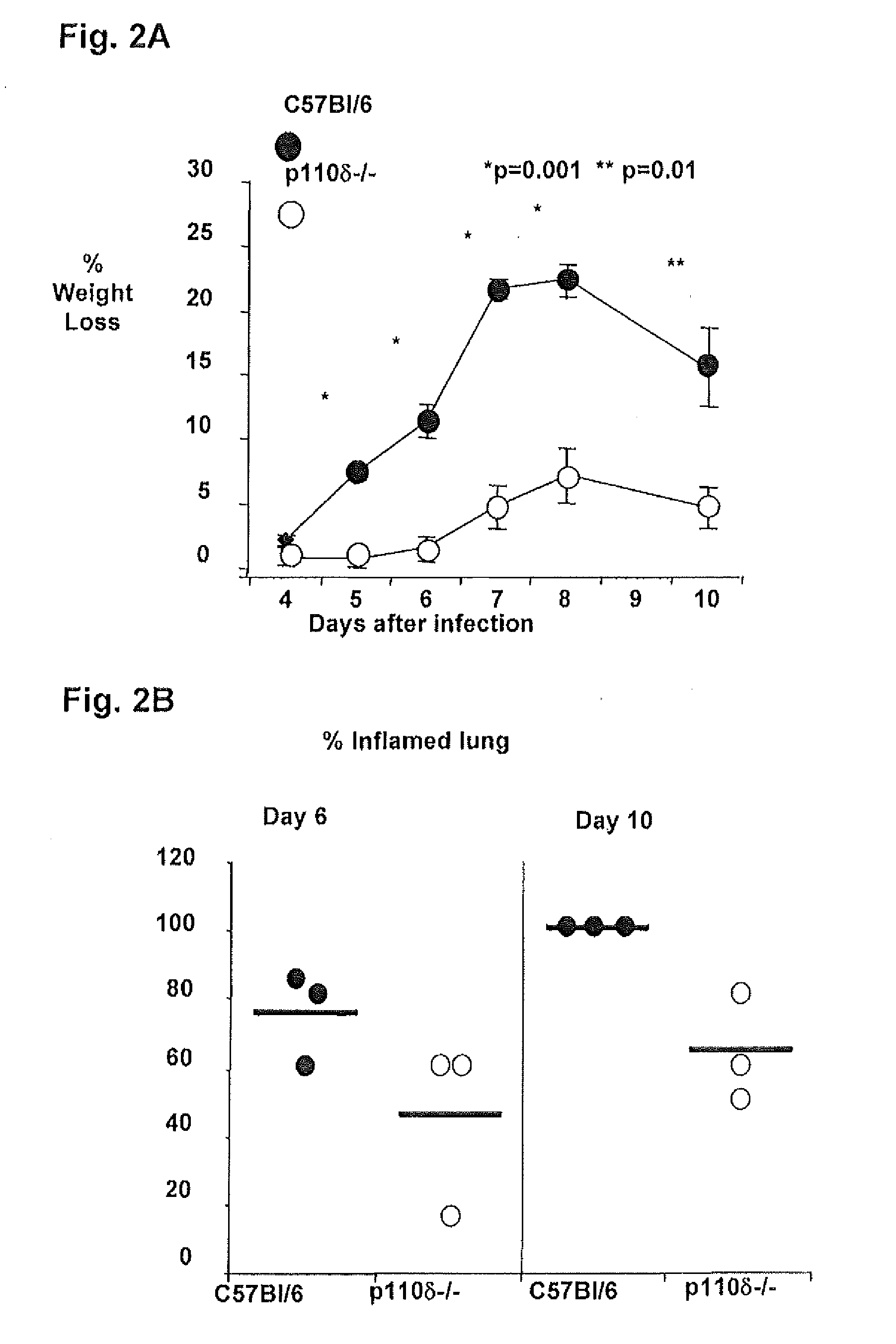
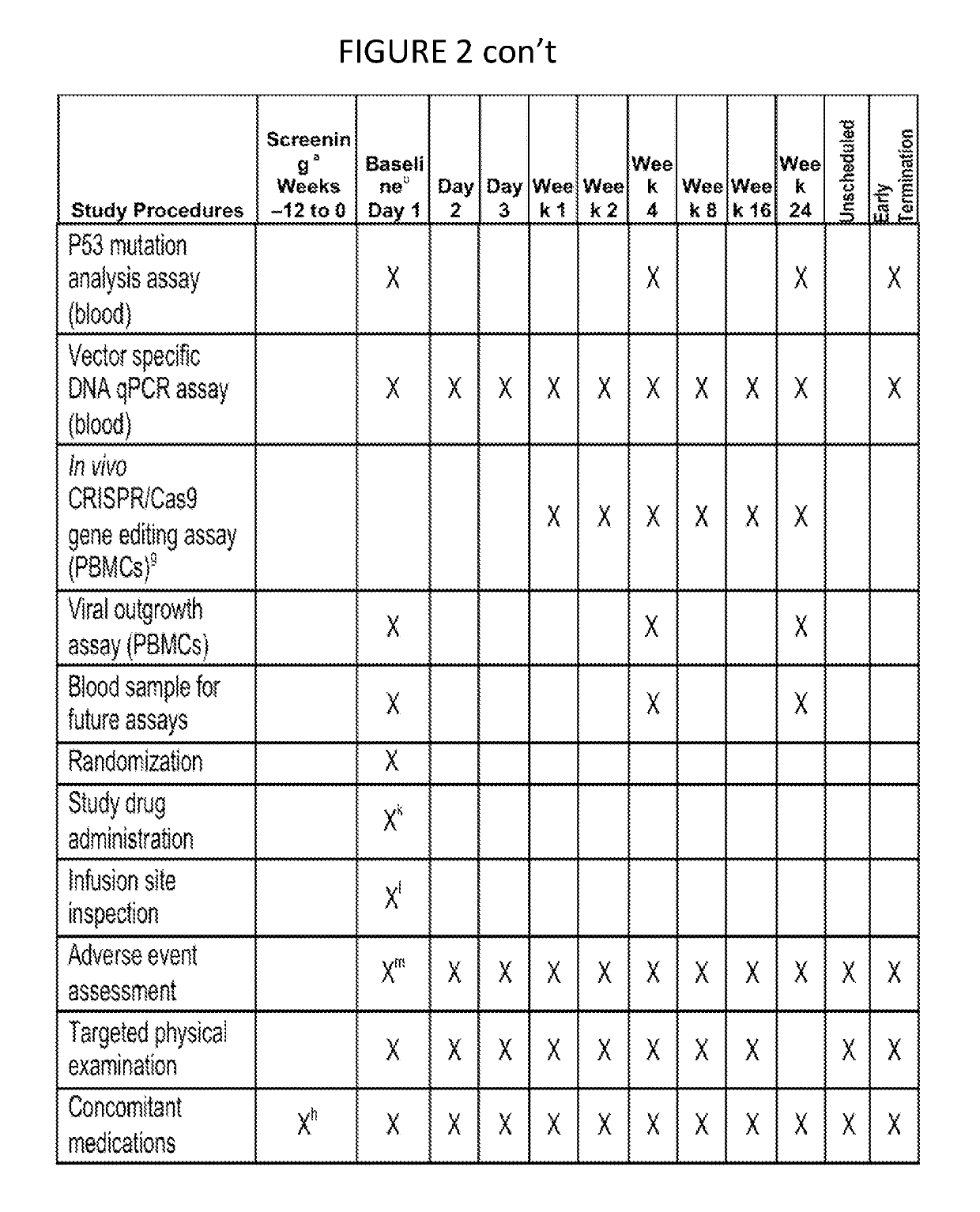
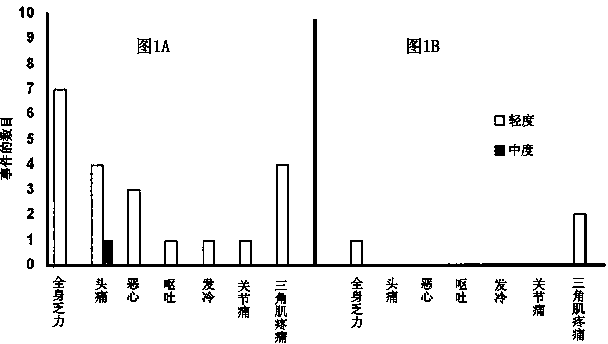
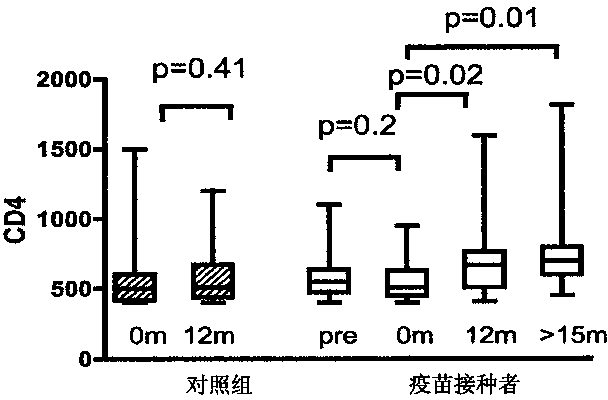
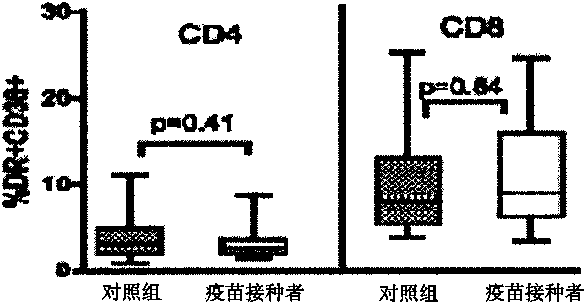
Patents
Literature
48 results about "Antiretroviral treatment" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antiretroviral treatments are usually provided in a combination of three antiviral drugs to suppress the disease, at best, and prevent further progression of the HIV virus infection. This works best, particularly at early stages of the disease.
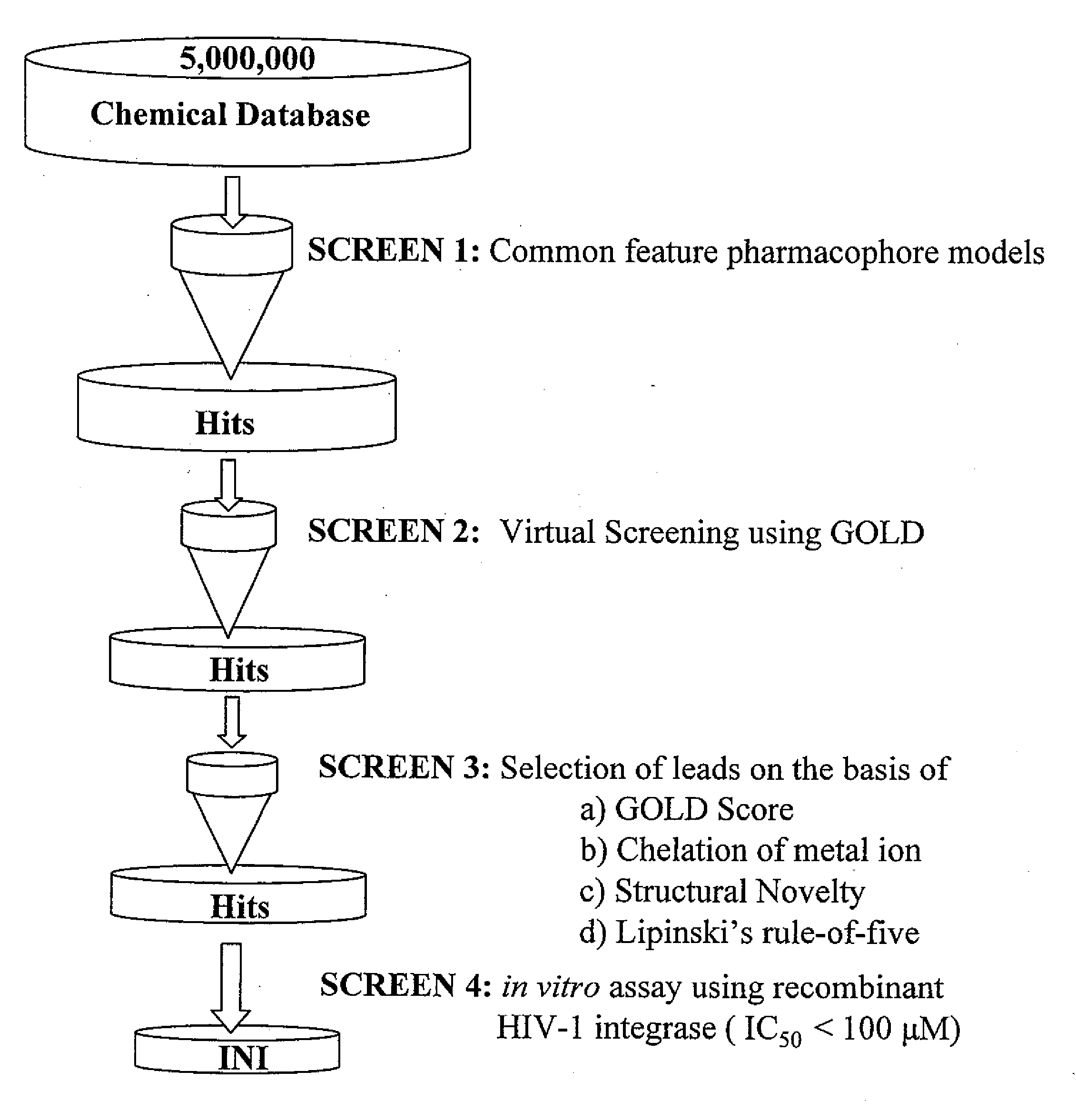
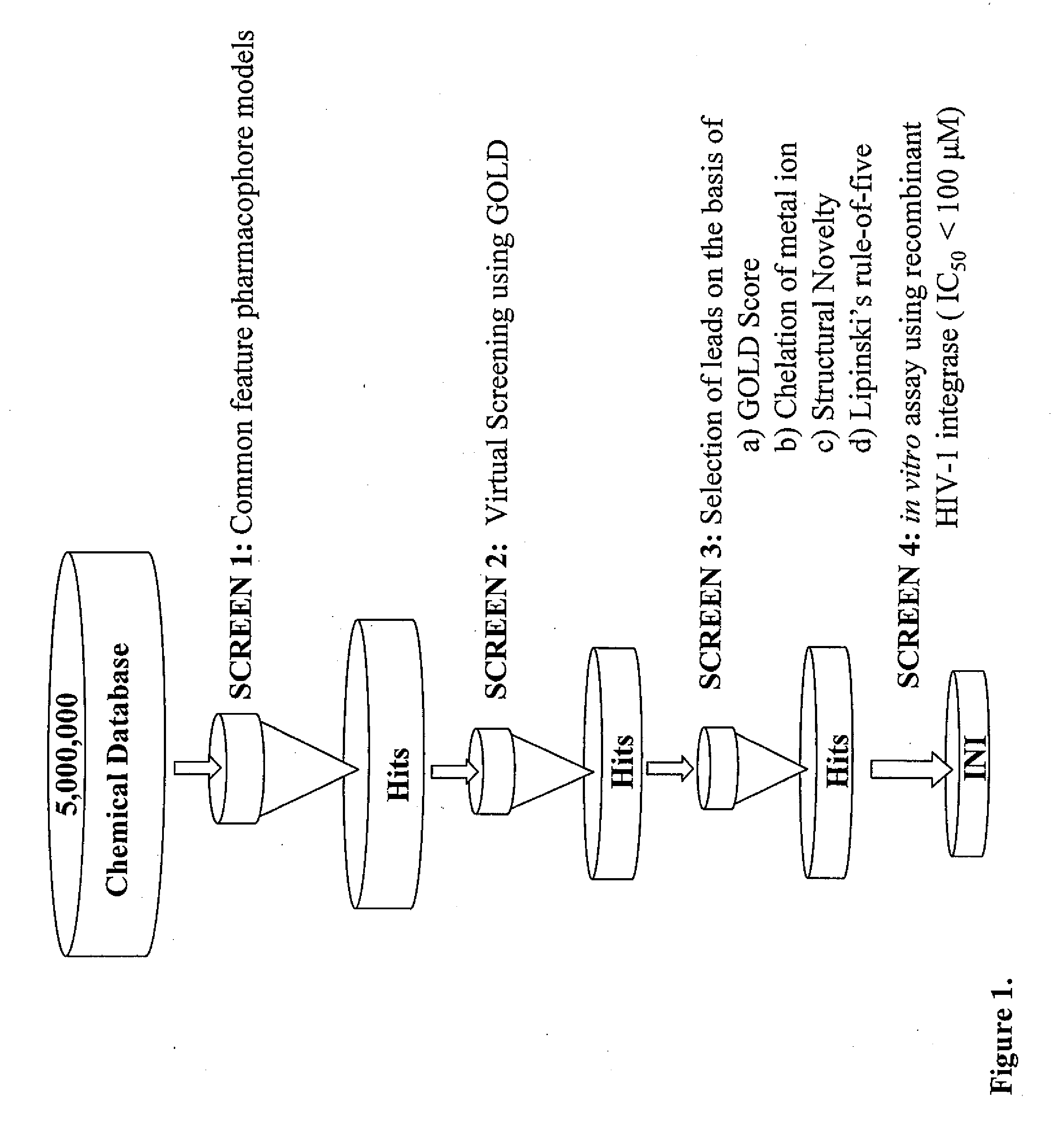
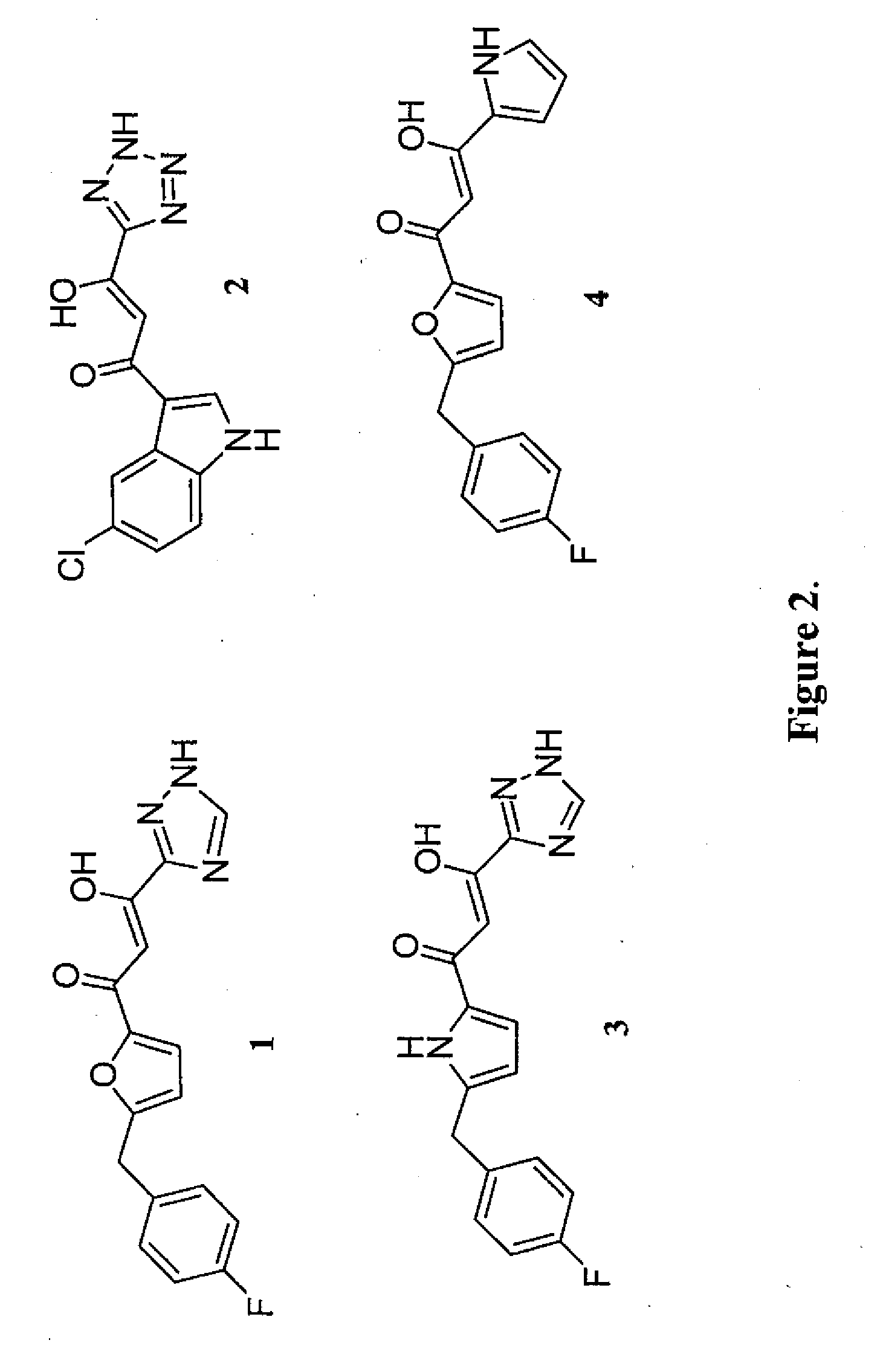
Compounds with hiv-1 integrase inhibitory activity and use thereof as Anti-hiv/aids therapeutics
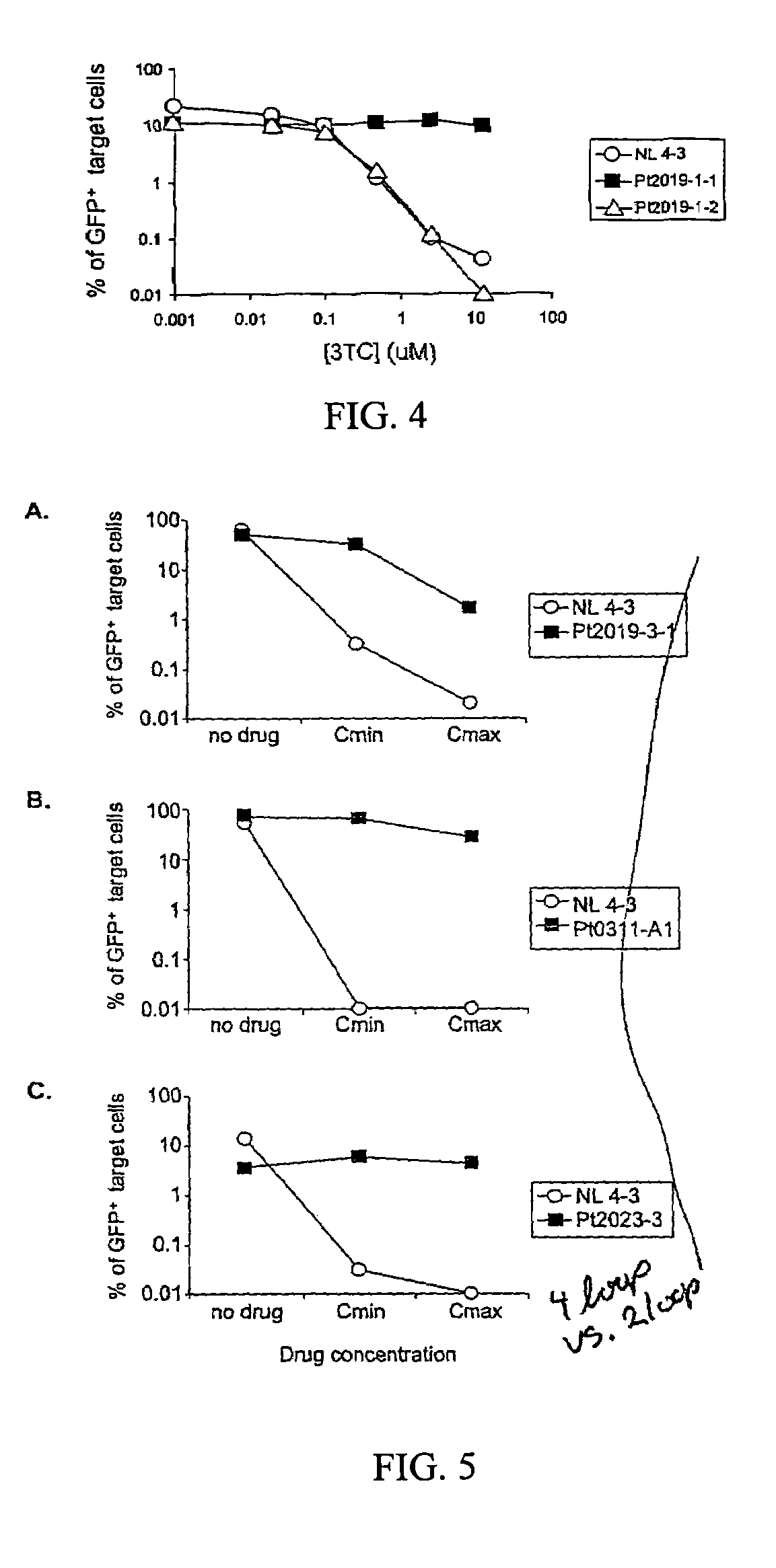
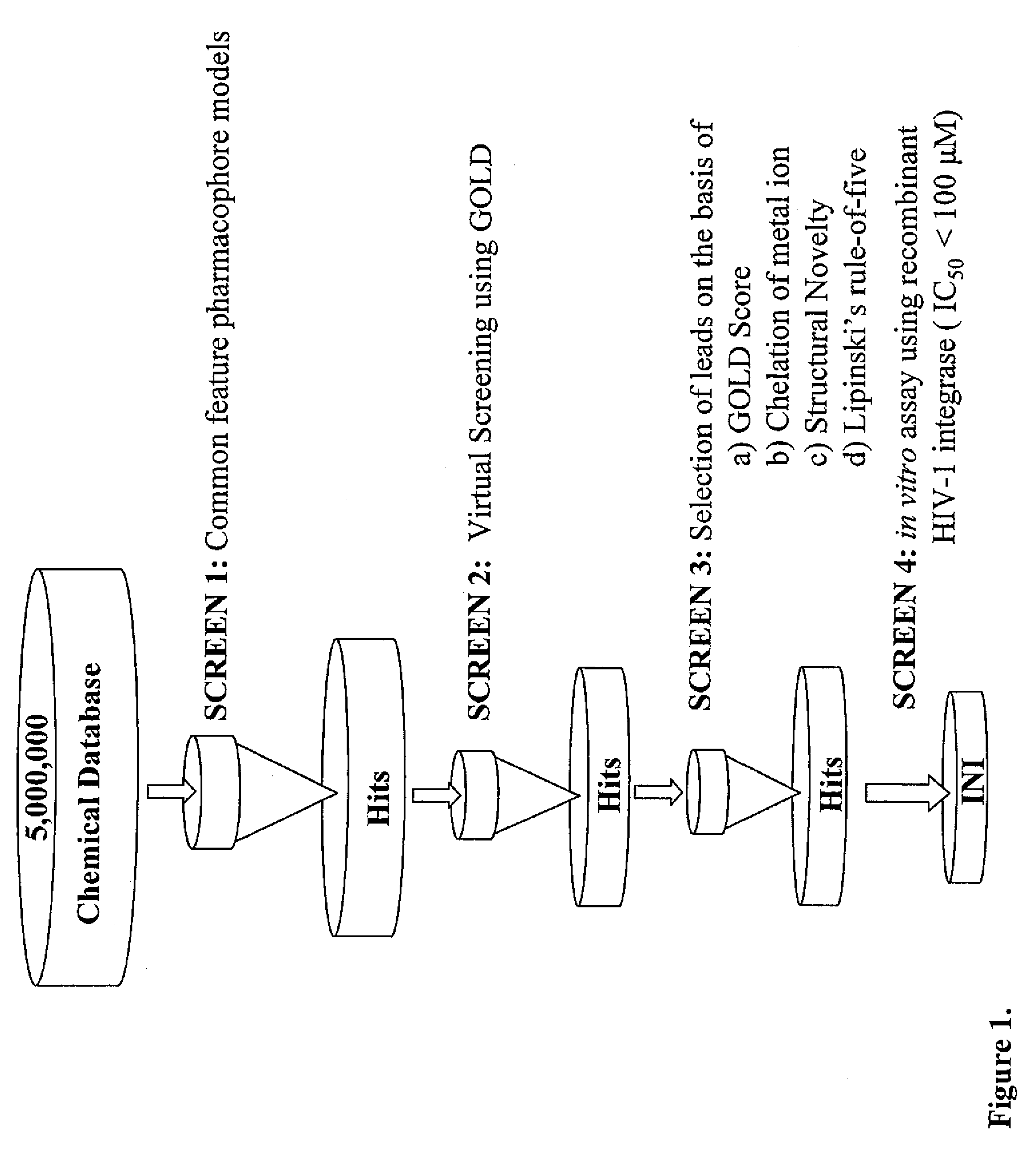
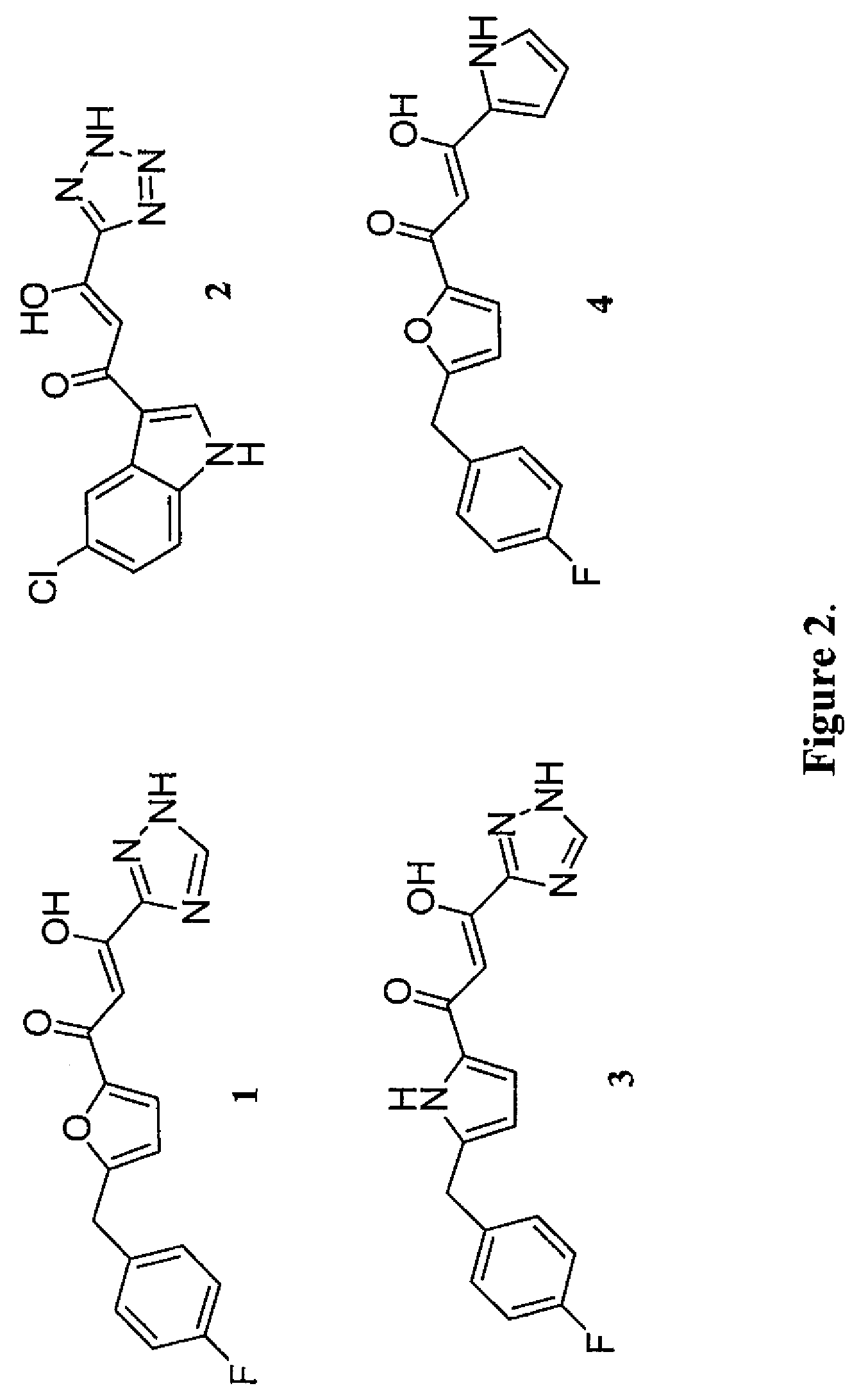
Pharmacophore models to be used in drug design and discovery are provided. An in silico protocol and in vitro assays are presented. Compounds and their pharmaceutically acceptable salts with HIV-1 integrase inhibitory and anti-HIV activity and use thereof in the treatment of HIV / AIDS and related infections either alone or in combination with all the known antiretroviral therapeutics are described.
Owner:UNIV OF SOUTHERN CALIFORNIA
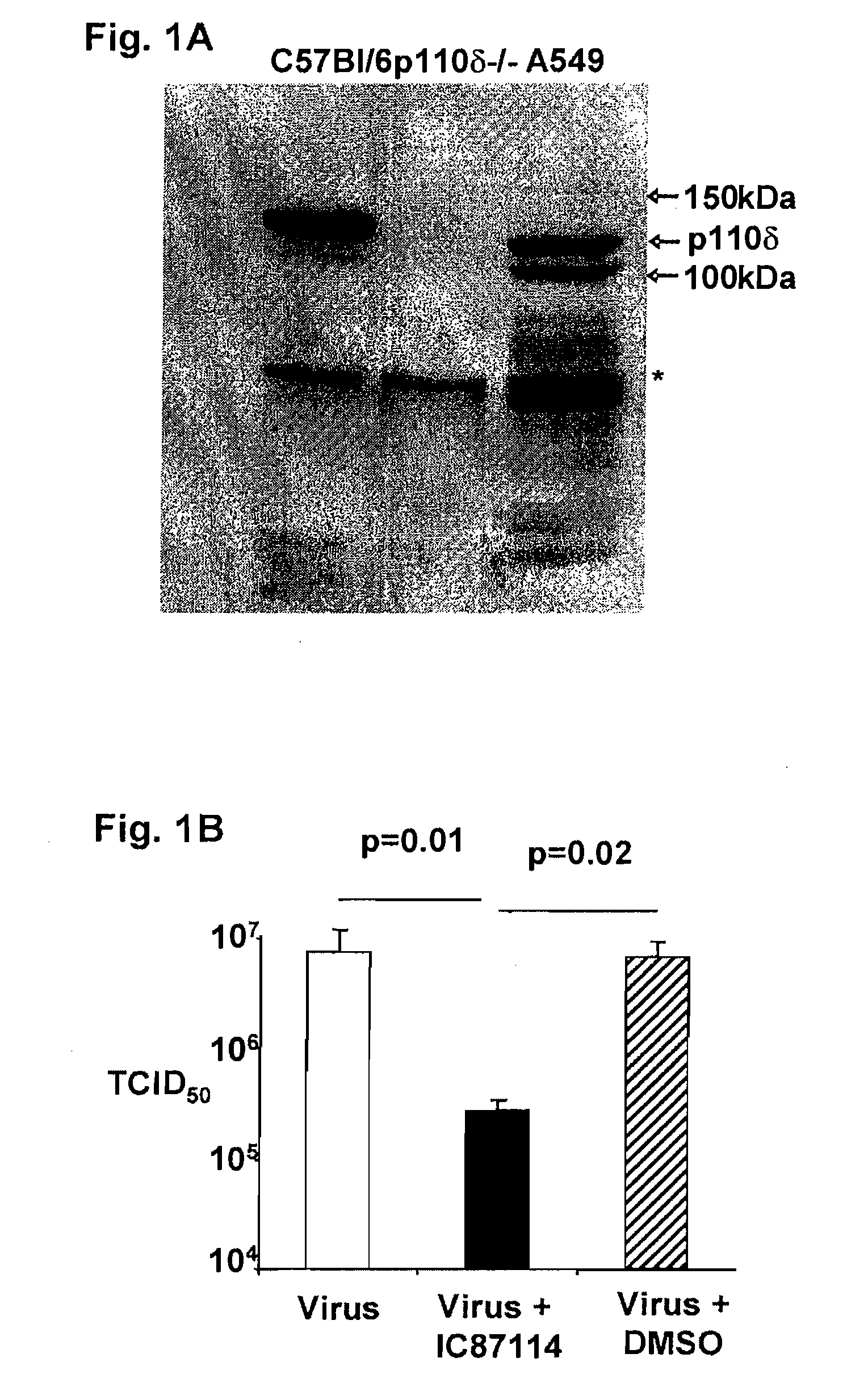
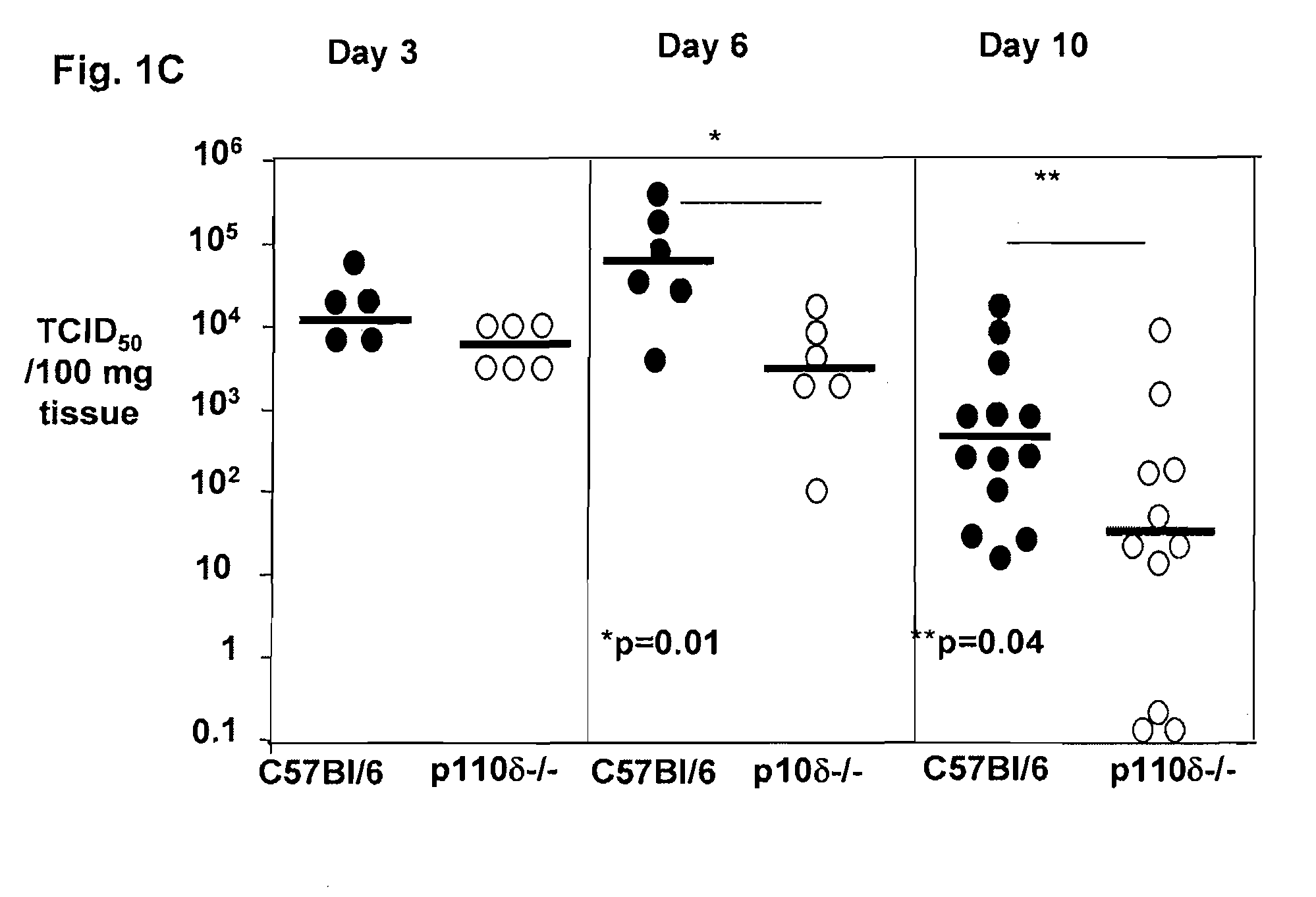
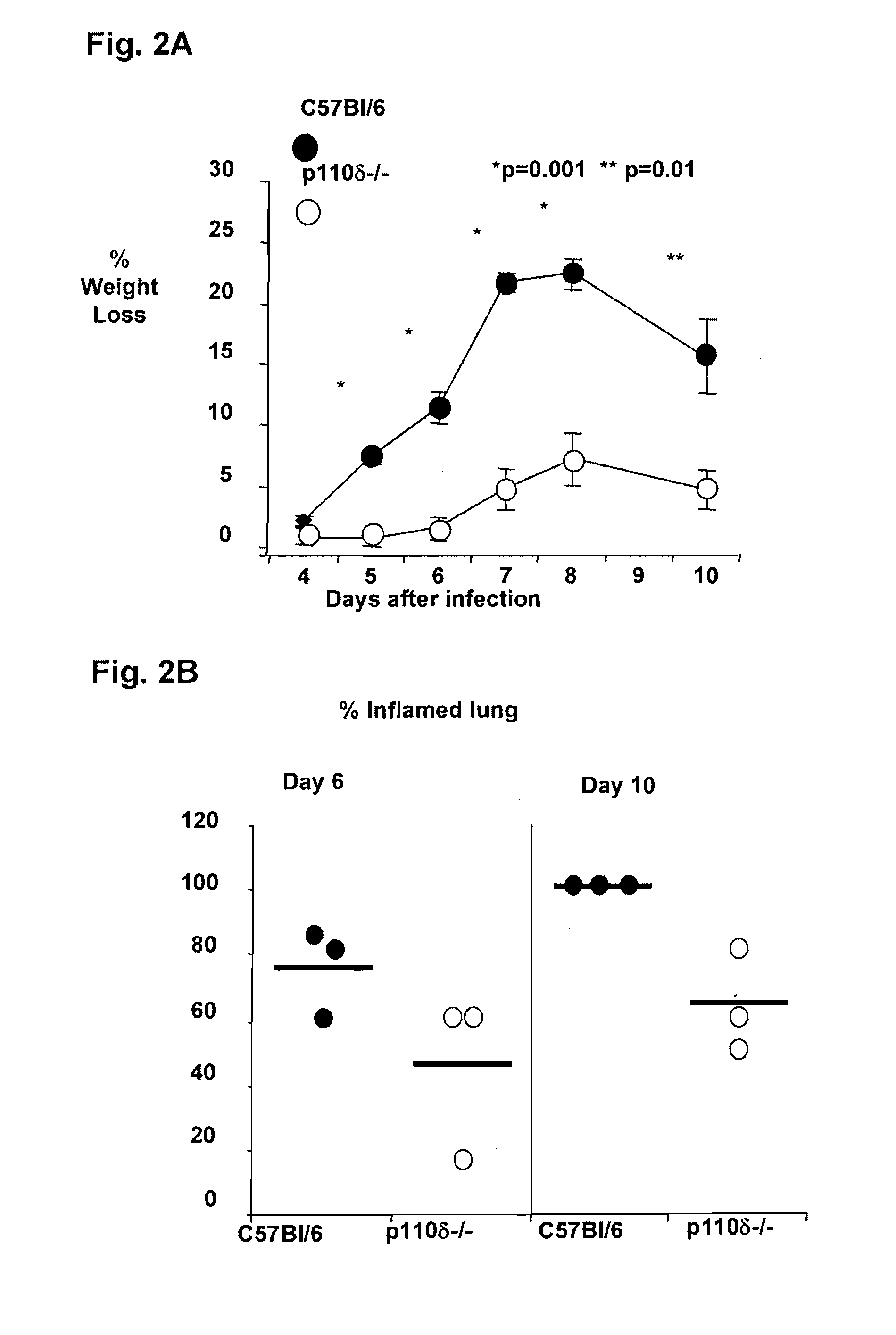
Role of PI3K p110 delta Signaling in Retroviral Infection and Replication
InactiveUS20110135655A1Avoid infectionSsRNA viruses negative-senseBiocideVirus-RetrovirusRetroviral infection
The invention includes compositions and methods for regulating PI3K p110 delta as an anti-retroviral therapy. The invention includes inhibiting p110 delta, a component of PI3K p110 delta signaling pathway, or any combination thereof in a cell as an anti-retroviral therapeutic approach for treating a retroviral infection, for example HIV. The invention includes a method of modulating PI3K p110 delta in a cell infected with a retrovirus by contacting the cell with an effective amount of a composition comprising an inhibitor of PI3K p110 delta.
Owner:DREXEL UNIV +1
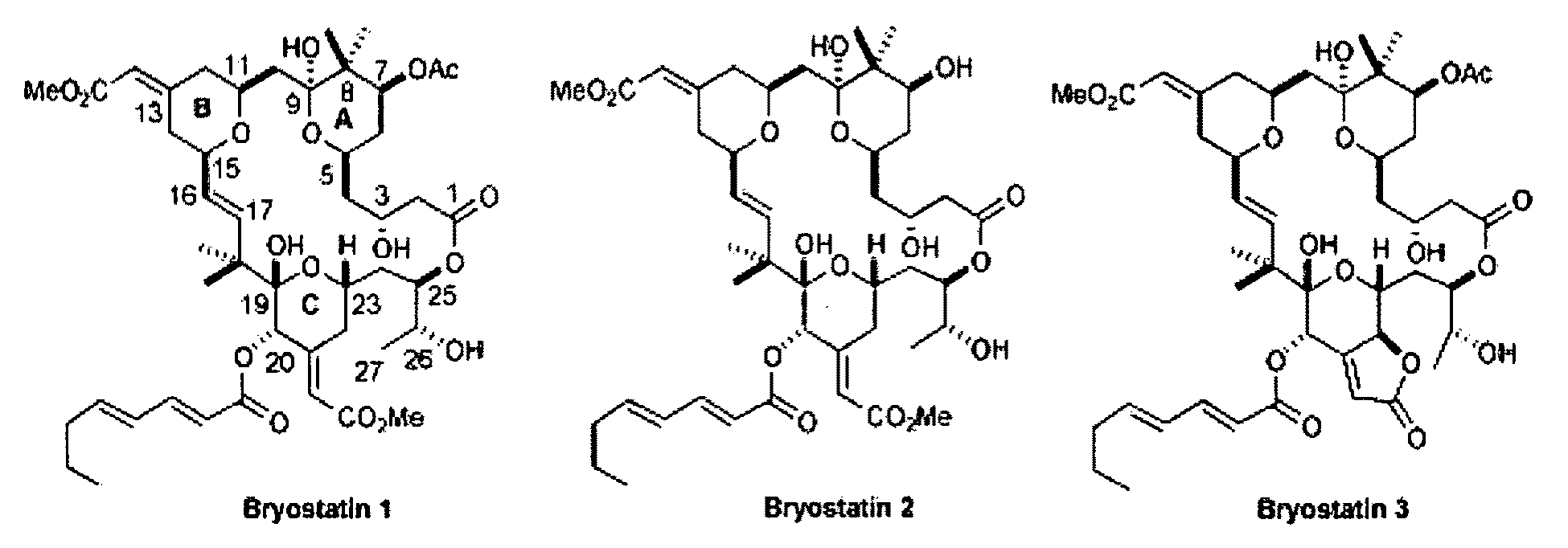
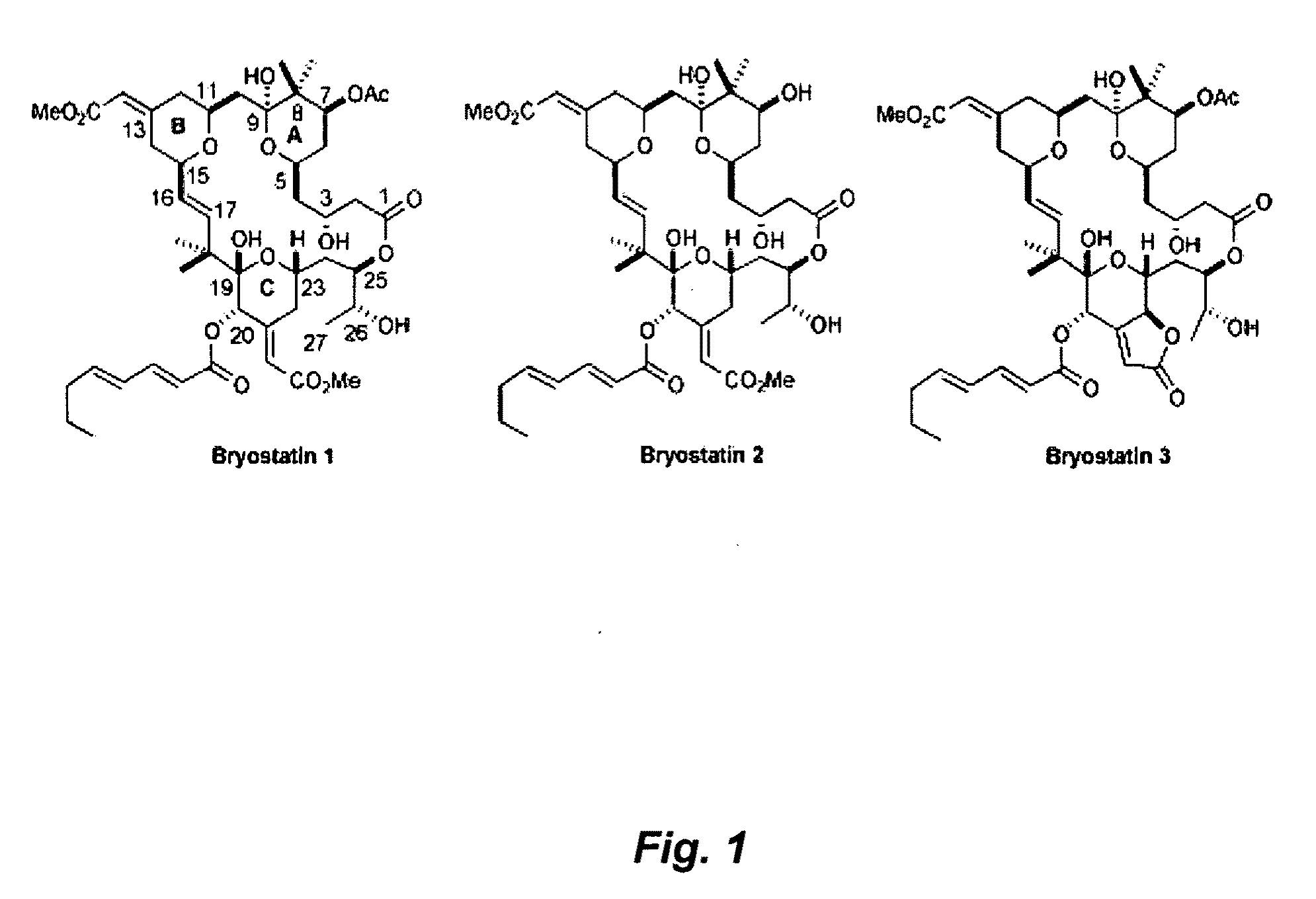
Combination therapy comprising the use of protein kinase C modulators and Histone Deacetylase inhibitors for treating HIV-1 latency
InactiveUS20100166806A1Adverse propertyPrevent HIV-1-induced cytotoxicityBiocideOrganic chemistryReverse transcriptaseHydroxamic acid
The invention relates to a combination of treatments, more particularly a combination treatment for HIV-1 infection. The present invention is directed to the use of bryostatin-1 and their natural and synthetic derivatives for AIDS therapy, in particular to the use of bryostatins in combination with other active drugs such as Histone Deacetylases (HDACs) inhibitors and anti-retrovirals, for the treatment of HIV-1 latency. According to the present invention, we provide a combination therapy for the treatment of HIV-1 latency which employs bryostatin-1 (and analogues) and one of the following HDAC inhibitors; valproic acid, butyrate derivatives, hydroxamic acids and benzamides. While HDACi can be used in continuous dosing protocol, bryostatins can be used following a cyclical dosing protocol. Bryostatins can be formulated in pharmaceutical acceptable carriers including nanoparticles, phospholipids nanosomes and / or biodegradable polymer nanospheres. This combination therapy needs to be used in patients treated with antiretroviral therapy (HIV-1 protease inhibitors, HIV-1 reverse transcriptase inhibitors, HIV-1 integrase inhibitors, CCR5 co-receptor inhibitors and fusion inhibitors).
Owner:APHIOS
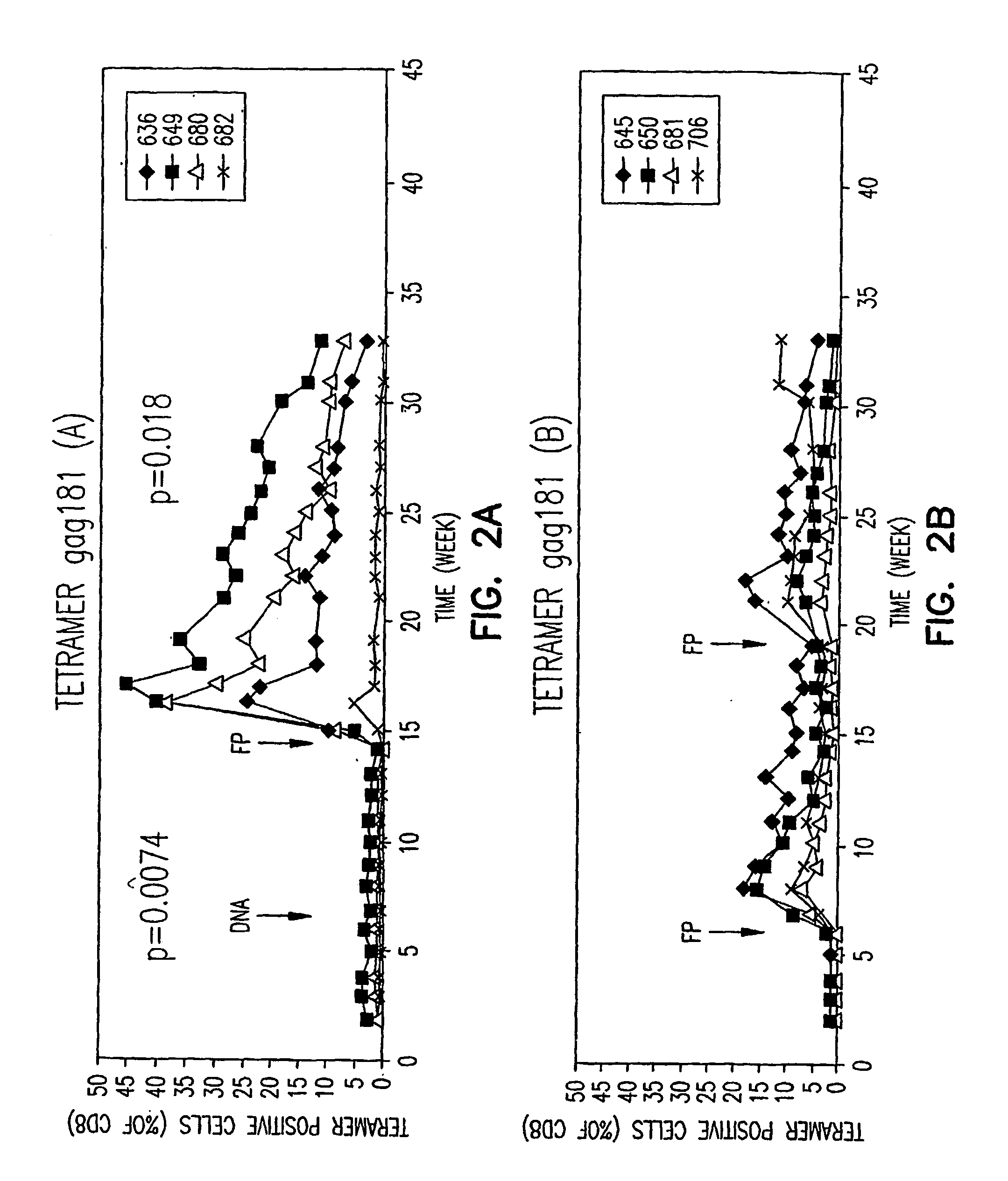
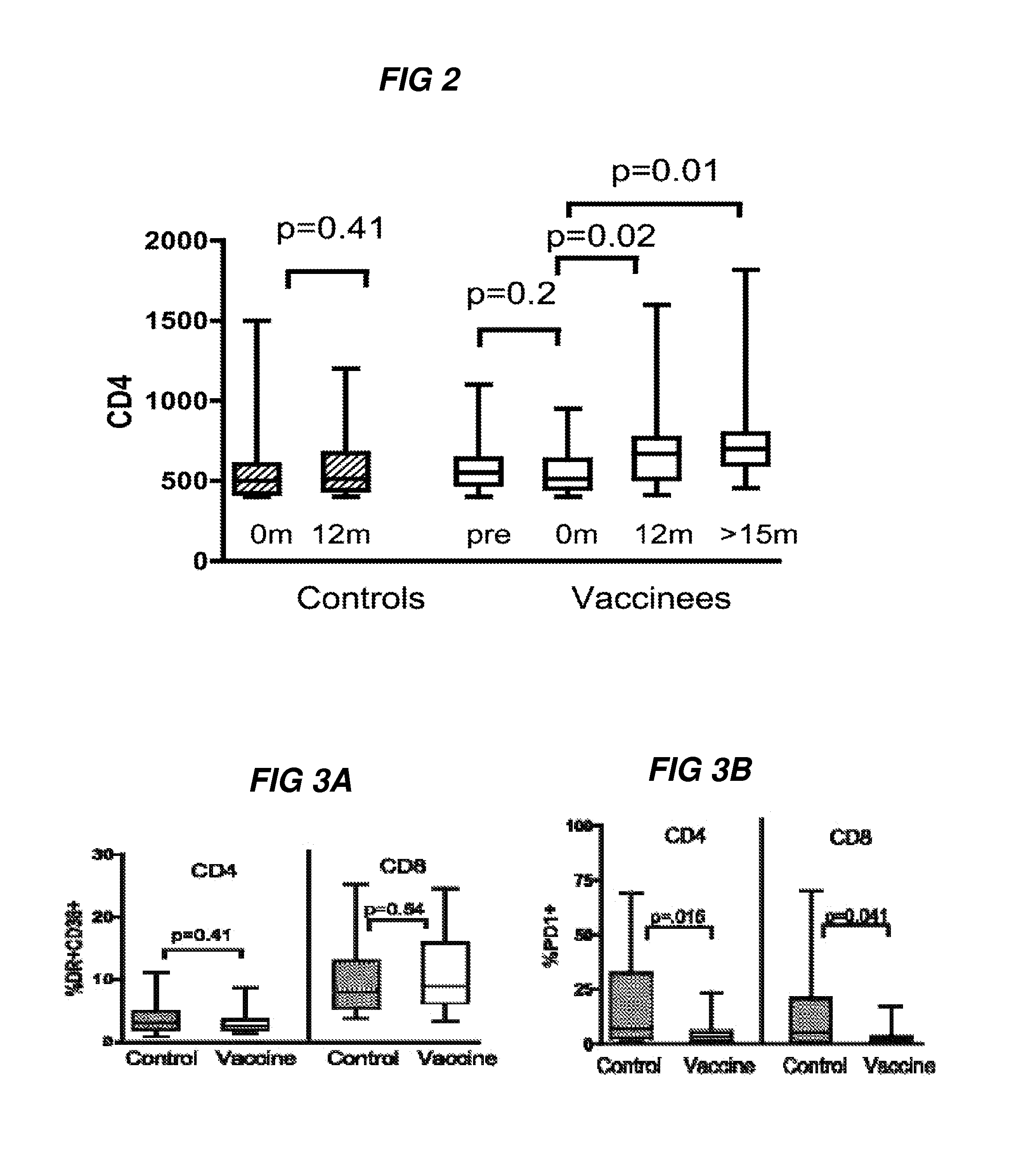
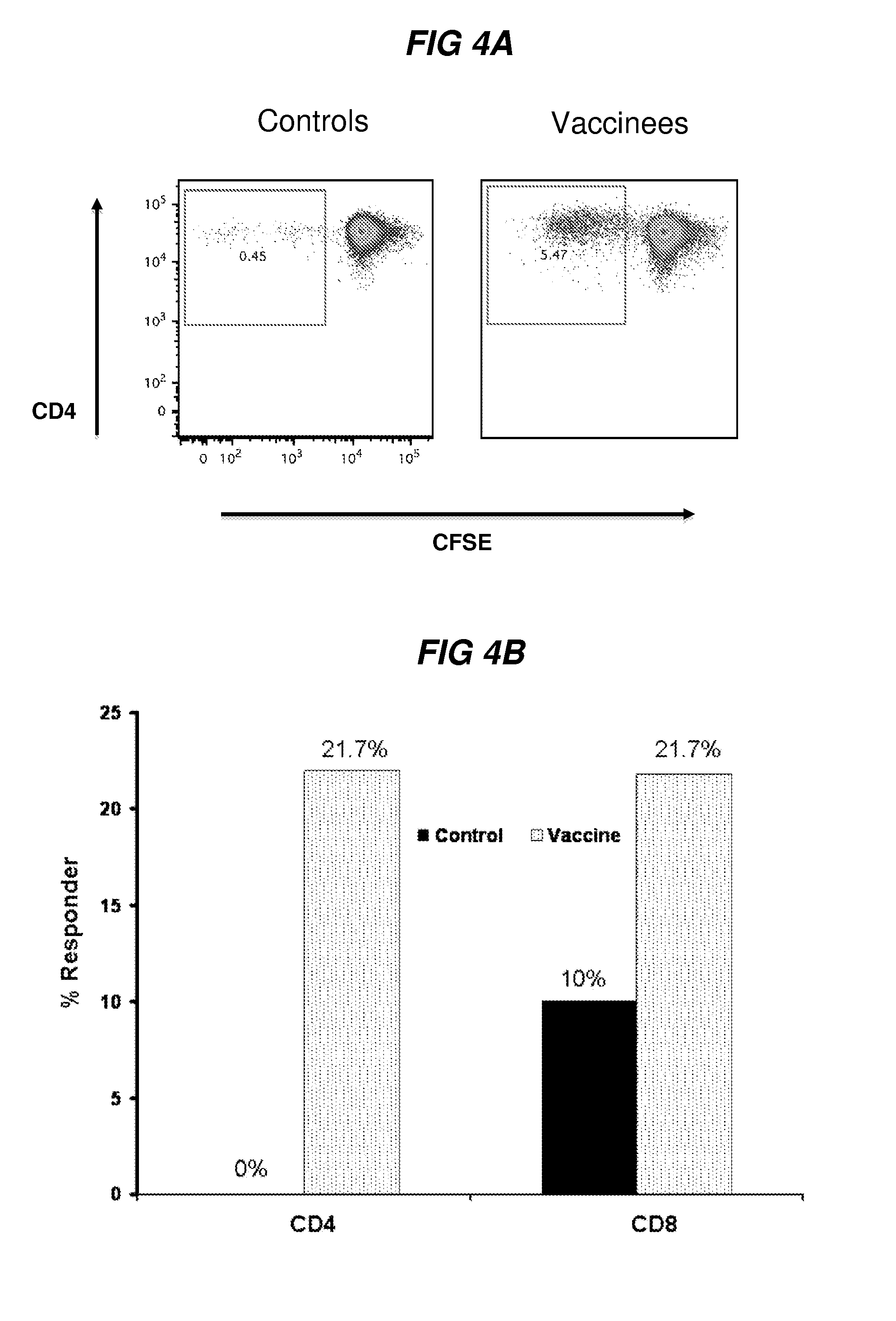
Methods for inducing an immune response against human immunodeficiency virus infection in subjects undergoing antiretroviral treatment
ActiveUS20180064803A1Measurable immune responseMaintain viremic controlViral antigen ingredientsAntiviralsImmunodeficiency virusVaccinia
Methods for inducing an immune response against Human Immunodeficiency Virus (HIV) in HIV-infected subjects undergoing antiretroviral therapy (ART) are described. The methods include administering an adenovirus vector primer vaccine and a modified vaccinia virus (MVA) vector booster vaccine encoding mosaic HIV antigens.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY +3
Highly sensitive method for detection of viral HIV DNA remaining after antiretroviral therapy of aids patients
InactiveUS20110027774A1High sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsNucleotideImmunodeficiency virus
Methods for detecting polynucleotides, especially the DNA replicated from samples obtained from subjects infected with pathogenic viruses such as human immunodefiency virus, by detecting electromagnetic signals (“EMS”) emitted by such polynucleotides, and methods for improving the sensitivity of the polymerase chain reaction (“PCR”).
Owner:MONTAGNIER LUC
Use of dsrnas in strategic therapeutic intervention of highly active antiretroviral therapy
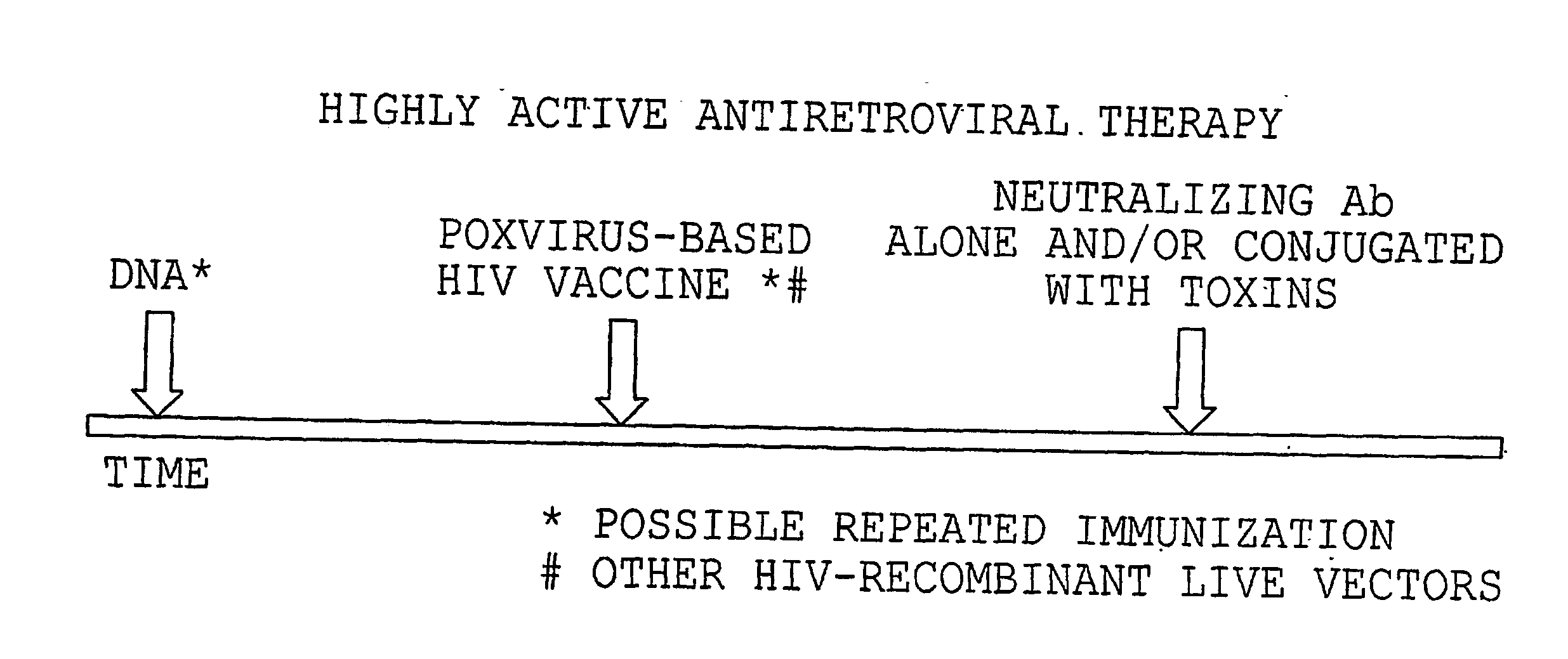
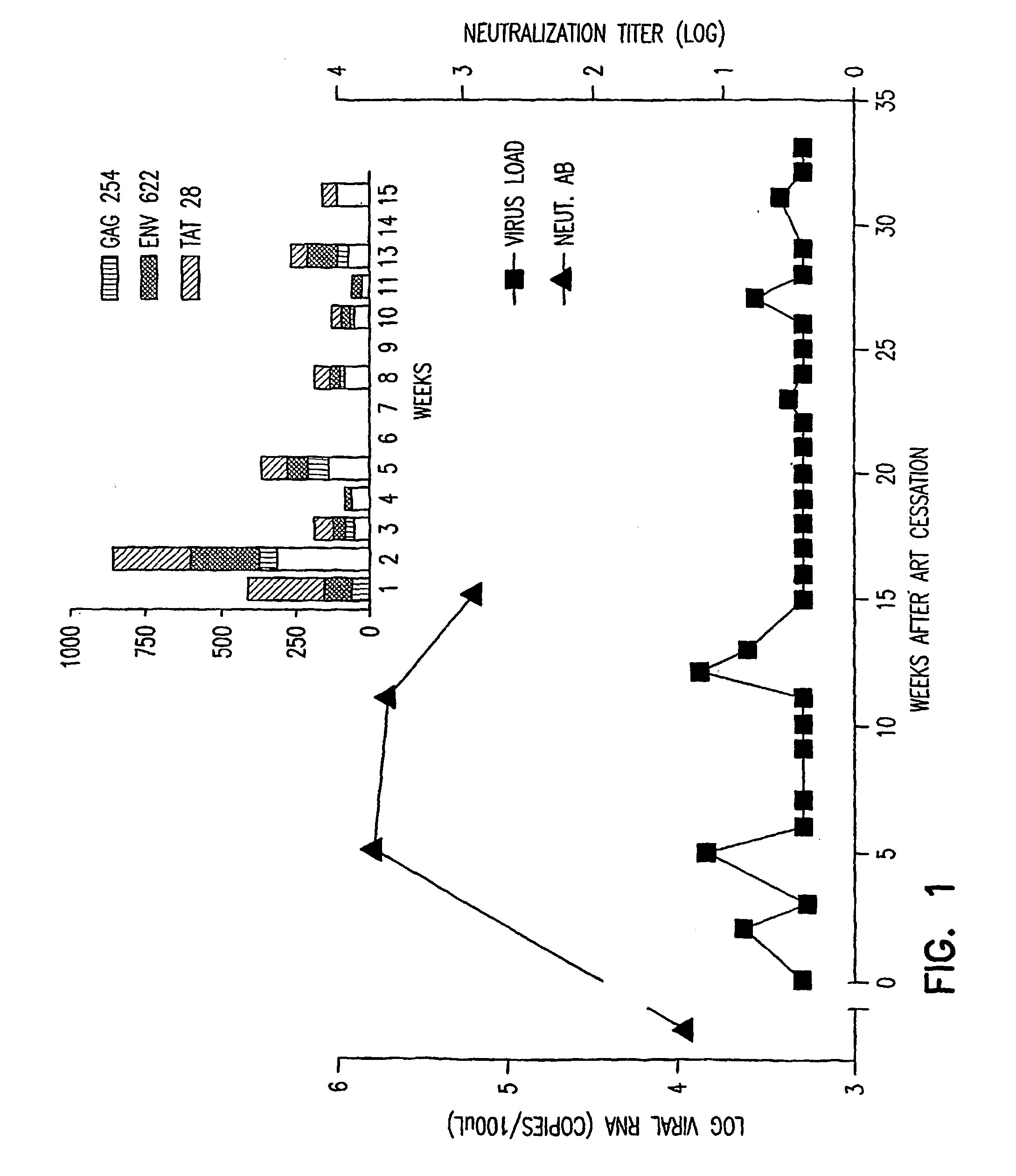
In the treatment of HIV administration of dsRNA at an appropriate stage in highly active antiretroviral (HAART) therapy of HIV allows for the discontinuation of HAART by increasing the time to HIV rebound after stopping HAART.
Owner:HEMISPHERX BIOPHARMA
Highly sensitive method for detection of viral HIV DNA remaining after antiretroviral therapy of aids patients
InactiveUS20130143205A1High sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsNucleotideImmunodeficiency virus
Owner:MONTAGNIER LUC
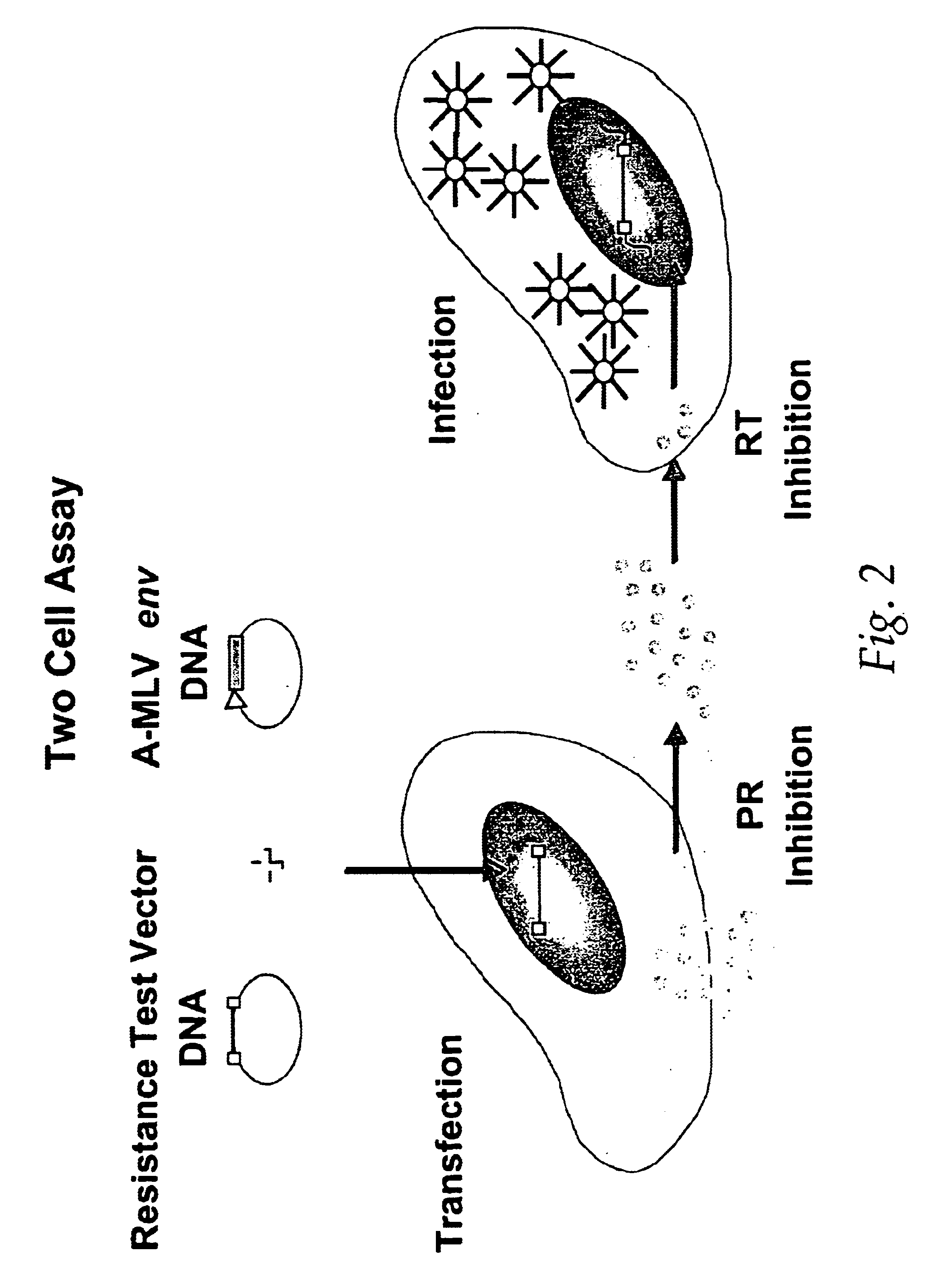
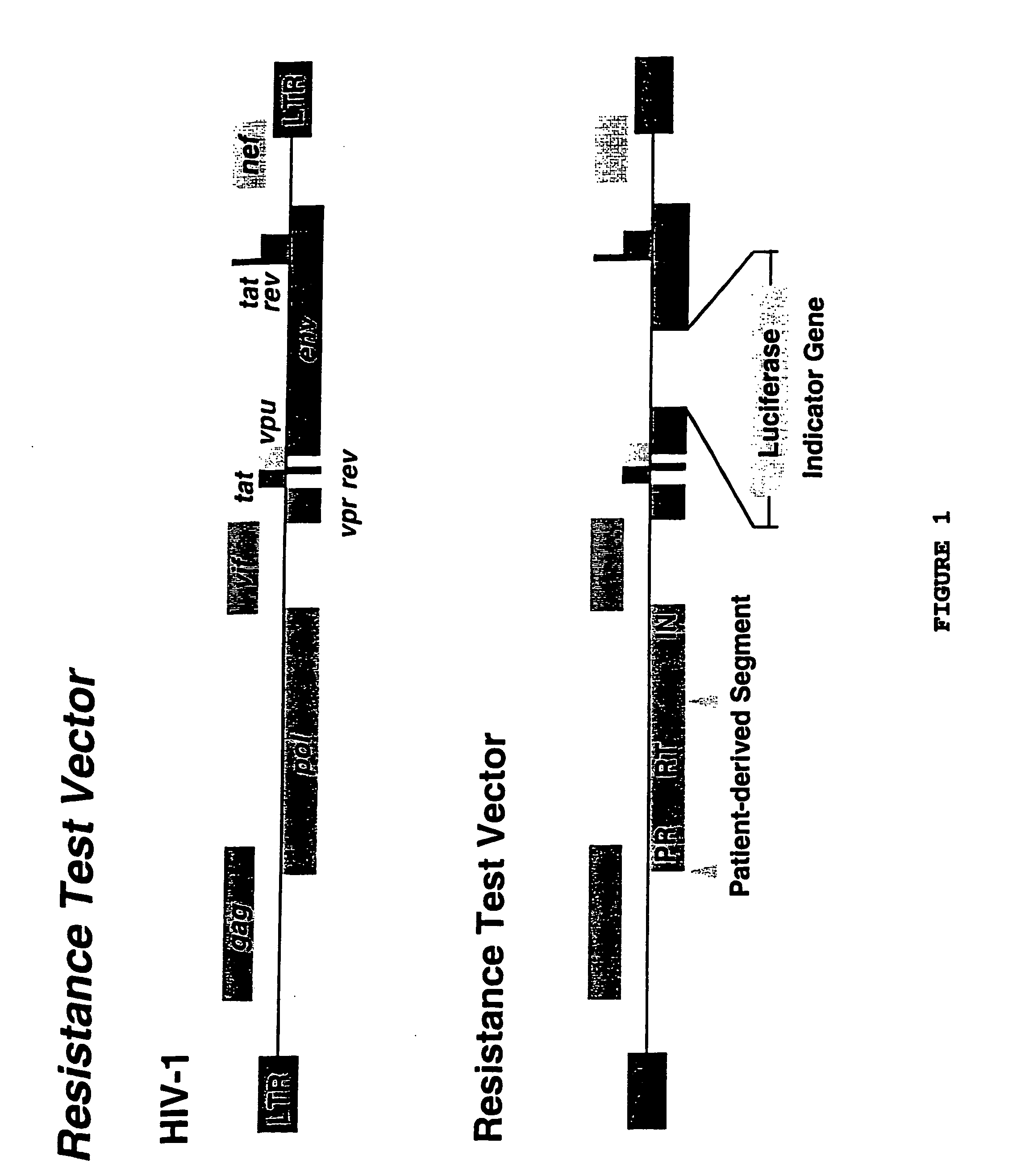
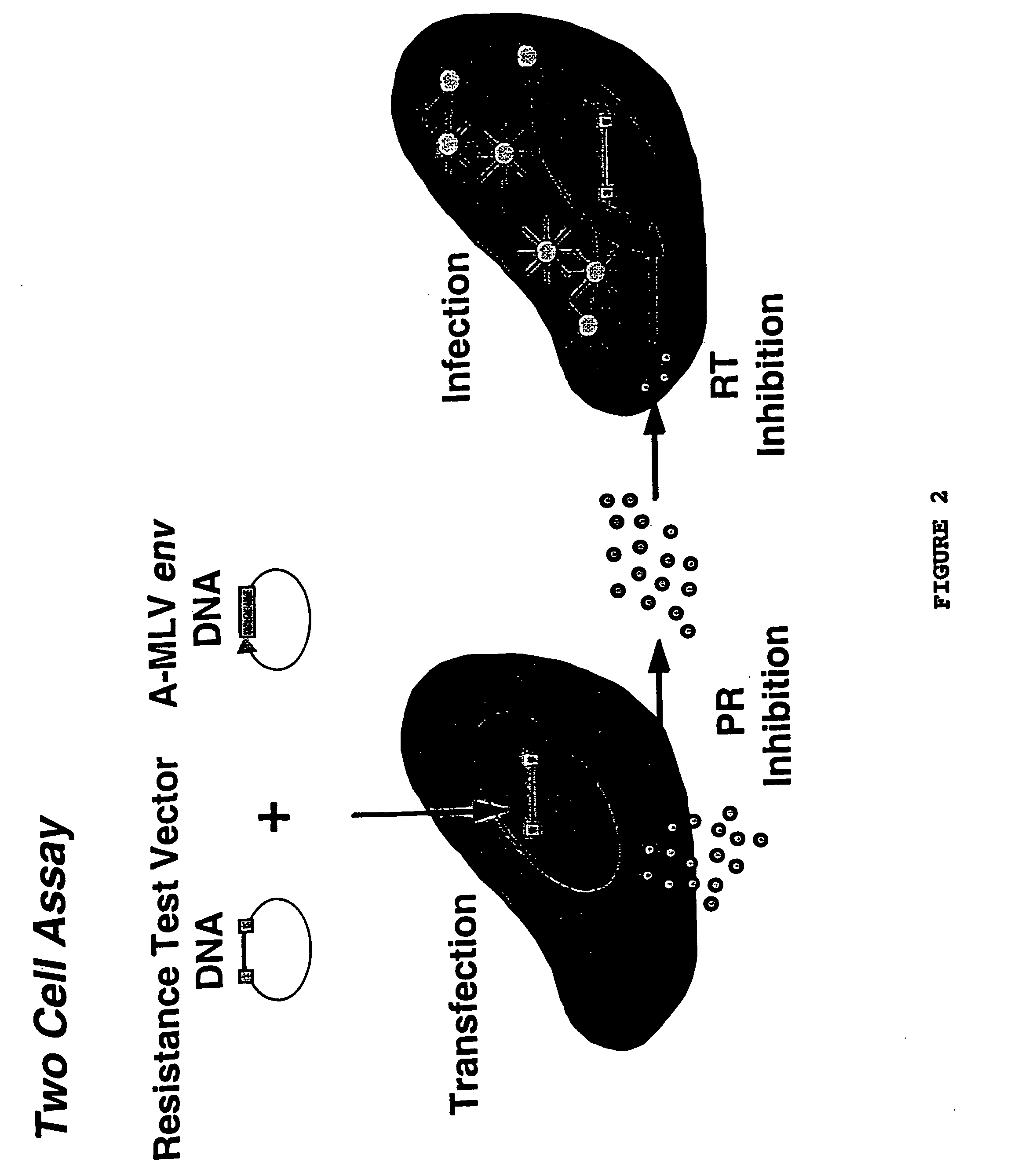
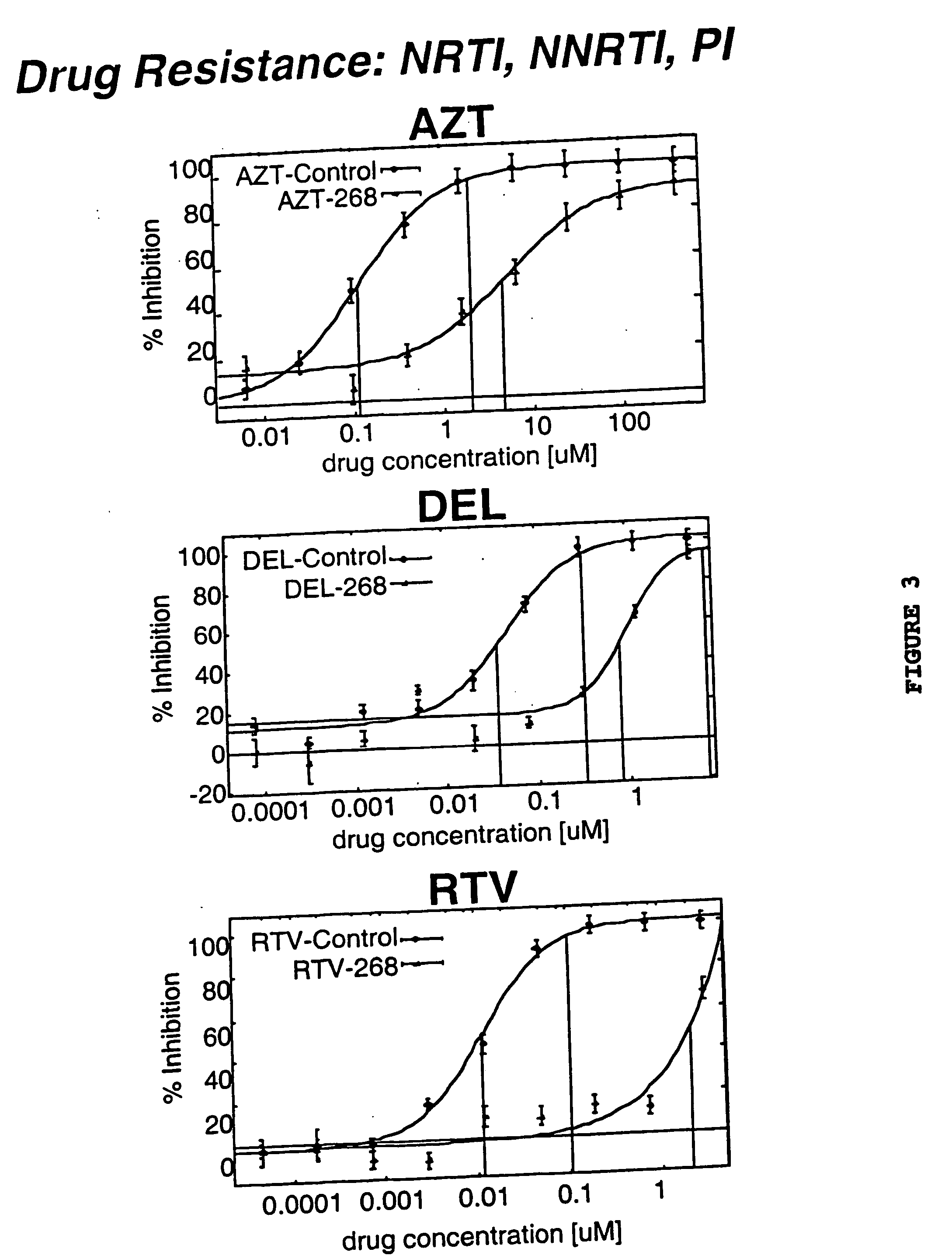
Single cell analysis of HIV replication capacity and drug resistance
ActiveUS20050244818A1Less-effective in controllingAccurate countVectorsMicrobiological testing/measurementAssayResistant virus
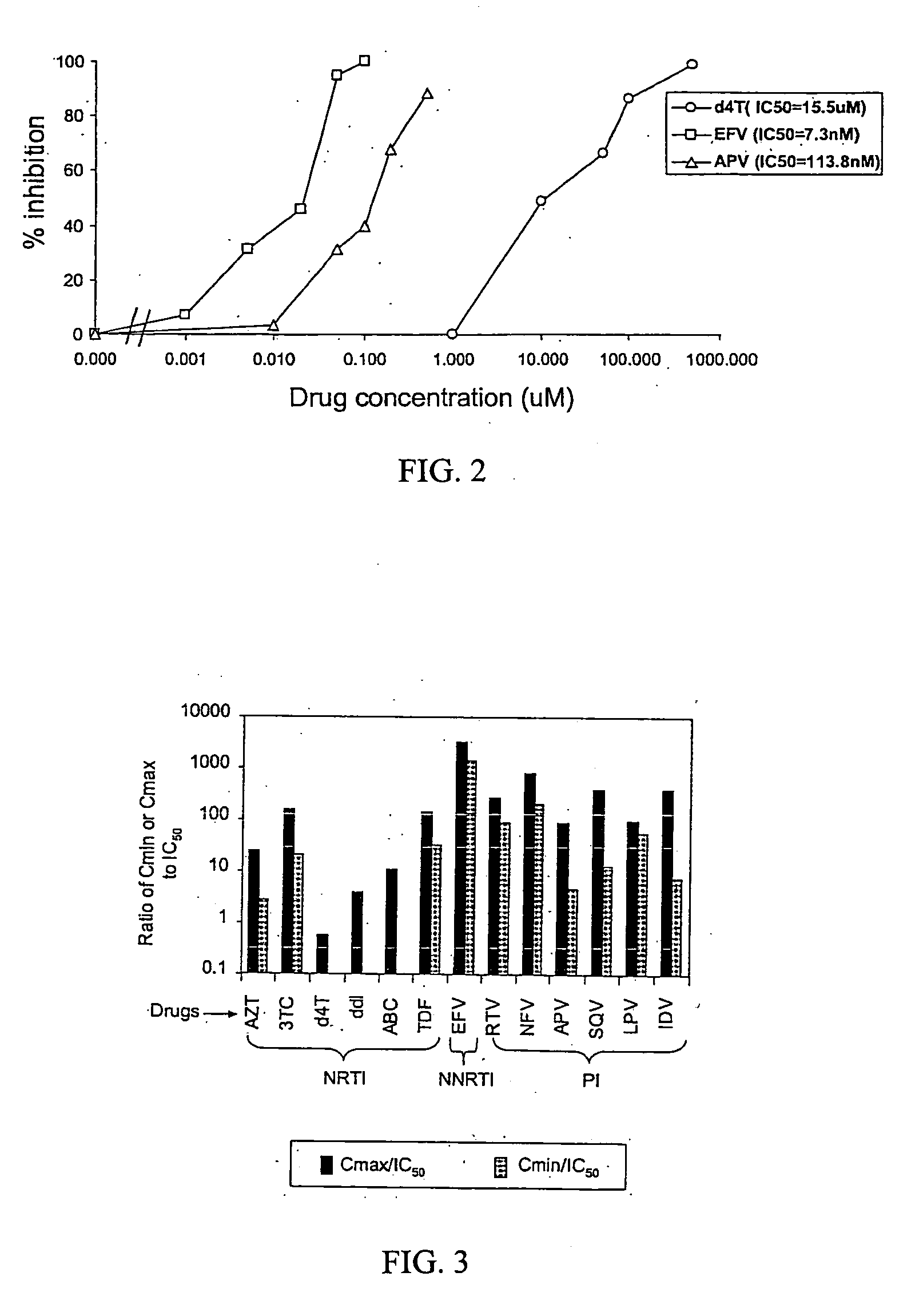
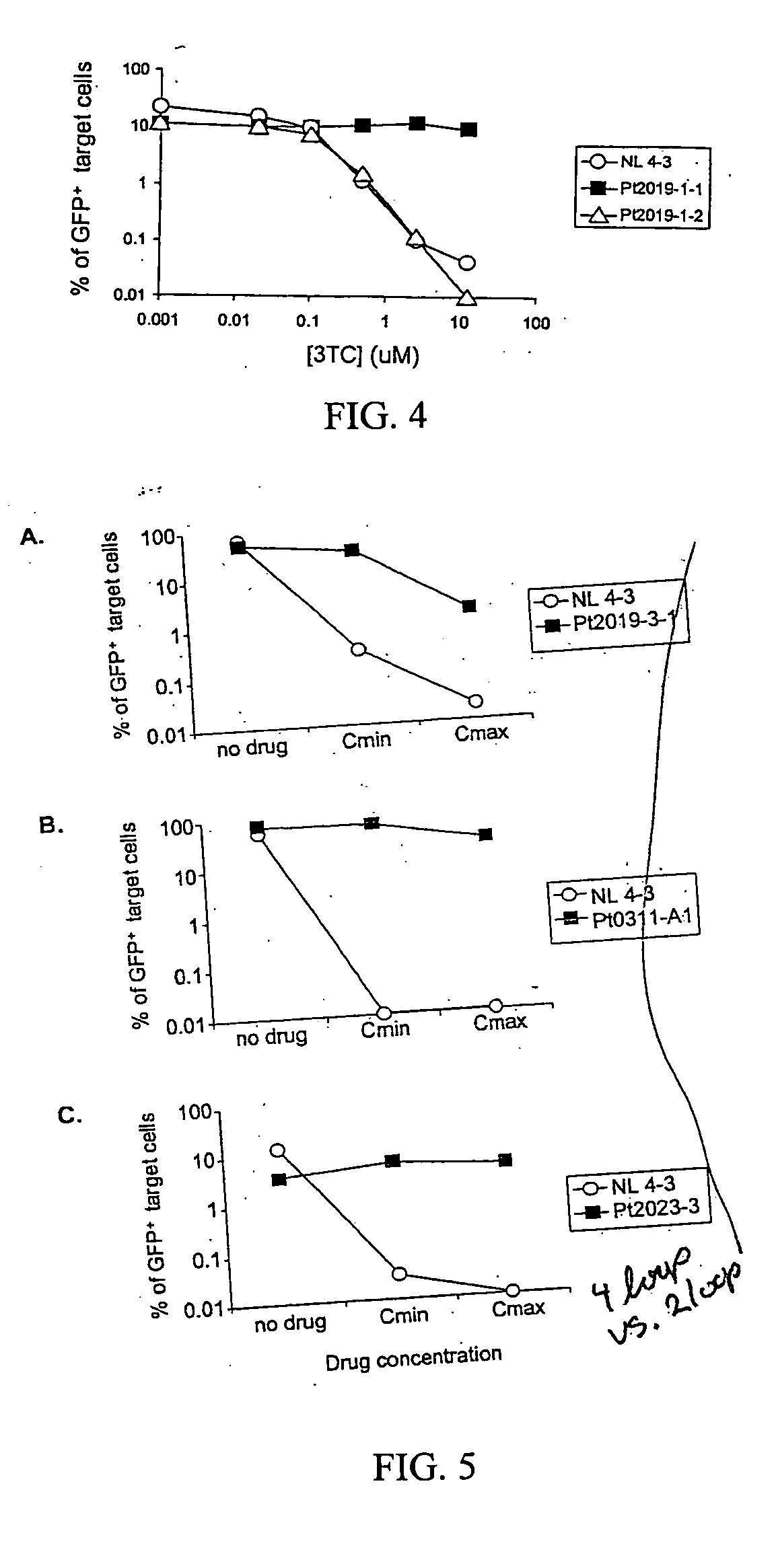
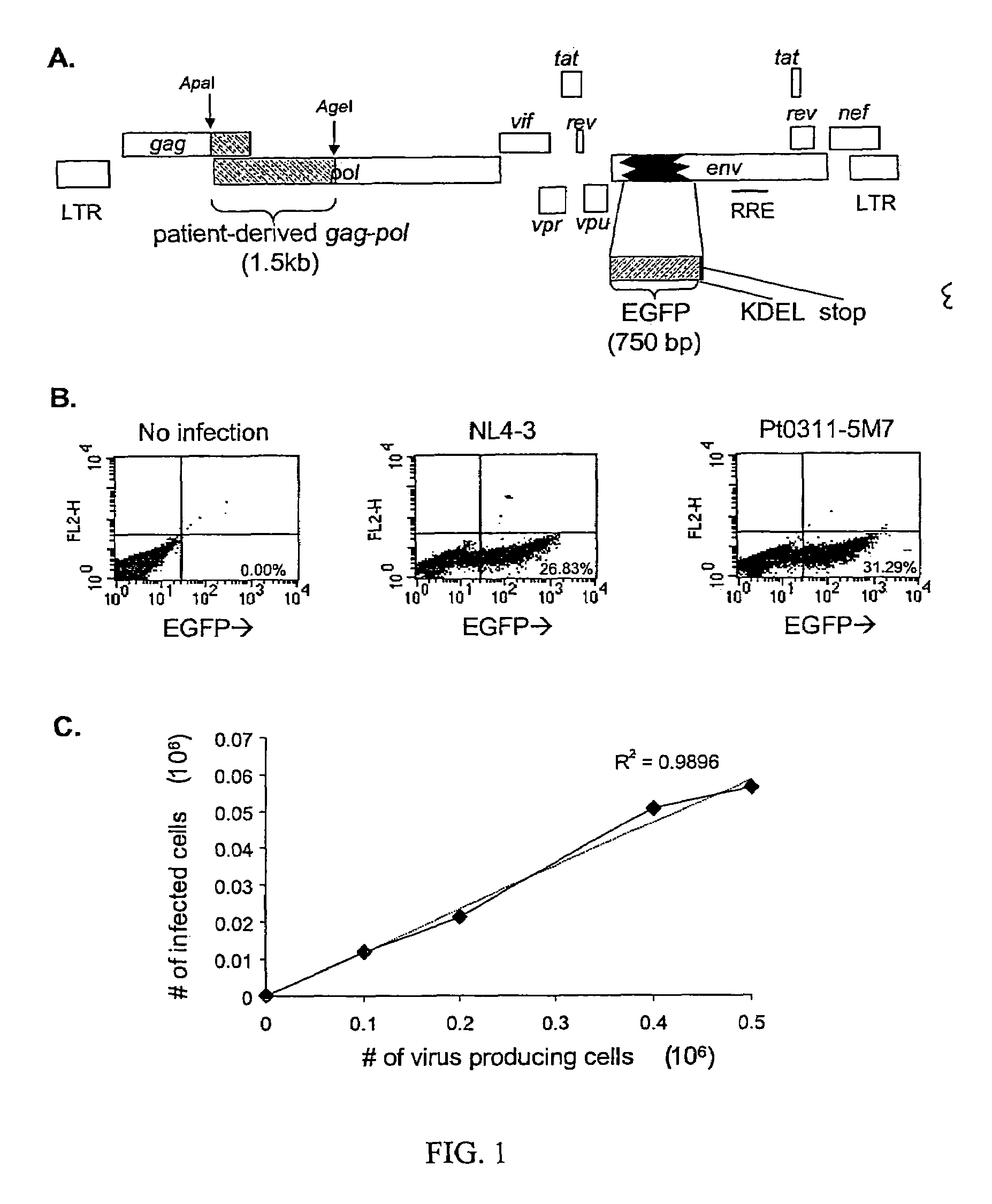
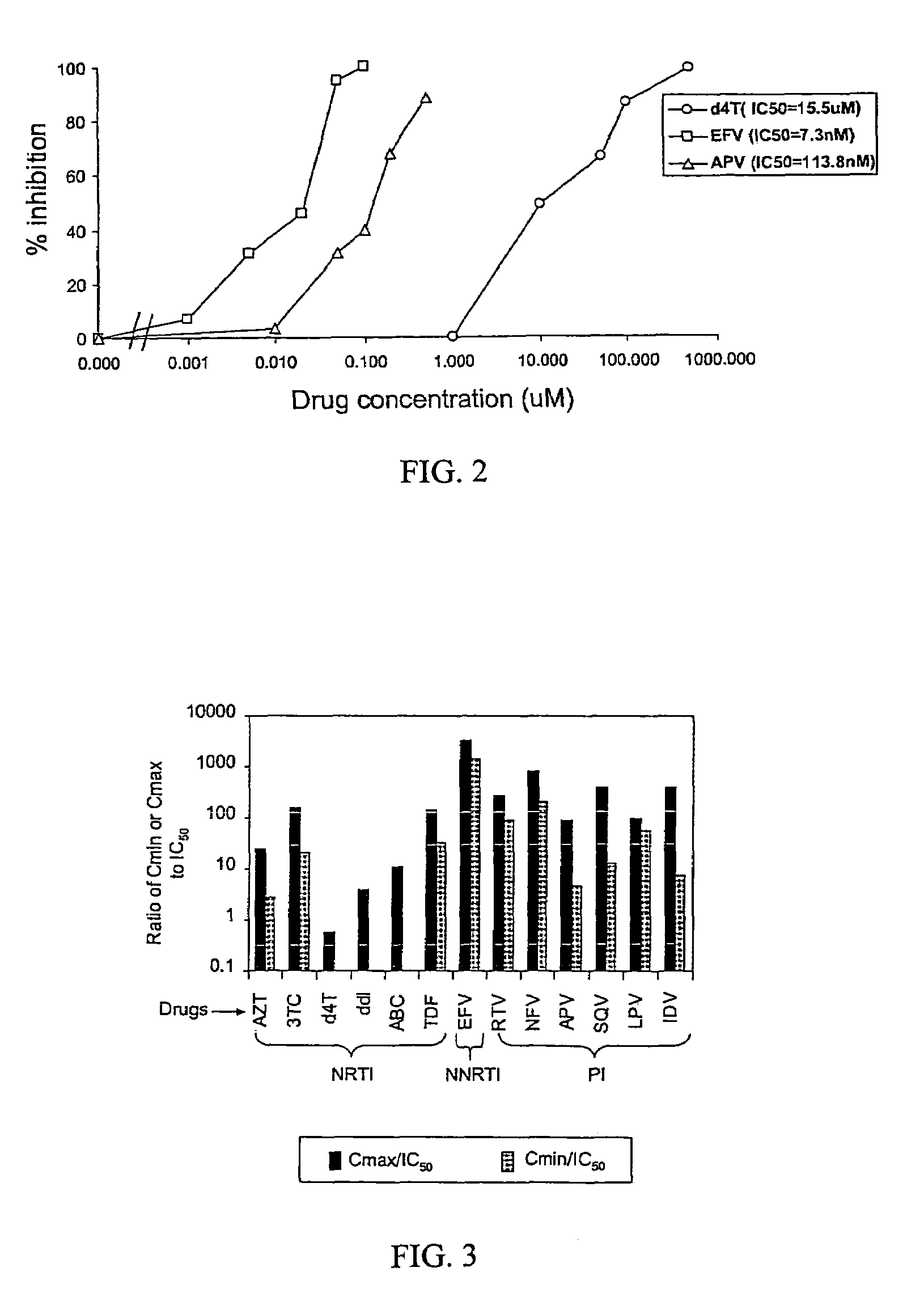
A novel single-cell-level phenotypic assay is described, which can simultaneously analyze HIV-1 drug susceptibility and intrinsic replication capacity. This allows quantitative dissection of the functions of antiretroviral drugs into suppression of viral replication and selection of resistant viruses with diminished replication capacities. The disclosed assay provides a tool for the rational evaluation of treatment decisions for patients failing antiretroviral therapy and is expected to be an important part in clinical management of HIV.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Single cell analysis of HIV replication capacity and drug resistance
ActiveUS7468274B2Less-effective in controllingAccurate countVectorsMicrobiological testing/measurementResistant virusViral replication
A novel single-cell-level phenotypic assay is described, which can simultaneously analyze HIV-1 drug susceptibility and intrinsic replication capacity. This allows quantitative dissection of the functions of antiretroviral drugs into suppression of viral replication and selection of resistant viruses with diminished replication capacities. The disclosed assay provides a tool for the rational evaluation of treatment decisions for patients failing antiretroviral therapy and is expected to be an important part in clinical management of HIV.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Detection of DNA sequences as risk factors for HIV infection
A method for identifying a risk factor for diseases, disorders or conditions, such as those caused by human immunodeficiency virus, using the polymerase chain reaction and specific primers. Methods for treating patients having these diseases, disorders or conditions by antimicrobial treatment of the risk factor by combined antiviral and antibacterial treatment or by sustaining or stimulating the subject's immune system. Methods for screening biological products including red blood cell preparations. Primers and methods for detecting nucleic acids or microbial agents associated with red blood cells, such as those associated with red blood cells in subjects infected with HIV and undergoing antiretroviral therapy.
Owner:MONTAGNIER LUC
Compounds with HIV-1 integrase inhibitory activity and use thereof as anti-HIV/AIDS therapeutics
Pharmacophore models to be used in drug design and discovery are provided. An in silico protocol and in vitro assays are presented. Compounds and their pharmaceutically acceptable salts with HIV-1 integrase inhibitory and anti-HIV activity and use thereof in the treatment of HIV / AIDS and related infections either alone or in combination with all the known antiretroviral therapeutics are described.
Owner:UNIV OF SOUTHERN CALIFORNIA
Method for mutation detection in hiv using pol sequencing
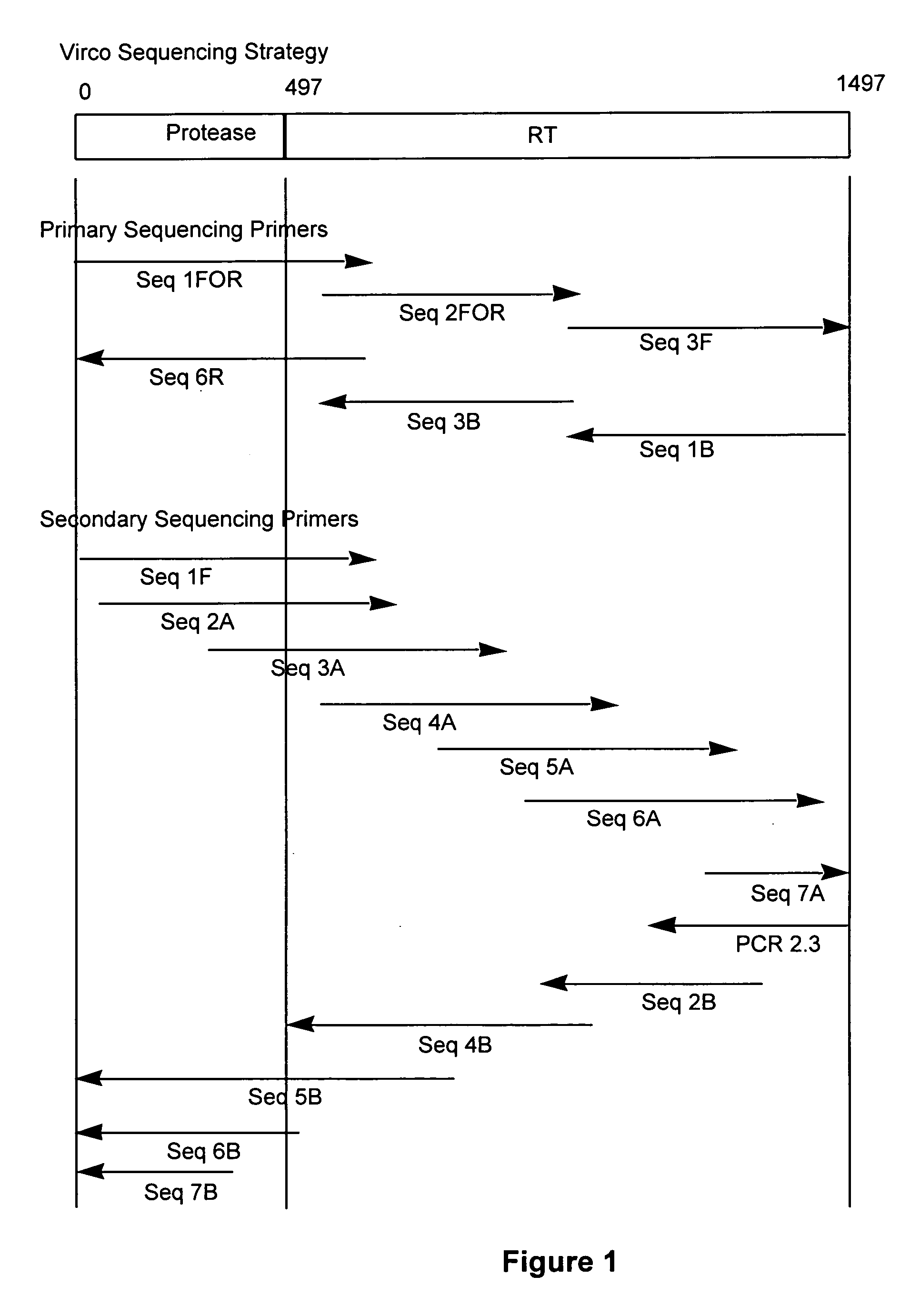
InactiveUS20050058981A1Sugar derivativesMicrobiological testing/measurementMutation detectionOligonucleotide
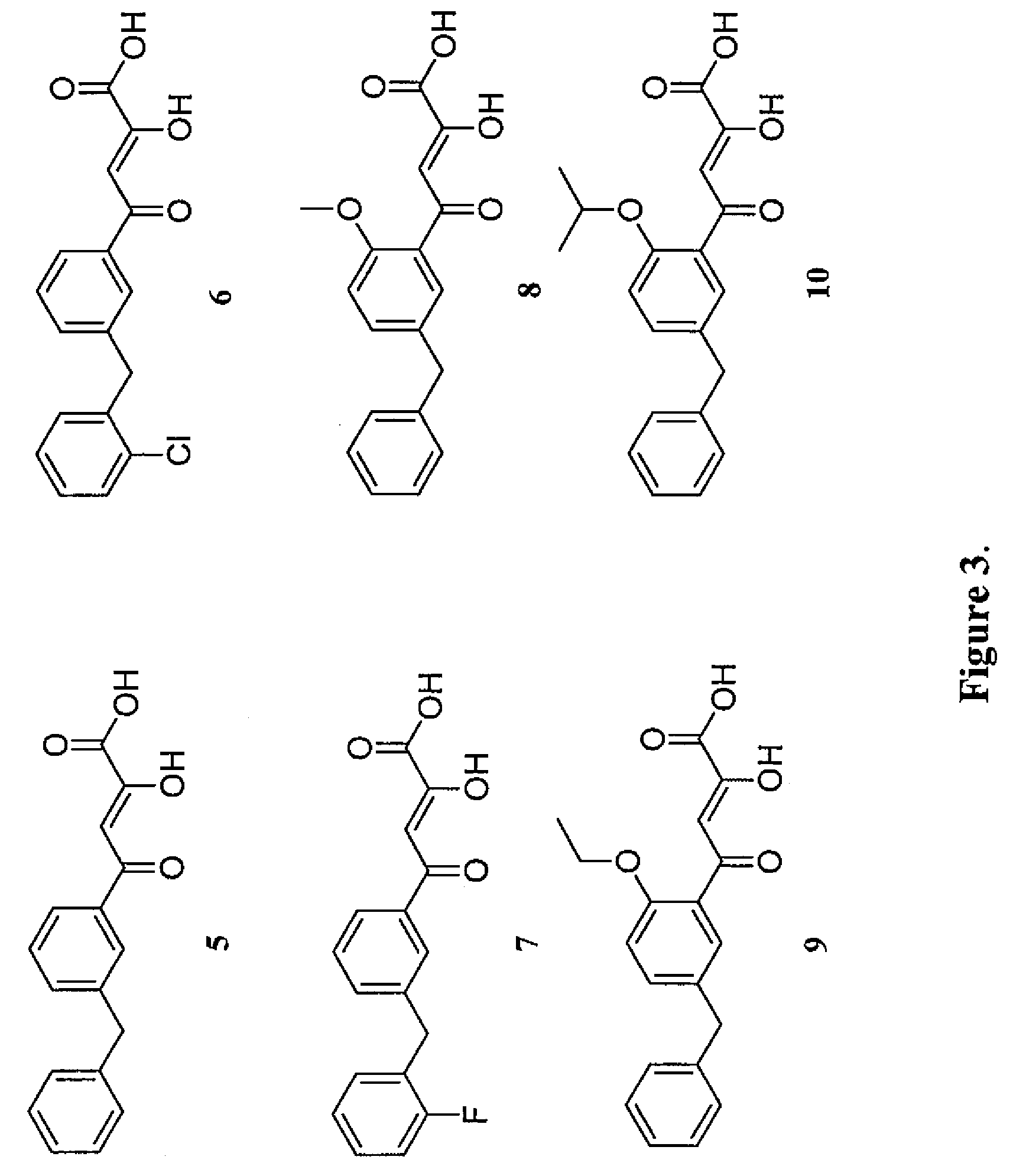
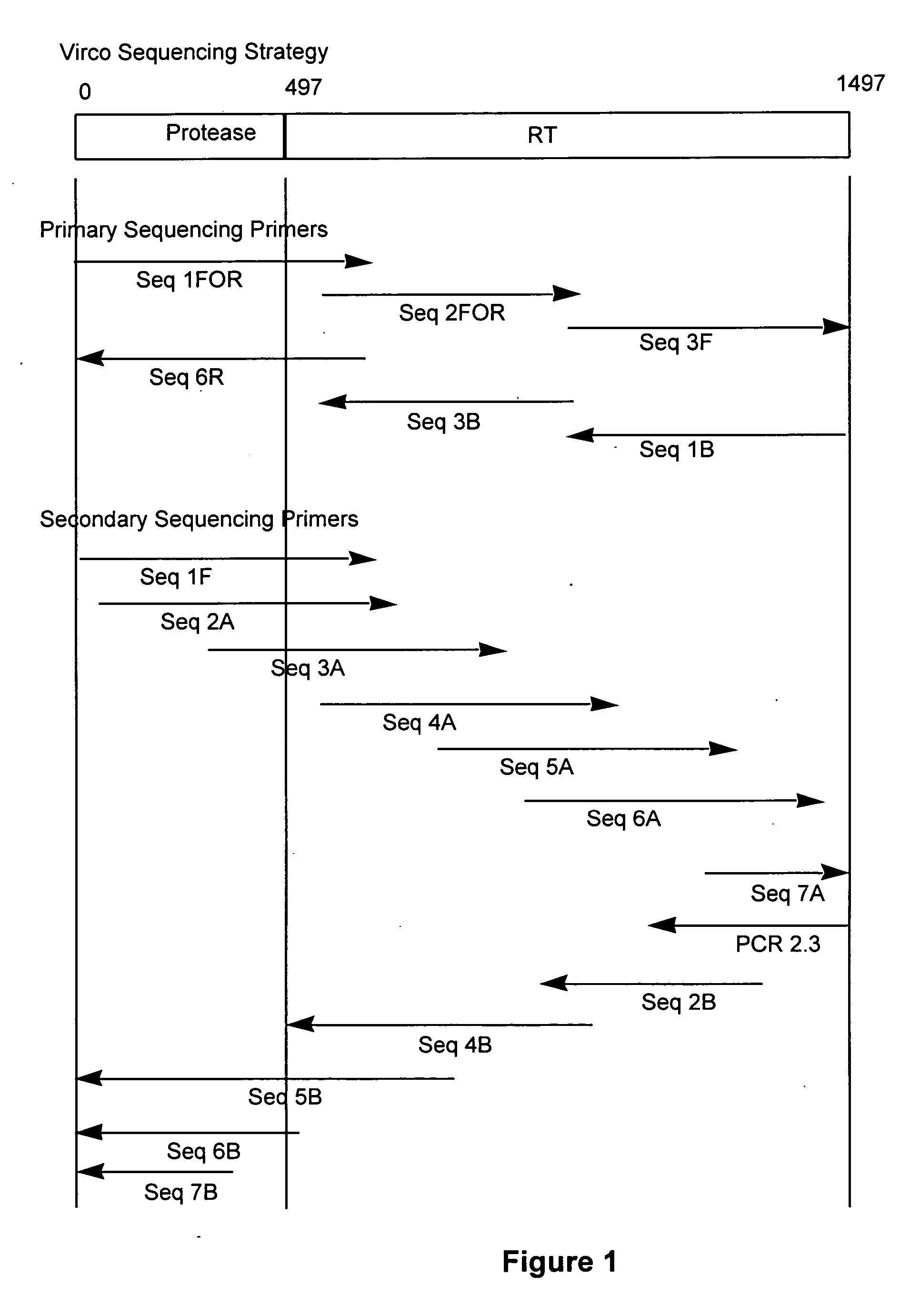
The present invention relates to a method for mutation analysis of the HIV pol gene of HIV virions comprising amplifying virion RNA or DNA via nested PCR using outer primers as represented in SEQ ID No. 1 and 2, amplifying said PCR product via nested PCR using a 5′ and 3′ primer chosen from the inner primers SEQ ID No. 3, 4, 5, and 6, and sequencing this secondary obtained PCR product using at least one sequencing primer chosen from any of SEQ ID No. 7 to 12 or variants thereof. In the alternative, at least one secondary sequencing primer may be used chosen from any of SEQ ID No. 13 to 24. The benefit of the sequences present in the invention resides in the fact that, with the aid of the oligonucleotides, the sequences of all presently known HIV subtypes and all mutations of the pol gene presently known to yield resistance towards antiretroviral therapy can be determined. The present invention also relates to kits for performing such a method as well as primers for performing the same.
Owner:VIRCO NV
Polymerase chain reaction assays for monitoring antiviral therapy and making therapeutic decisions in the treatment of acquired immunodeficiency syndrome
InactiveUS20030118986A1Inhibit deteriorationConducive to survivalBioreactor/fermenter combinationsBiological substance pretreatmentsRegimenImmunodeficiency virus
The present invention relates to methods of monitoring, via polymerase chain reaction, the clinical progression of human immunodeficiency virus infection and its response to antiretroviral therapy. According to the invention, polymerase chain reaction assays may be used to predict immunological decline and to identify, at an early stage, patients whose infection has become resistant to a particular antiretroviral drug regimen.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Compositions and Methods for Preventing or Treating Influenza Virus Infection
The invention includes compositions and methods for regulating PI3K p110 delta as an anti-retroviral therapy. The invention includes inhibiting p110 delta, a component of PI3K p110 delta signaling pathway, or any combination thereof in a cell as an anti-retroviral therapeutic approach for treating a retroviral infection, for example HIV. The invention includes a method of modulating PI3K p110 delta in a cell infected with a retrovirus by contacting the cell with an effective amount of a composition comprising an inhibitor of PI3K p110 delta.
Owner:DREXEL UNIV
Immunotherapy regimens in hiv-infected patients
InactiveUS20060094006A1Good curative effectImprove satisfactionViral antigen ingredientsMicrobiological testing/measurementImproved methodImmunotherapy
This invention relates to an improved method of maintaining an immuno-protective response in persons infected with a retrovirus during early infection or after highly active anti-retroviral therapy.
Owner:SANOFI PASTEUR LTD +1
Methods for inducing an immune response against human immunodeficiency virus infection in subjects undergoing antiretroviral treatment
Methods for inducing an immune response against Human Immunodeficiency Virus (HIV) in HIV-infected subjects undergoing antiretroviral therapy (ART) are described. The methods include administering an adenovirus vector primer vaccine and either a Modified Vaccinia Ankara virus (MVA) vector booster vaccine or adenovirus booster vaccine in combination with isolated HIV envelope polypeptides.
Owner:JANSSEN VACCINES & PREVENTION BV +1
Method for the non-surgical treatment of lipodystrophy in a retrovirus-infected individual
InactiveUS20140024630A1Reducing fat tissueOrganic active ingredientsMetabolism disorderSurgical treatmentNon surgical treatment
The present invention refers to a method for the non-surgical treatment of a lypodistrophy caused by an antiretroviral therapy in an individual infected by a retrovirus, such as HIV, and undergoing said antiretroviral therapy said method comprising the step of administering an effective amount of a adipocitolytic composition comprising deoxycholic acid and / or a pharmaceutically acceptable salt thereof in a retrovirus-infected individual affected by said lipodistrophy.
Owner:MOTOLESE PASQUALE
Treatment of lipodystrophy
InactiveUS7022693B2Effective treatmentConvenient treatmentBiocidePeptide/protein ingredientsRenin–angiotensin systemAntiretroviral treatment
An inhibitor of the renin-angiotensin system is useful for the treatment or prevention of the lipodystrophy syndrome, e.g. in AIDS patients also receiving anti-retroviral therapy.
Owner:ARK THERAPEUTICS
Methods for inducing an immune response against human immunodeficiency virus infection in subjects undergoing antiretroviral treatment
ActiveUS10307477B2Viral antigen ingredientsVirus peptidesAcute human immunodeficiency virus infectionImmunodeficiency virus
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY +3
Means and methods for monitoring non-nucleoside reverse transcriptase inhibitor antiretroviral therapy and guiding therapeutic decisions in the treatment of HIV/AIDS
InactiveUS7037644B1Microbiological testing/measurementNucleoside Reverse Transcriptase InhibitorGenotype Assay
This invention relates to antiviral drug susceptibility and resistance tests to be used in identifying effective drug regimens for the treatment of human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS) and further relates to the means and methods of monitoring the clinical progression of HIV infection and its response to antiretroviral therapy, particularly non-nucleoside reverse transcriptase inhibitor therapy using phenotypic susceptibility assays or genotypic assays.
Owner:MONOGRAM BIOSCIENCES
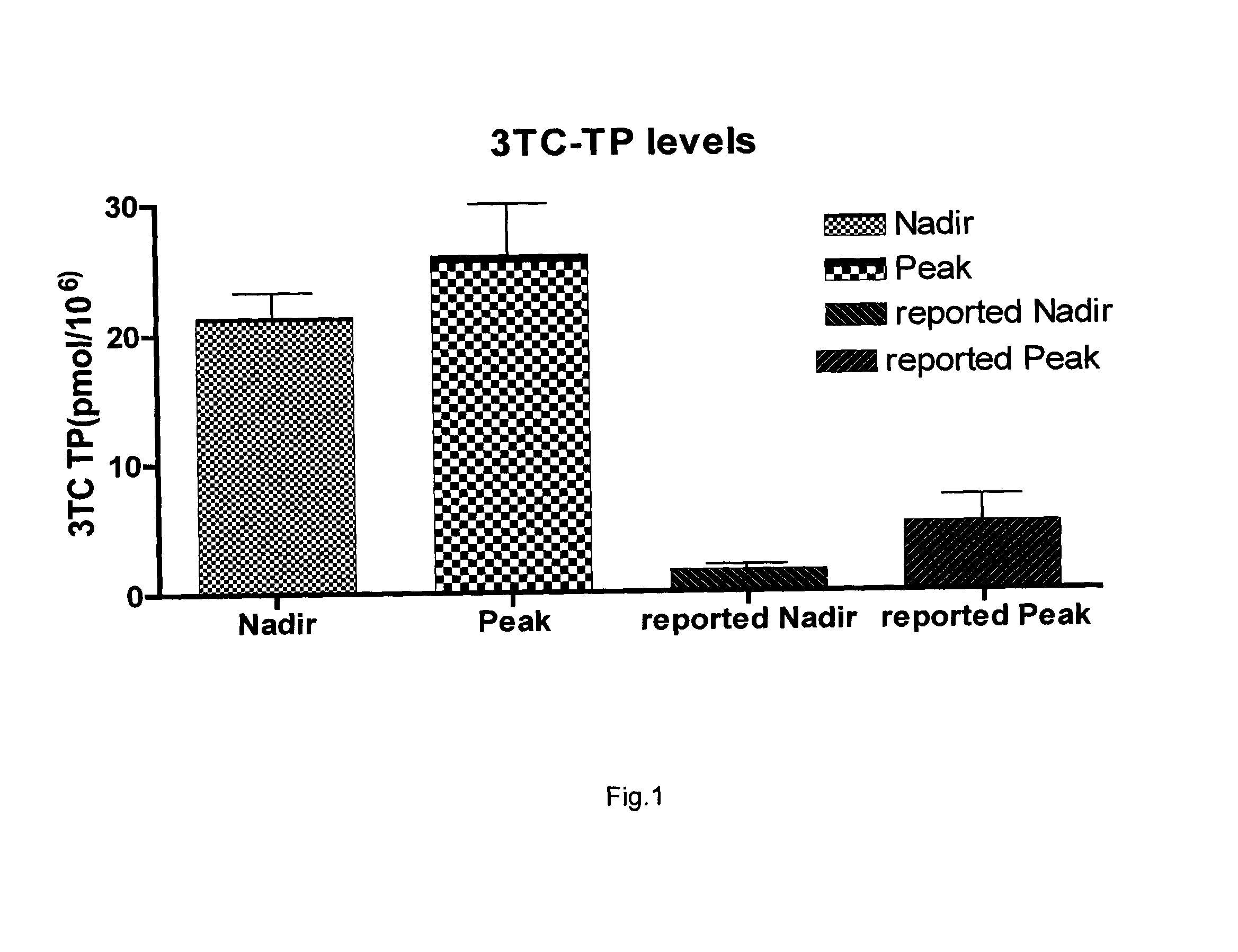
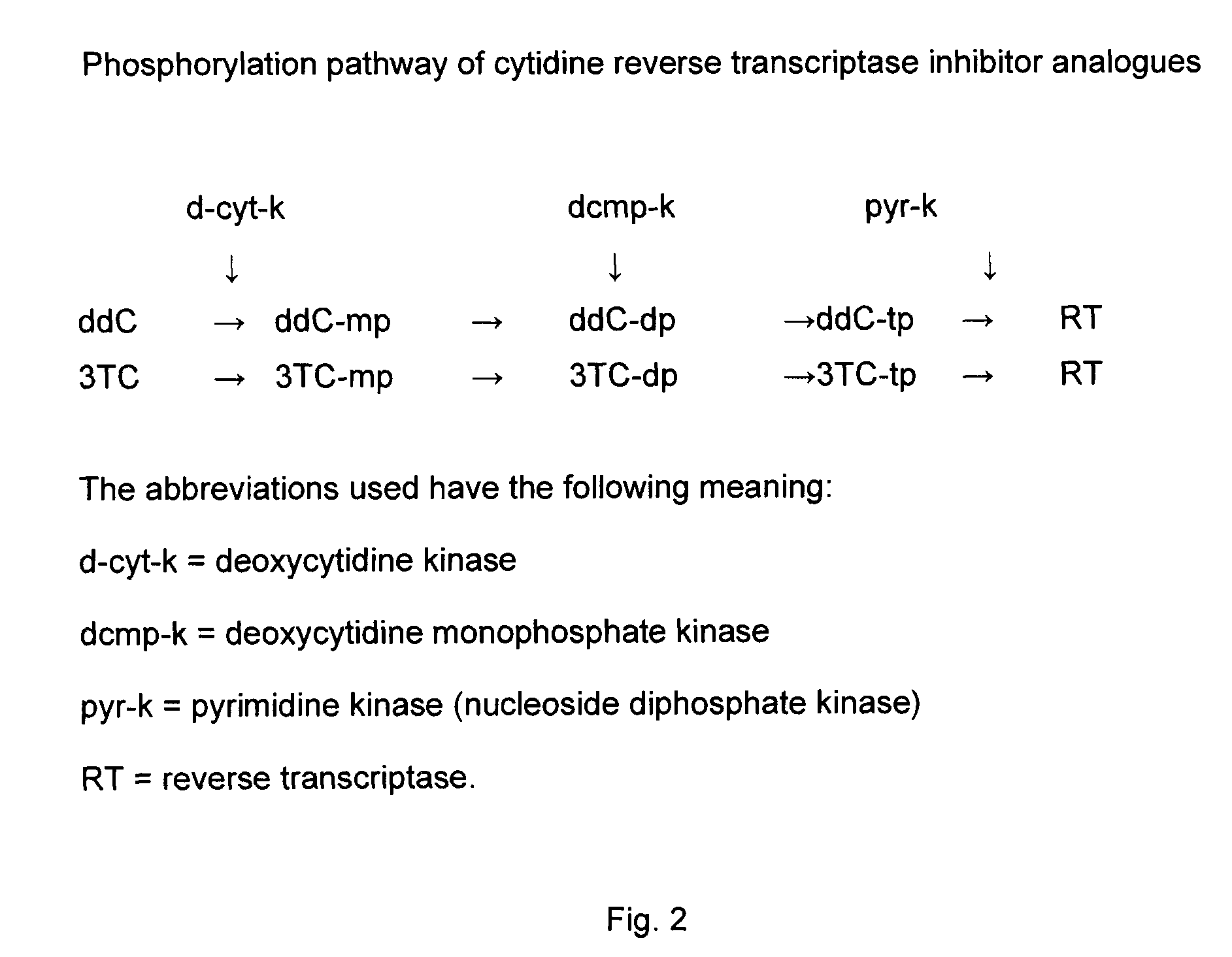
Zalcitabine (Ddc) Boosted Lamivudine (3Tc) Compositions for Antiretroviral Therapy
ActiveUS20080214590A1High activityImprovement of TC antiretroviral activityOrganic active ingredientsBiocideTolerabilitySide effect
Boosted cytidine analogue reverse transcriptase inhibitor antiretroviral compound is a new therapeutic anti HIV option, in combination with another drug such as a NRTI or a protease inhibitor. It's heightened and sustained antiretroviral potency is due to the increased intracellular level of 3TC triphosphate, the active form of 3TC. This effect is obtained by combining 3TC, in usual doses, with a reduced dose of ddC, in the same pharmaceutical formulation. The product could be administered twice or even once daily, which is convenient, and does not increase the pill burden for the patient. The reduced ddC dosage prevents the occurrence of ddC related side effects. Other cytidine derivatives (racemic or negative enantiomers) could have the same effects as ddC and could probably be combined with 3TC, and have the same effect. On the other hand, low dose ddC may also increase the intracellular levels of other cytidine derivatives as it does for 3TC. Boosted cytidine analogue reverse transcriptase inhibitor antiretroviral compound could also be formulated in combination with another drug such as another NRTI (e.g. abacavir) or any protease inhibitor in the same capsule or tablet. This approach offers a dual anti-HIV therapy that is as efficacious as the routine triple therapy. In this way the HIV treatment cost could be significantly reduced which is imperative for resource-poor settings. This new formulation is convenient and well tolerated with no additional toxicity than that of the combining drug (NRTI or protease inhibitor) and 3TC. Moreover, this will enable a larger number of patients to benefit from the already known 3TC effects. It will also increase the 3TC effects in those organs or HIV sanctuaries with usually reduced 3TC concentrations or activity. It could be indicated in both the initial as well as in salvage HIV therapy. It could also be used for therapy optimization or simplification. Moreover, in combination with another NRTI such as abacavir, or even alone, it could be beneficial for reducing the HIV harm in resource-poor settings.
Owner:TOMA EMIL
Therapeutic immunization in HIV infected subjects to augment antiretroviral treatment
InactiveUS20130115237A1Stimulate immune responseEnhanced inhibitory effectBacterial antigen ingredientsViral antigen ingredientsAntigenVirus
The present invention generally relates to HIV compositions and methods of use. One aspect of the present invention relates to a composition comprising a pharmaceutically acceptable carrier and an antigen preparation, the antigen preparation comprising an HIV polypeptide or fragment thereof and a Bacillus anthracis lethal factor (LF) polypeptide, such as a LFn polypeptide. In some embodiments, the LF polypeptide can be fused or otherwise associated with the HIV polypeptide. Other aspect of the present invention relate to use of vaccine comprising a HIV polypeptide and a Bacillus anthracis lethal factor (LF) polypeptide in methods to enhance efficacy traditional antiretroviral therapy.
Owner:VACCINE TECH
Means and methods for monitoring non-nucleoside reverse transcriptase inhibitor antiretroviral therapy and guiding therapeutic decisions in the treatment HIV-AIDS
InactiveUS20050130134A1Increase in delavirdine susceptibilityLittle and no changeBiocideMicrobiological testing/measurementNucleoside Reverse Transcriptase InhibitorGenotype Assay
This invention relates to antiviral drug susceptibility and resistance tests to be used in identifying effective drug regimens for the treatment of human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS) and further relates to the means and methods of monitoring the clinical progression of HIV infection and its response to antiretroviral therapy, particularly non-nucleoside reverse transcriptase inhibitor therapy using phenotypic susceptibility assays or genotypic assays.
Owner:VIROLOGIC INCORPORATED
Method for mutation detection in HIV-1 using pol sequencing
The present invention relates to a method for mutation analysis of the HIV pol gene of HIV virions comprising amplifying virion RNA or DNA via nested PCR using outer primers as represented in SEQ ID No. 1 and 2, amplifying said PCR product via nested PCR using a 5′ and 3′ primer chosen from the inner primers SEQ ID No. 3, 4, 5, and 6, and sequencing this secondary obtained PCR product using at least one sequencing primer chosen from any of SEQ ID No. 7 to 12 or variants thereof. In the alternative, at least one secondary sequencing primer may be used chosen from any of SEQ ID No. 13 to 24. The benefit of the sequences present in the invention resides in the fact that, with the aid of the oligonucleotides, the sequences of all presently known HIV subtypes and all mutations of the pol gene presently known to yield resistance towards antiretroviral therapy can be determined. The present invention also relates to kits for performing such a method as well as primers for performing the same.
Owner:VIRCO NV
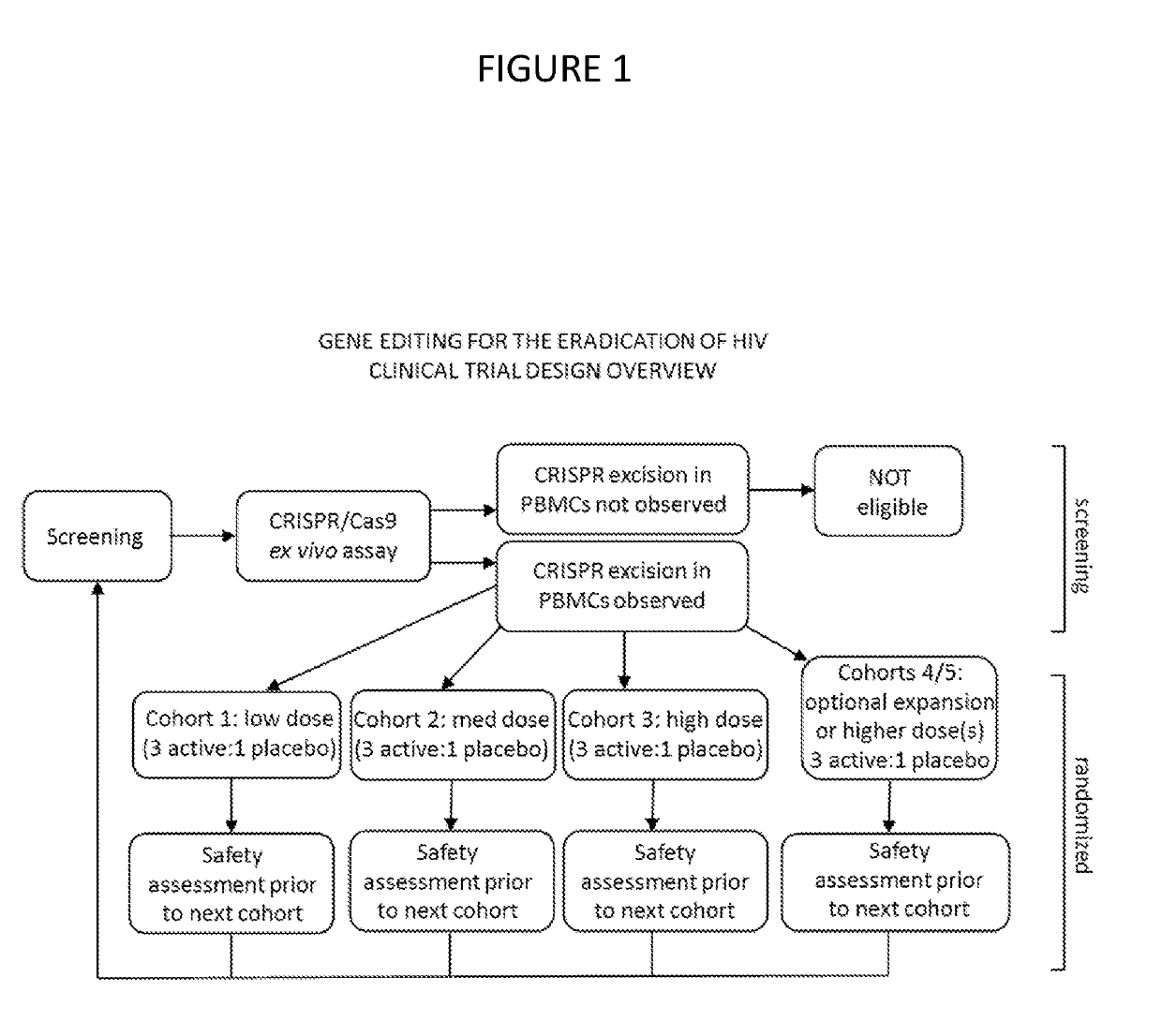
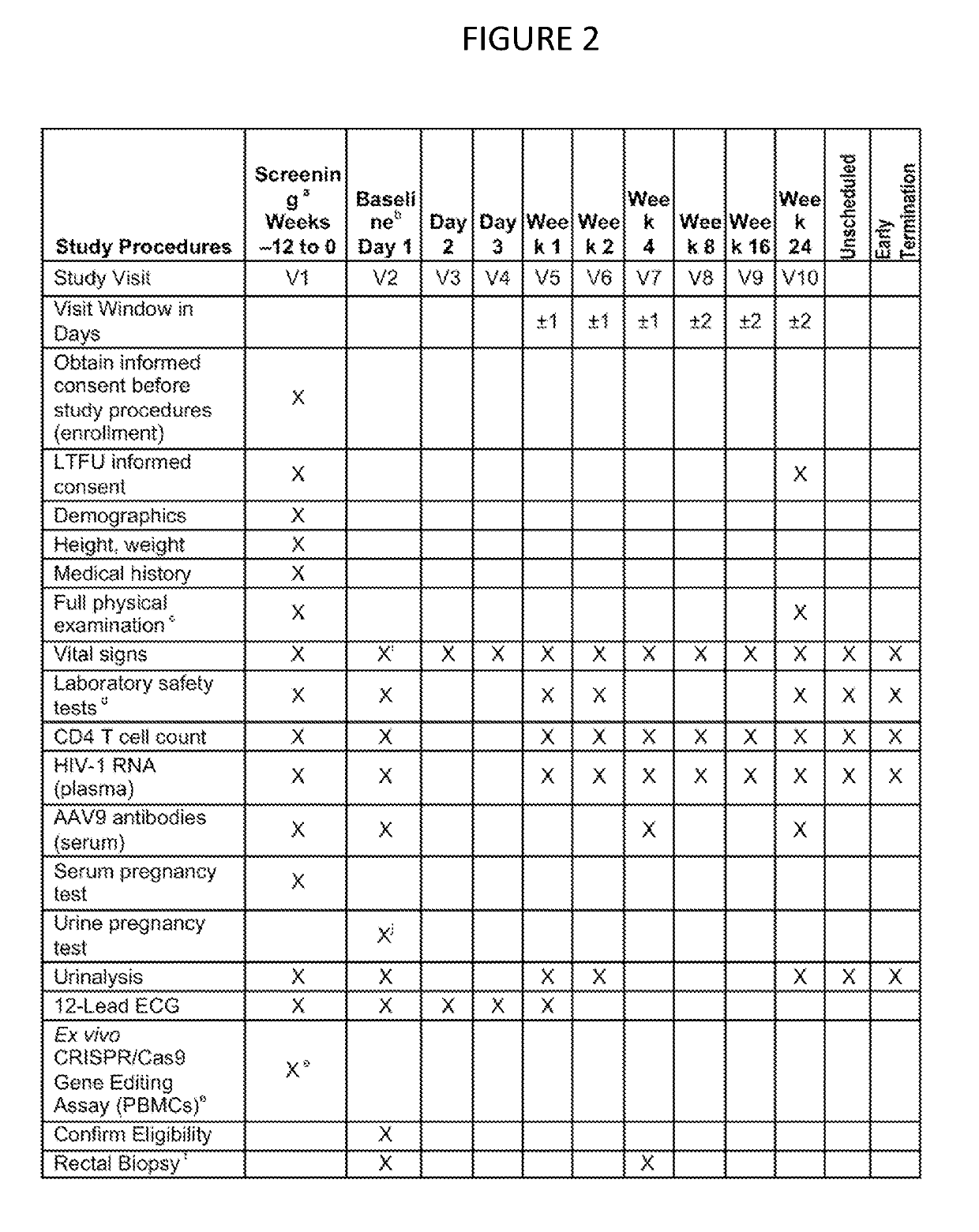
HIV clinical plan
InactiveUS20190194652A1Reduce viral loadReduce loadHydrolasesMicrobiological testing/measurementAssayClinical trial
A method of performing a clinical trial for a gene editing or gene excising system for treating HIV in humans, by recruiting HIV infected individuals currently receiving highly active antiretroviral therapy (HAART) that is effective in lowering viral load and entering qualified individuals as participants in a clinical trial, administering the gene editing or gene excising system treatment to the participants in Phase 1a, Phase 1b, and Phase 1c, and performing assays to confirm HIV viral genome excision from the participants' cells. A method of performing a clinical trial for a gene editing or gene excising system for treating a latent viral infection in humans.
Owner:EXCISION BIOTHERAPEUTICS INC
Therapeutic immunization in hiv infected subjects receiving stable antiretroviral treatment
The present invention generally relates to HIV compositions and methods of use. One aspect of the present invention relates to a composition comprising a pharmaceutically acceptable carrier and an antigen preparation, the antigen preparation comprising an HIV polypeptide or fragment thereof and a Bacillus anthracis lethal factor (LF) polypeptide, such as a LFn polypeptide. In some embodiments, the LF polypeptide can be fused or otherwise associated with the HIV polypeptide. Other aspect of the present invention relate to use of vaccine comprising a HIV polypeptide and a Bacillus anthracis lethal factor (LF) polypeptide in methods to enhance efficacy traditional antiretroviral therapy.
Owner:VACCINE TECH
Detection of DNA sequences as risk factors for HIV infection
A method for identifying a risk factor for diseases, disorders or conditions, such as those caused by human immunodeficiency virus, using the polymerase chain reaction and specific primers. Methods for treating patients having these diseases, disorders or conditions by antimicrobial treatment of the risk factor by combined antiviral and antibacterial treatment or by sustaining or stimulating the subject's immune system. Methods for screening biological products including red blood cell preparations. Primers and methods for detecting nucleic acids or microbial agents associated with red blood cells, such as those associated with red blood cells in subjects infected with HIV and undergoing antiretroviral therapy.
Owner:MONTAGNIER LUC
Combination and uses and treatments thereof
PendingUS20200113838A1Improve stabilityStability benefitOrganic active ingredientsAntiviralsPharmaceutical drugPharmaceutical medicine
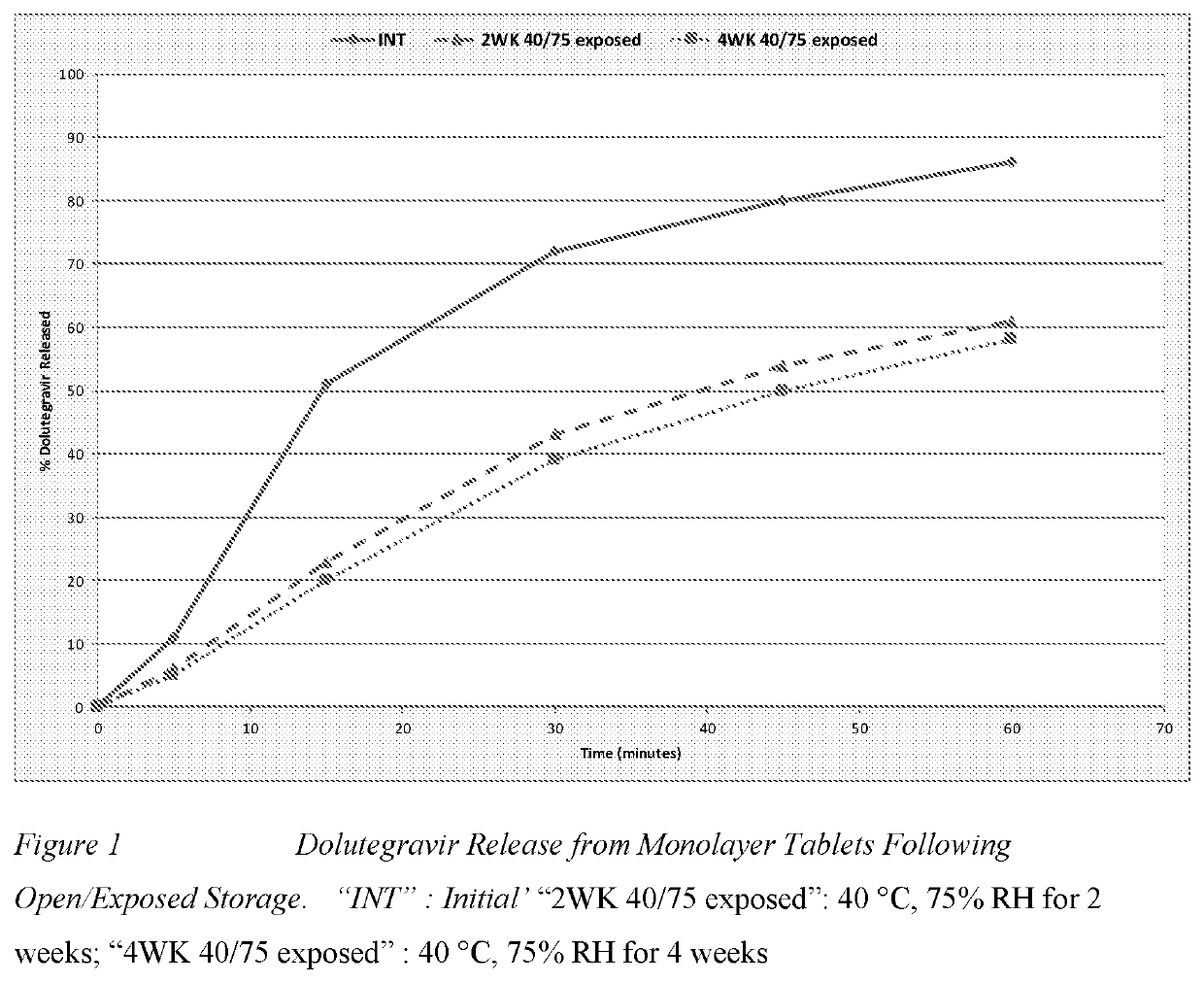
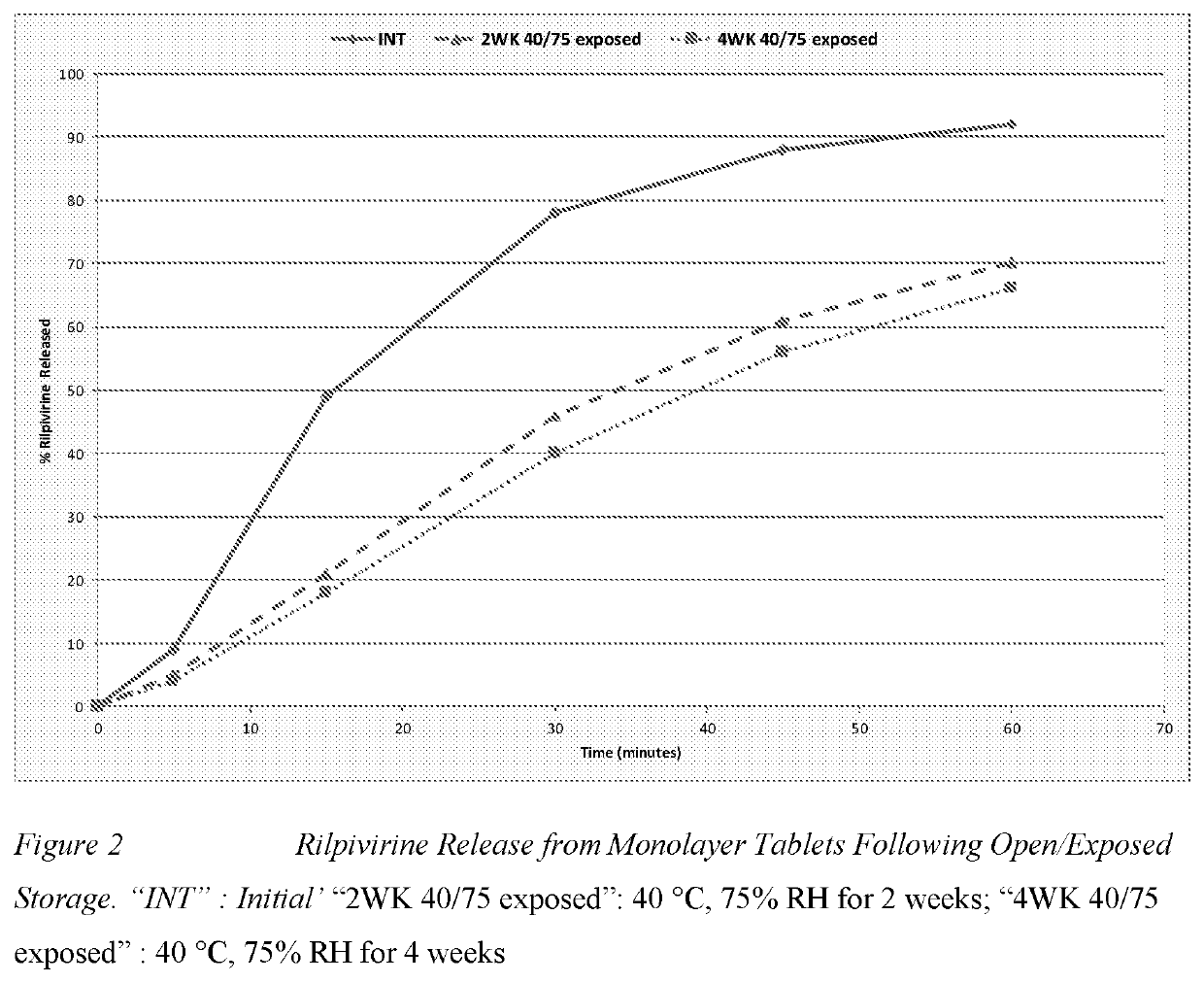
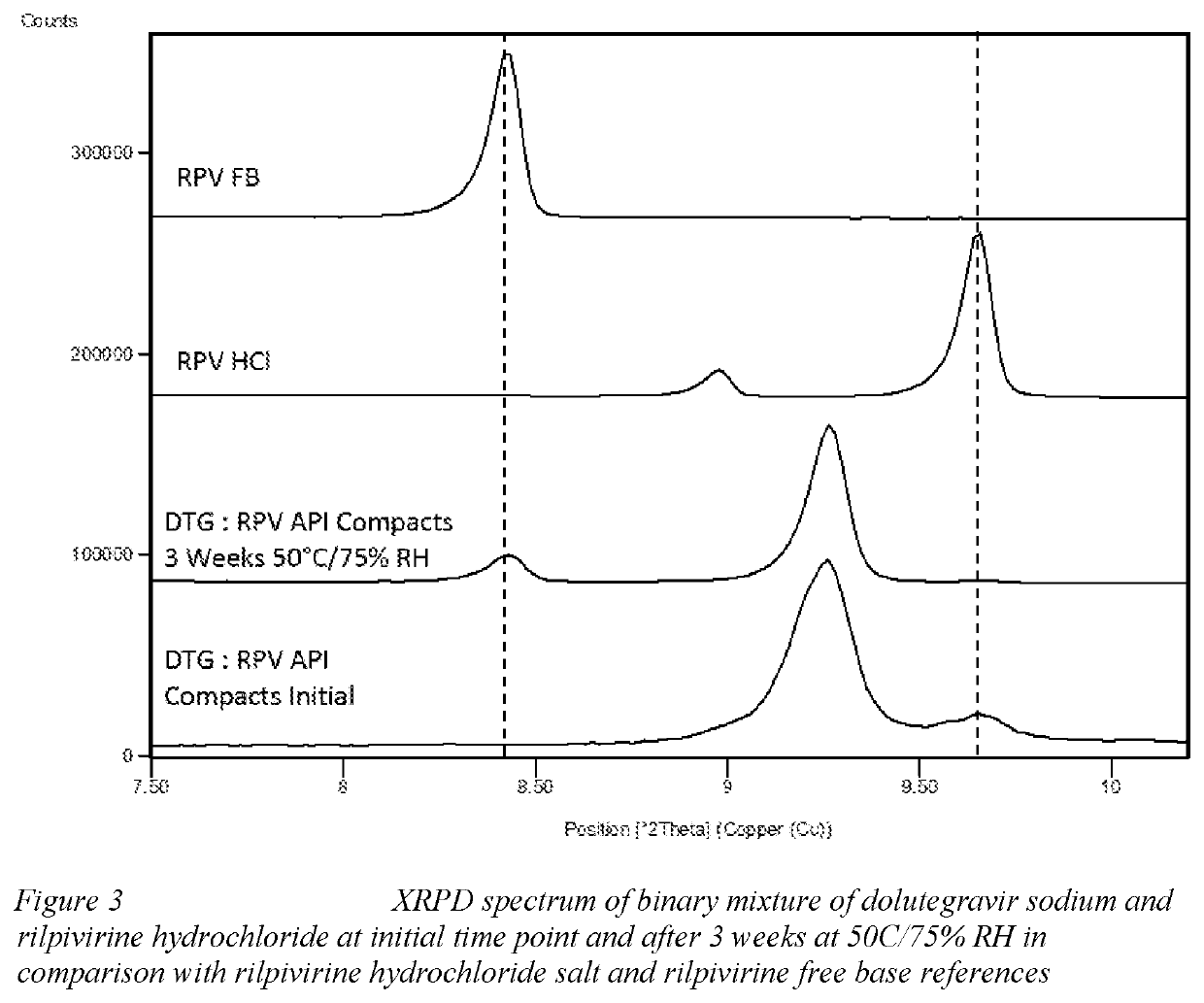
Methods are provided for treating or preventing human immunodeficiency virus-1 (HIV-1) or human immunodeficiency virus-2 (HIV-2) in a virologically suppressed patient in need thereof comprising switching the patient from an antiretroviral treatment regimen comprising at least three antiretroviral agents to a treatment regimen comprising only two antiretroviral agents. In one aspect the two treatment regimen consists of dolutegravir, rilpivirine and at least one pharmaceutically acceptable excipient, diluent or carrier. In another aspect of the invention, there is provided a multilayer tablet comprising dolutegravir or a pharmaceutically acceptable salt thereof and rilpivirine or a pharmaceutically acceptable salt thereof.
Owner:VIIV HEALTHCARE CO +1
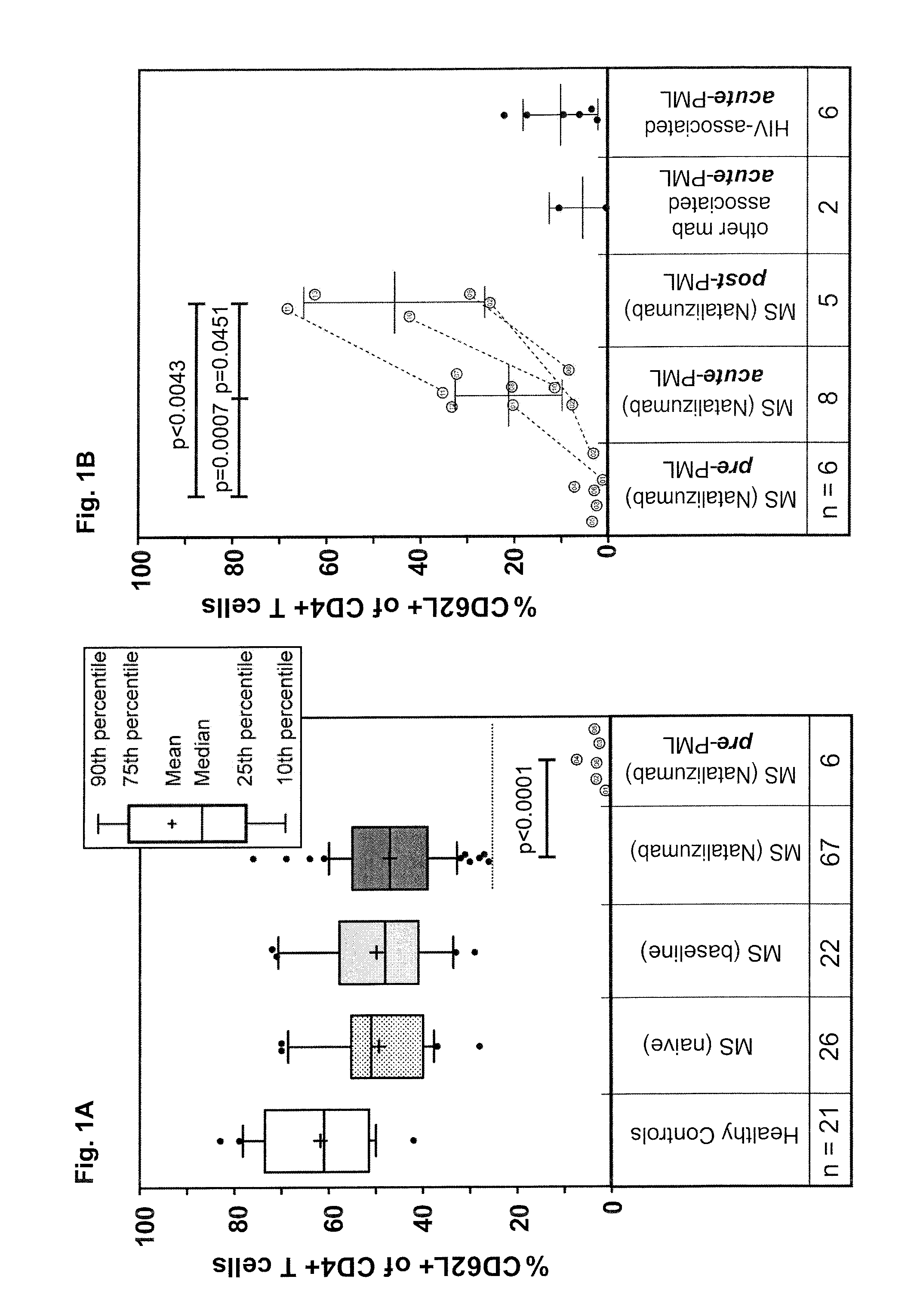
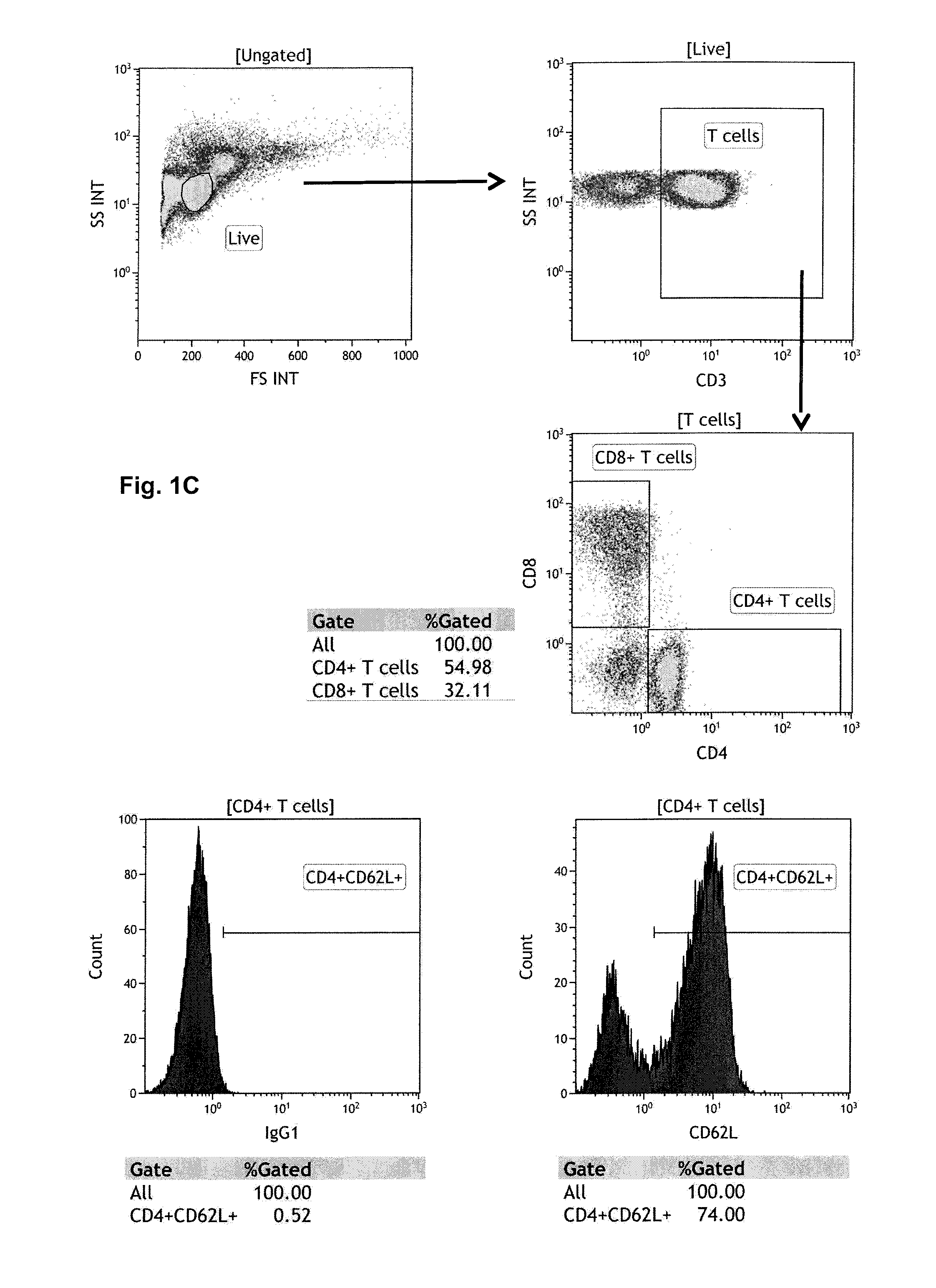
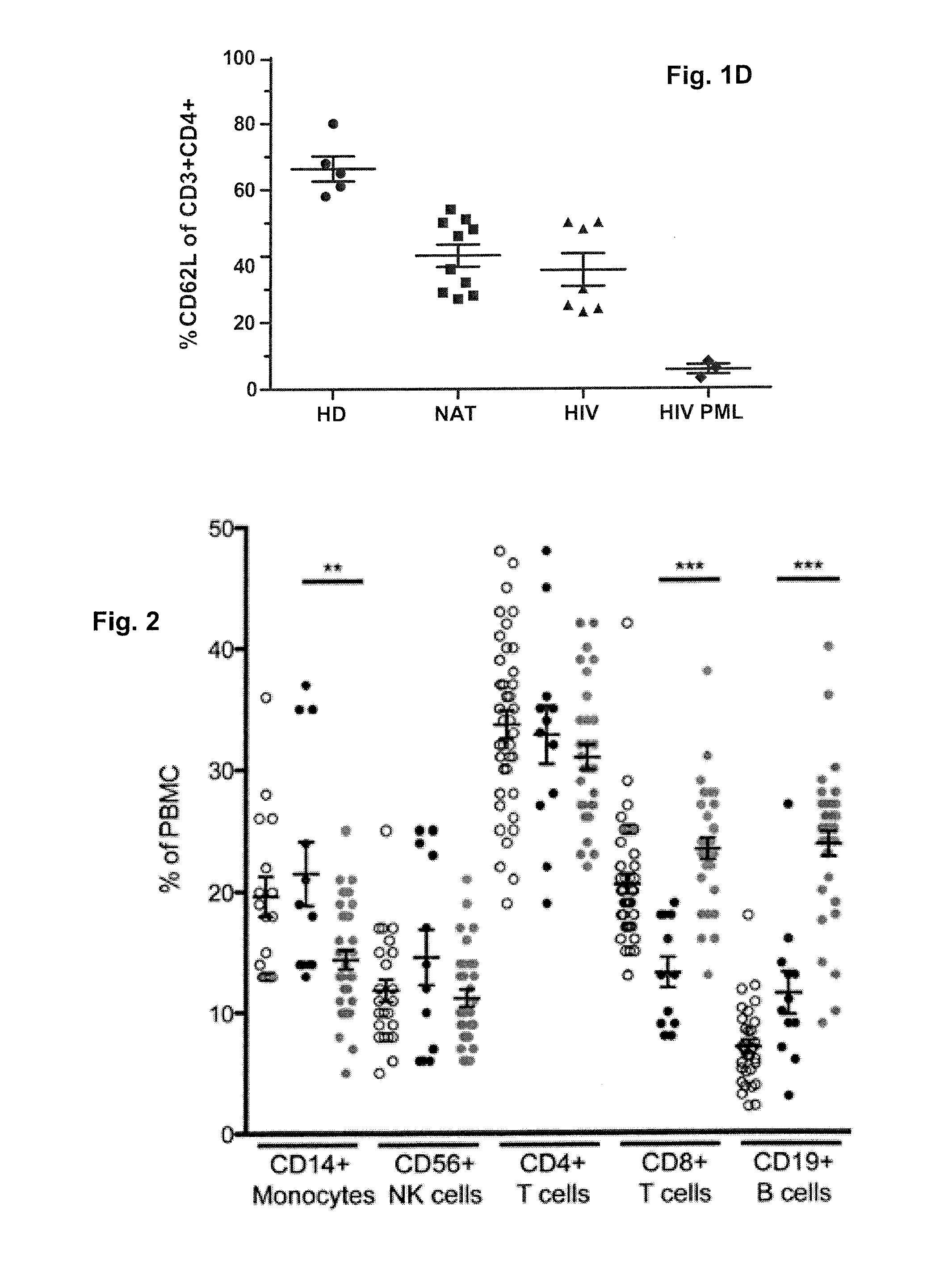
Assessment of pml risk and methods based thereon
InactiveUS20140315188A1Avoid it happening againMicrobiological testing/measurementMaterial analysisVLA-4Progressive multifocal leukoencephalopathy
The invention provides a method of assessing the risk of occurrence of progressive multifocal leukoencephalopathy (PML) in a subject as well as a method of stratifying a subject undergoing α4-integrin and / or VLA-4 blocking agent treatment for suspension of the treatment and a method of stratifying a subject undergoing Highly Active Antiretroviral Therapy (HAART) for alteration of HAART. These methods comprise detecting the level of P-selectin glycoprotein ligand-1 (PSGL-1) expressing T cells in a sample from the subject.
Owner:WESTFAELISCHE WILHELMS UNIV MUENSTER
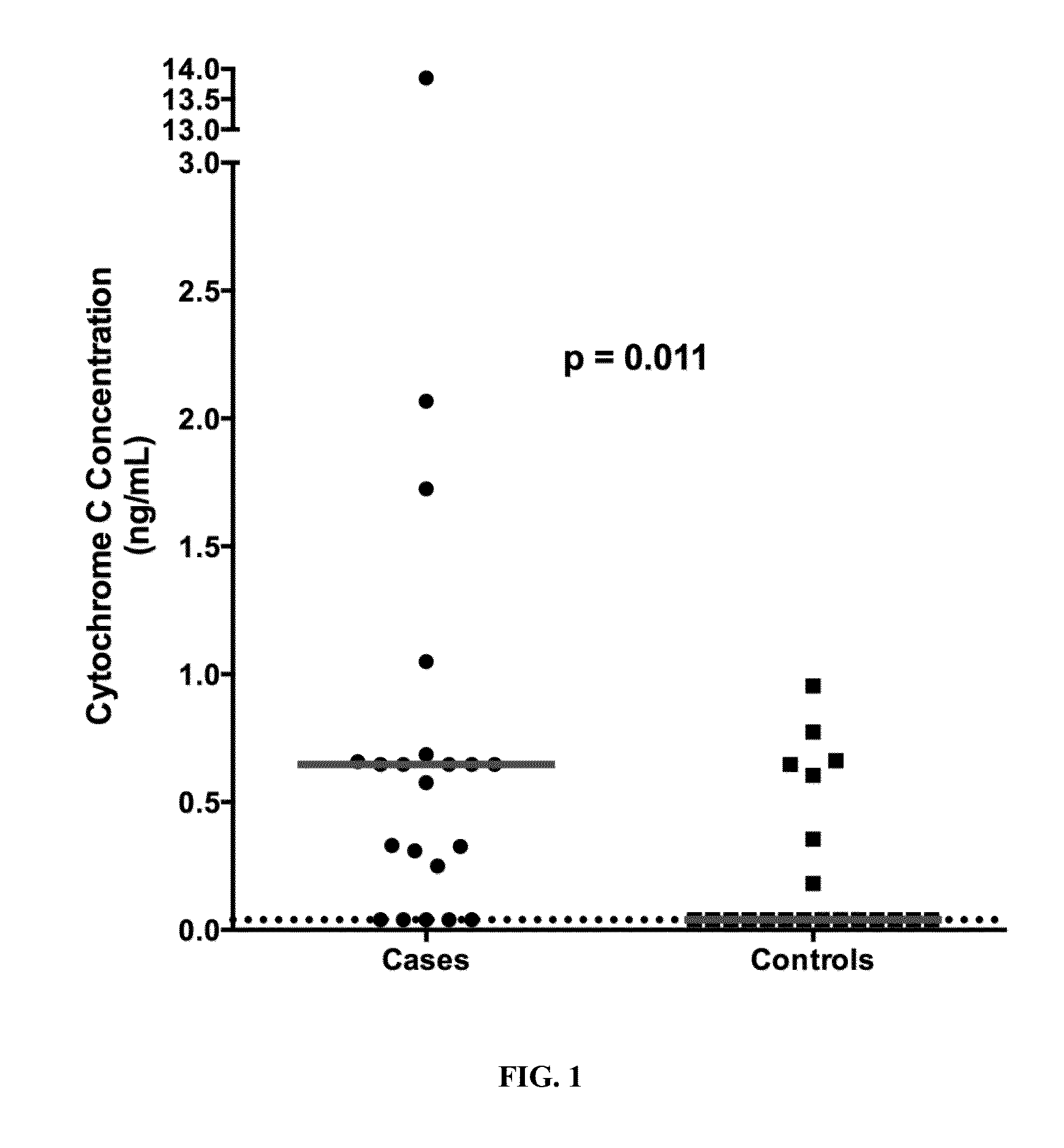
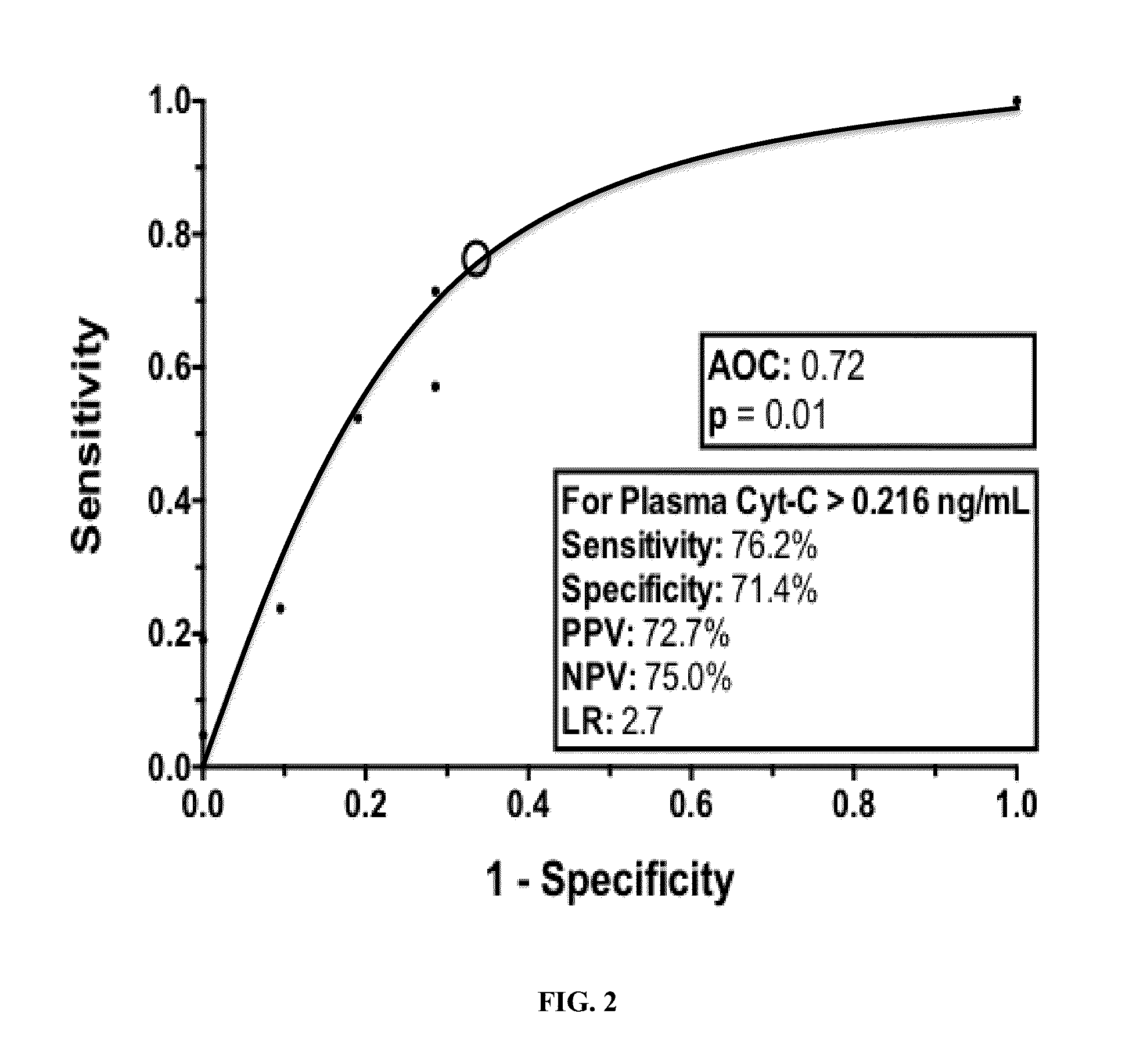
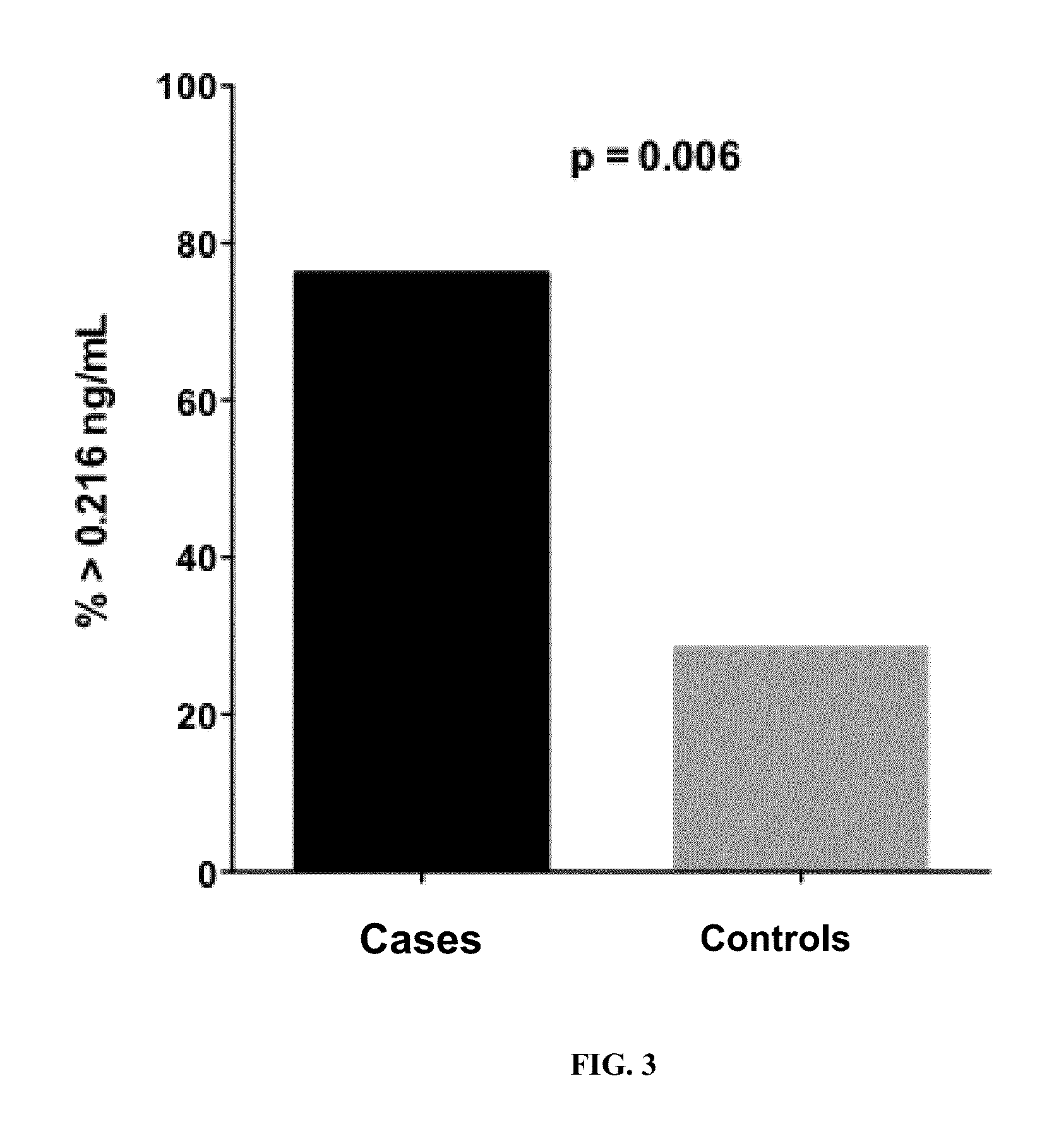
Plasma cytochrome c as a biomarker for mitochondrial toxicity during antiretroviral therapy
ActiveUS20150346213A1Detecting drug toxicityImprove the level ofLibrary screeningBiological material analysisImmunodeficiency virusMitochondrial toxicity
The present invention relates to the discovery that measurement of the level of cytochrome c (Cyt-C) in the plasma can be used as a diagnostic signature to predict antiretroviral therapy (ART) toxicity in human immunodeficiency virus (HIV) infected patients. Thus, in various embodiments described herein, the methods of the invention relate to methods of diagnosing a HIV patient with ART toxicity, methods of predicting a patient's risk of having or developing toxicity for ART, methods of assessing if a patient will benefit from a change in the treatment strategies by adjusting the dosage and / or changing the medication or even terminating of ART, and methods of predicting antiretroviral drugs propensity for causing mitochondrial toxicity. Furthermore, the invention encompasses a diagnostic kit for carrying out the aforementioned methods.
Owner:YALE UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com