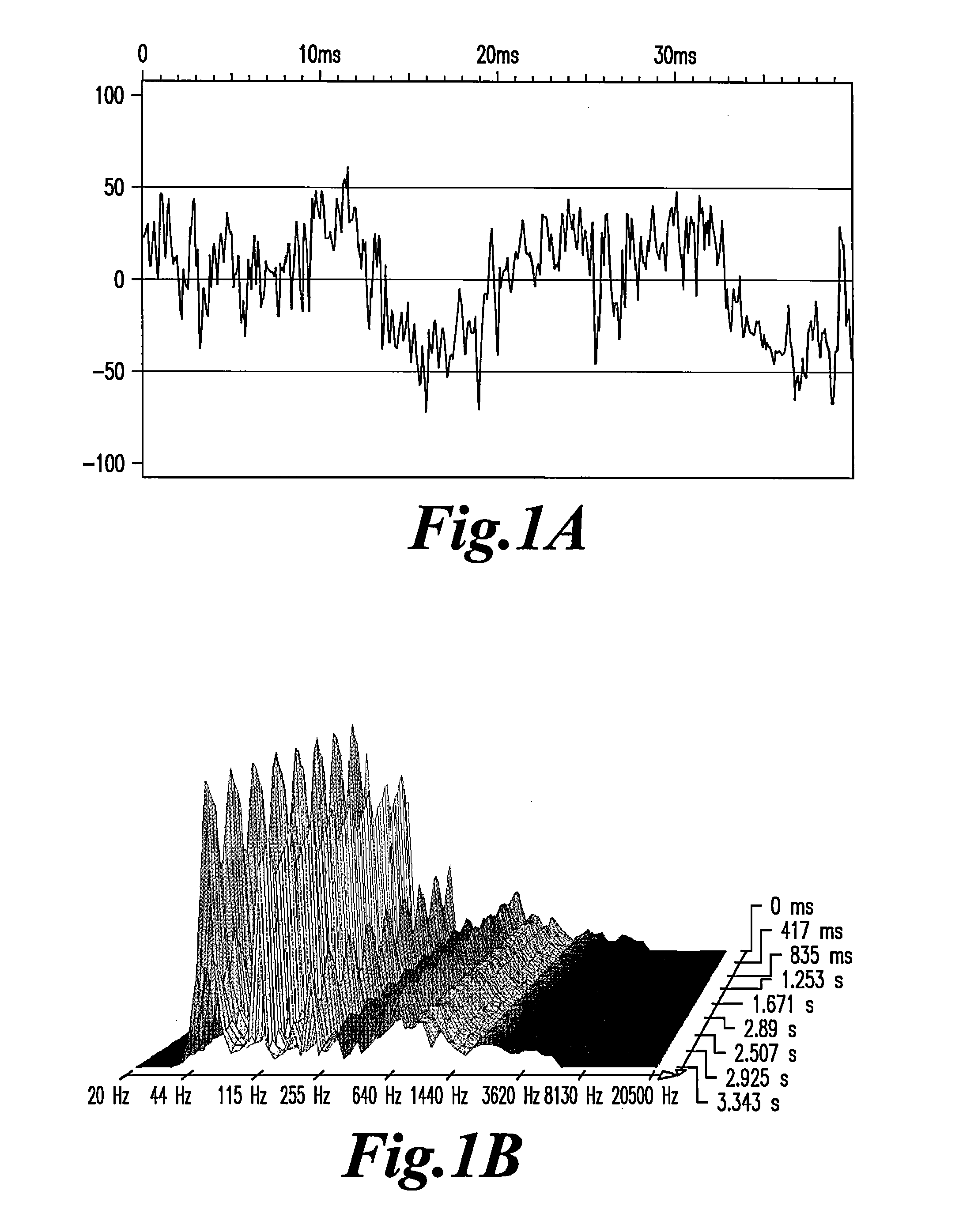
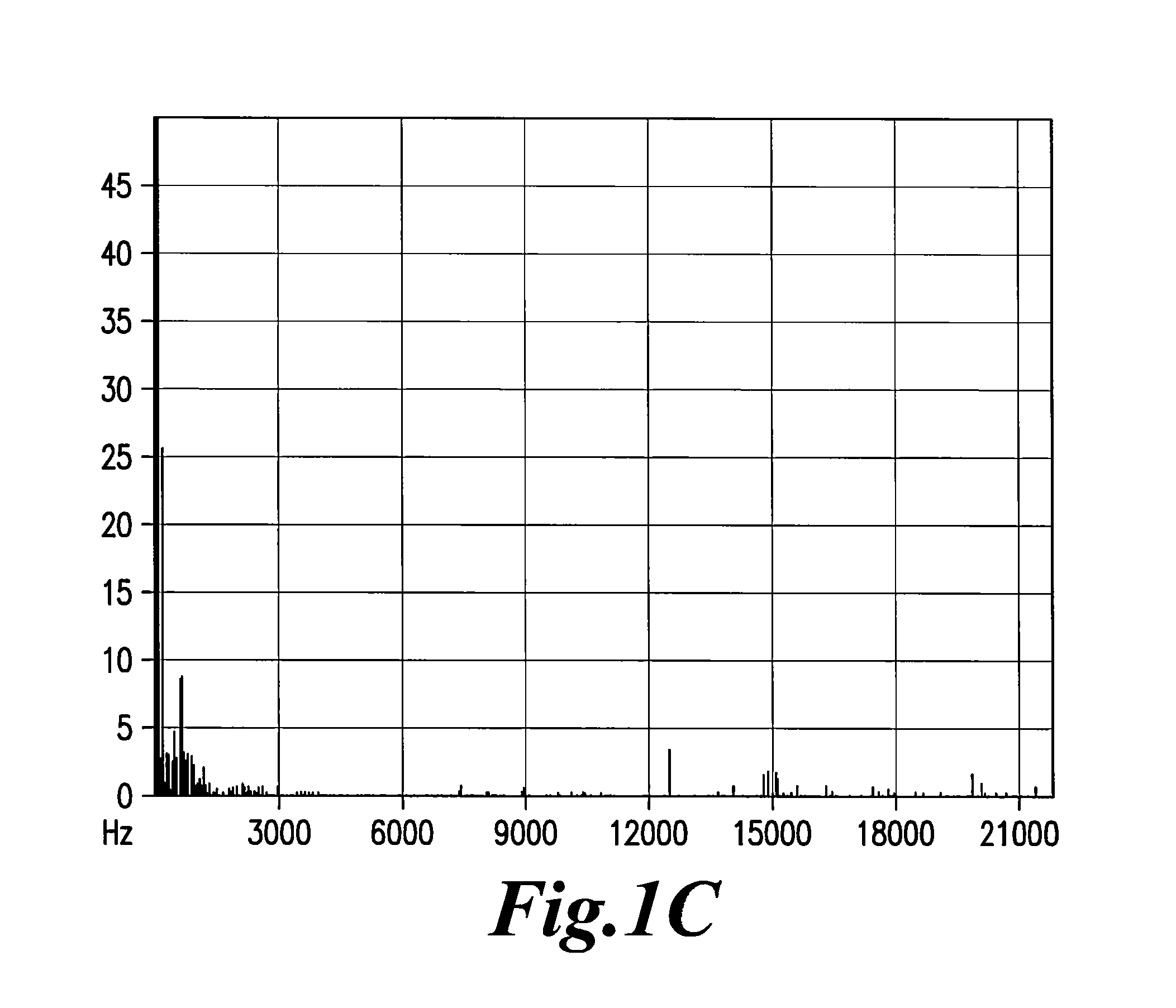
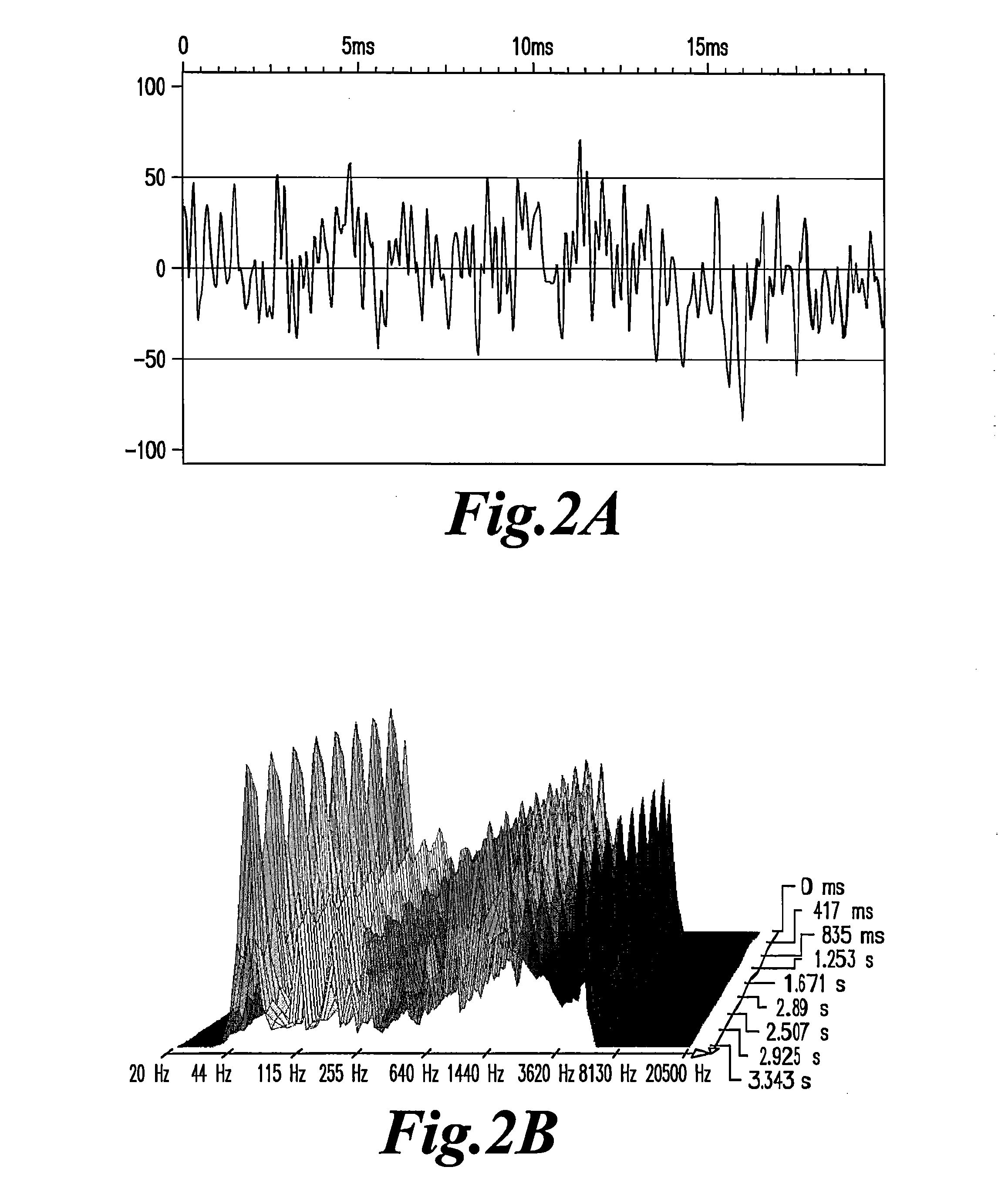
Highly sensitive method for detection of viral HIV DNA remaining after antiretroviral therapy of aids patients
a hiv dna and antiretroviral therapy technology, applied in the field of ##ds for detecting polynucleotides, can solve the problem that the classical inhibitors used in art cannot achieve the eradication of viral infection, and achieve the effect of improving the sensitivity of pcr
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Measurement of Electromagnetic Signals
[0045]The plasma or DNA solution [1-4 ng / ml] is dissolved in Phosphate Buffered Saline (PBS) at the concentration of 10−2, then filtered on Millipore 0.45 micrometer filter and the filtrate is refiltered on Anotop Whatman filter of 20 nanometer porosity. The filtrate is then diluted in distilled water in 1.5 ml Eppendorf conical plastic tubes in serial 1 part sample:9 parts diluent [decimal] dilutions ranging from 10−2 to 10−15 and strongly agitated on a vortex for at least 15 seconds.
[0046]Plasma is prepared by centrifugation of heparinized blood of patients presenting with conditions of: 1) Asymptomatic, untreated; 2) Symptomatic, not yet treated, with high virus load; or 3) Symptomatic, treated by antiretroviral therapy with no detectable virus load by commercial kits (<200 RNA copies / ml).
[0047]EMS was only detected in the plasma of the third category (30 out of 30), in plasma dilutions ranging from 10−5 to 10−8. Results with the two first ca...
example 2
The Decay with Time of EMS Production in Plasma Stored at +4° C.
[0050]The capacity to emit EMS in plasma can last for several days, sometimes for several weeks of storage, indicating a relative stability of the nanostructures that emit EMS in the plasma proteinic environment. In vitro studies indicated that filtration of the plasma (usually at the 1 / 100 dilution in PBS or saline) through 20 nM filters was a prerequisite for detecting the signals in further dilutions of water. In some rare cases, weaker signals can be detected at lower dilutions after filtration through 100 nM porosity filters. Positive signals were usually found in the range of the 10−3 to 10−9 dilutions.
example 3
Evidence that Positive Signals Come from DNA
[0051]Experiments were conducted to determine if nucleic acids carrying the genetic information for HIV, either residual viral RNA or proviral DNA, could be the sources of signals in the plasma of infected patients. Three groups of patients: infected and not treated in the asymptomatic stage; infected and not treated in the symptomatic stages; and infected and treated with ART with no detectable viral load.
[0052]Plasma was diluted 1 / 100 in PBS and the nucleic acids were extracted by the phenol-chloroform method. The solution was precipitated with ethanol and the precipitates were solubilized in water. The solution was filtered through a 20 nM filter at a concentration ranging from 1 ng / ml to 4 ng / ml.
[0053]EMS emissions were detected only in the group of patients treated by antiretroviral therapy and having an undetectable virus load. The signals were produced in the same range of aqueous dilutions than fresh plasma. Filtration of the origi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com