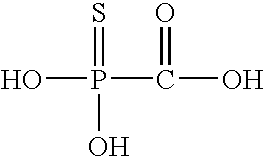
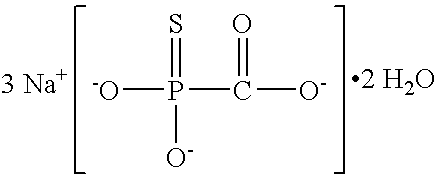
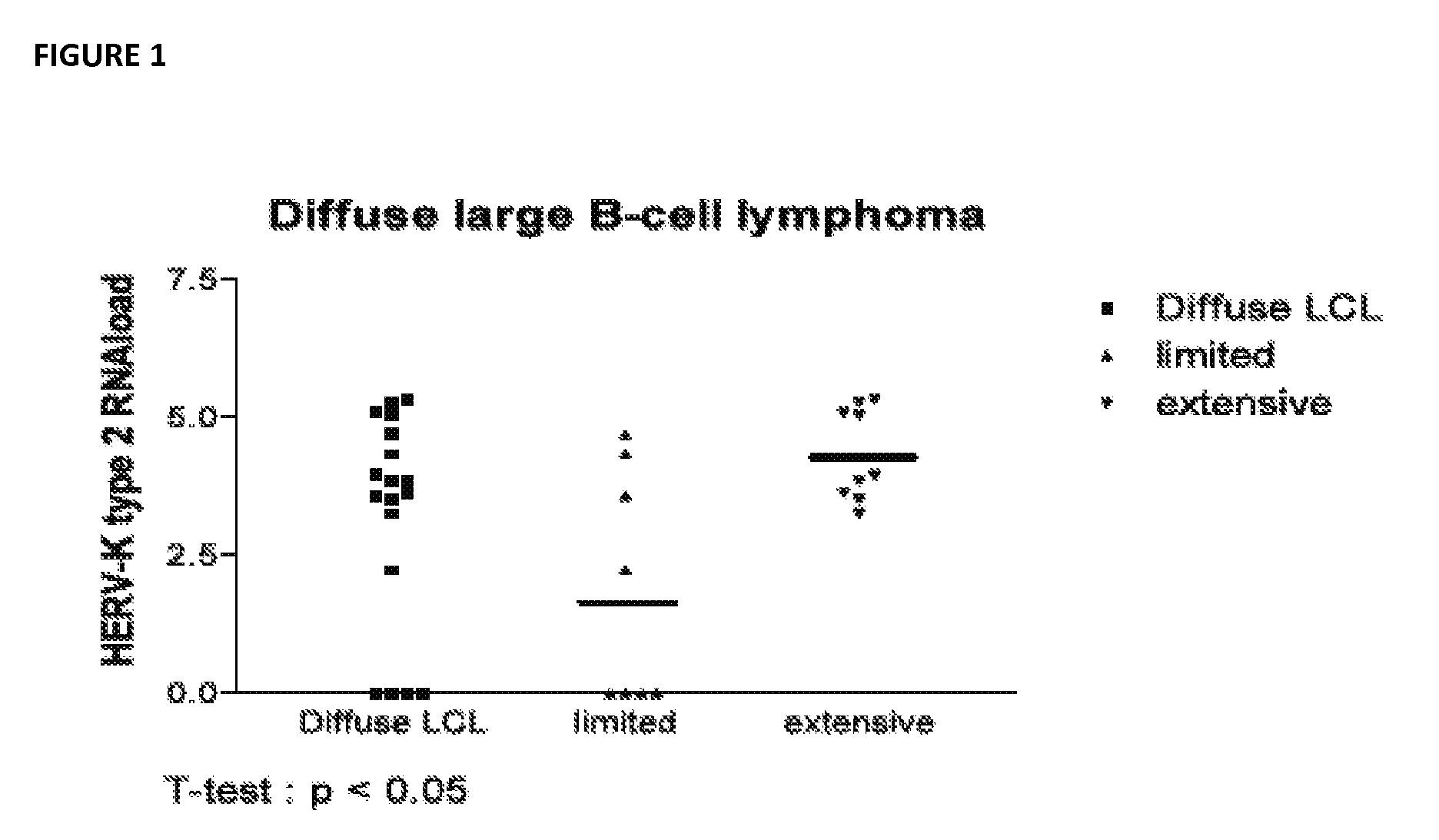Patents
Literature
46results about How to "Reduce viral load" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
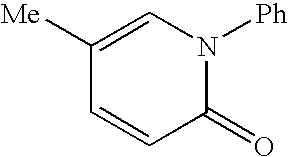
Azepane derivatives and methods of treating hepatitis B infections
ActiveUS9181288B2Reduce doseReduce frequencyPeptide/protein ingredientsGroup 5/15 element organic compoundsMedicineAzepane
Owner:NOVIRA THERAPEUTICS
Methods for treating viral infection using IL-28 and IL-29
ActiveUS7135170B2Reduction in viral infection levelReduce viral infectionBiocidePeptide/protein ingredientsInterferon therapyHematopoietic cell
IL-28A, IL-28B, IL-29, and certain mutants thereof have been shown to have antiviral activity on a spectrum of viral species. Of particular interest is the antiviral activity demonstrated on viruses that infect liver, such as hepatitis B virus and hepatitis C virus. In addition, IL-28A, IL-28B, IL-29, and mutants thereof do not exhibit some of the antiproliferative activity on hematopoietic cells that is observed with interferon treatment. Without the immunosuppressive effects accompanying interferon treatment, IL-28A, IL-28B, and IL-29 will be useful in treating immunocompromised patients for viral infections.
Owner:ZYMOGENETICS INC
Compositions and methods for targeted inactivation of HIV cell surface receptors
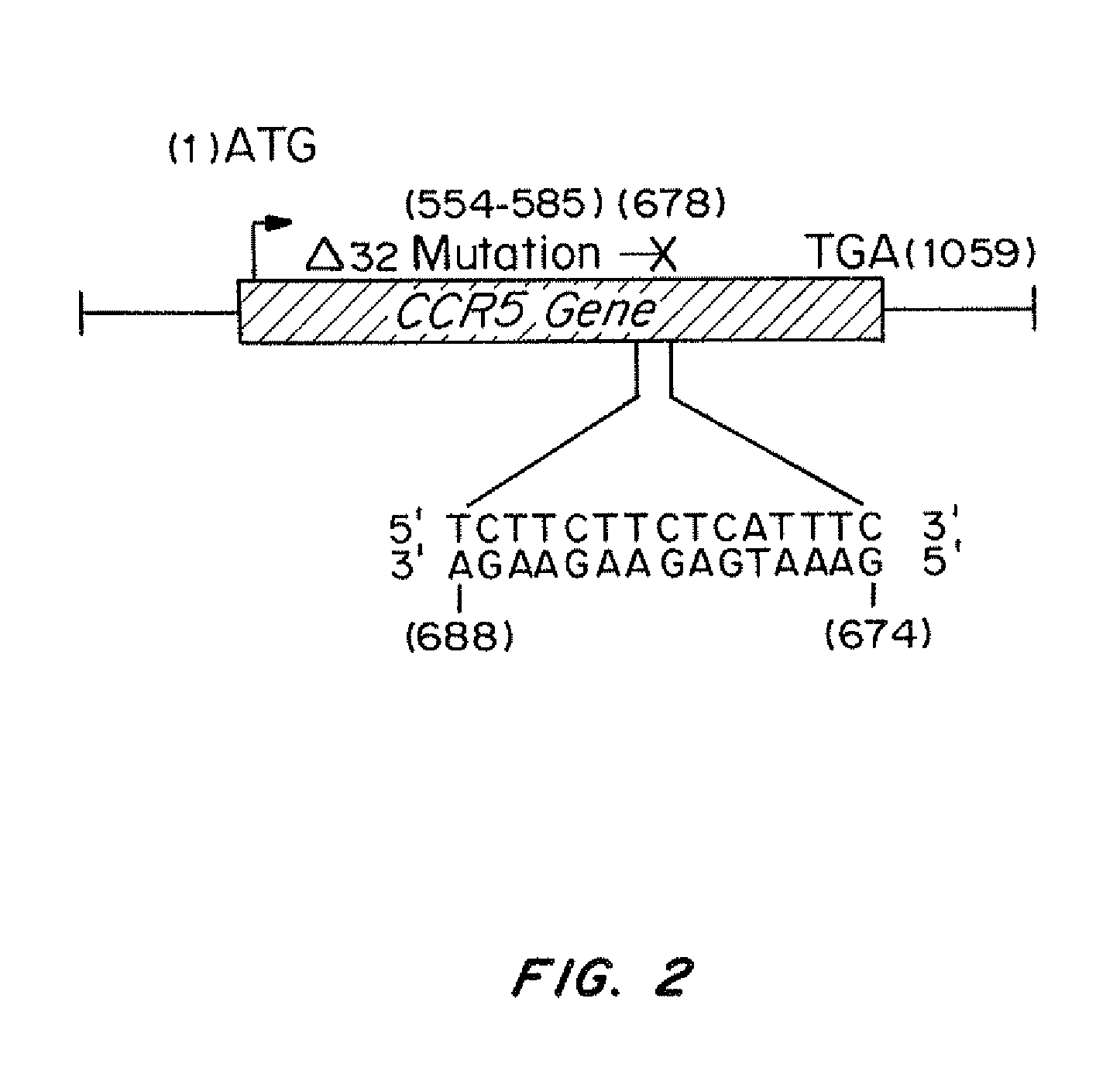
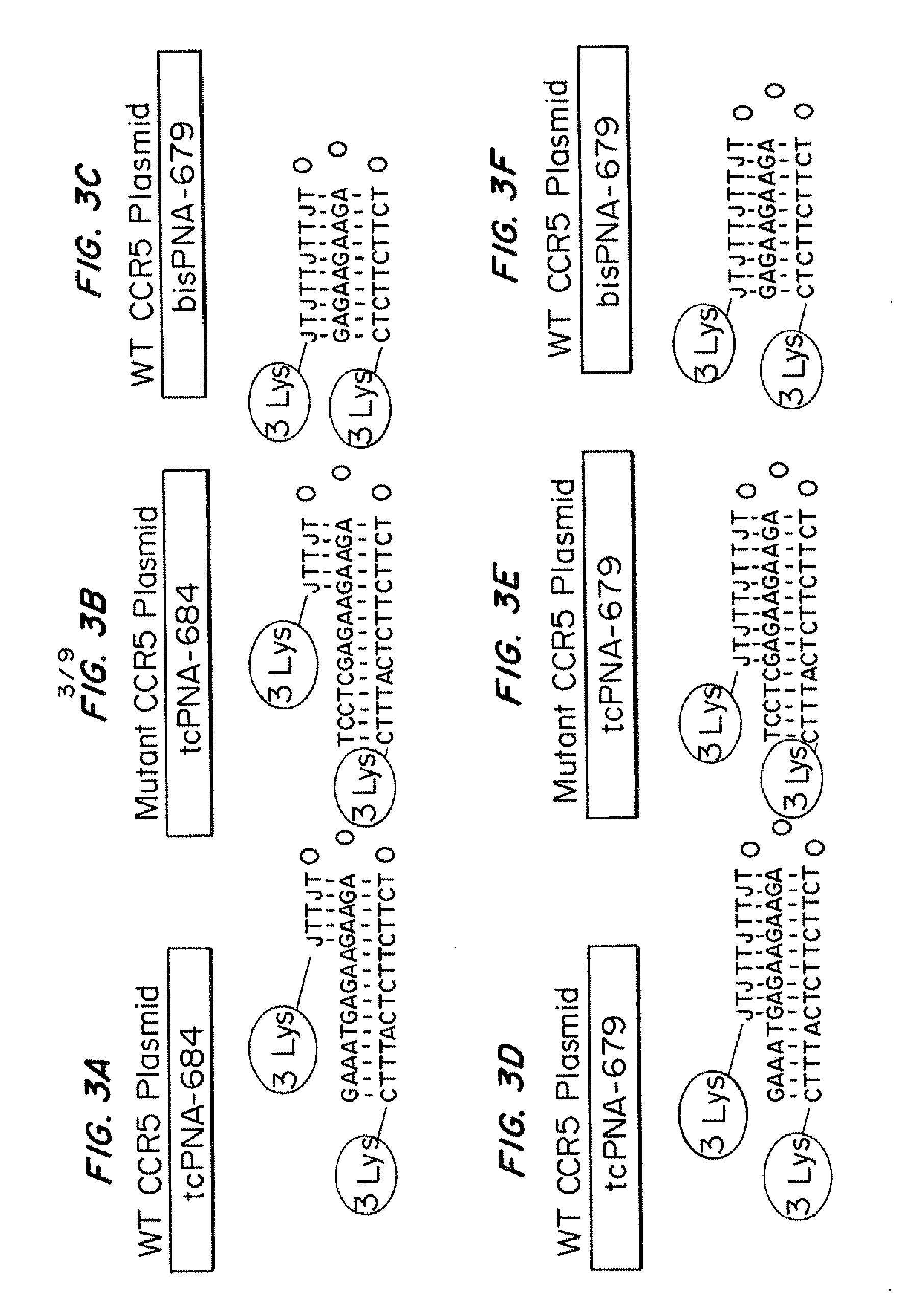
InactiveUS20110262406A1Avoid infectionReduce viral loadBiocideOrganic active ingredientsImmunodeficiency virusTail
Compositions for targeted mutagenesis of cell surface receptors for HIV and methods of their use are provided herein. The compositions include triplex-forming molecules that displace the polypyrimidine strand of target duplex and form a triple-stranded structure and hybrid duplex in a sequence specific manner with the polypurine strand of the target duplex. The triplex-forming molecules include a mixed-sequence “tail” which increases the stringency of binding to the target duplex, improves the frequency of modification at the target site, and reduces the requirement for a polypurine:polypyrimidine stretch. Methods for using the triplex-forming molecules in combination with one or more donor oligonucleotides for targeted modification of sites within or adjacent to genes that encodes cell surface receptors for human immunodeficiency virus (HIV) are also disclosed. Methods for ex vivo and in vivo prophylaxis and therapy of HIV infection using the disclosed compositions are also provided.
Owner:YALE UNIV
Methods for treating hcv
InactiveUS20110306541A1Reduce problemsReduce viral loadBiocideDigestive systemRegimenHepatitis C virus
Owner:GILEAD SCI INC
Method for modulating activity of HCV protease through use of a novel HCV protease inhibitor to reduce duration of treatment period
Methods are provided for using at least one novel hepatitis C (“HCV”) protease inhibitor in combination with at least one antiviral and / or immunomodulatory agent, which is different from the at least one HCV protease inhibitor, for treating a wide variety of diseases or disorders associated with hepatitis C virus by modulating the activity of HCV protease (for example HCV NS3 / NS4a serine protease) and reducing HCV viral load in a subject in a reduced treatment period. With the present invention, a hepatitis C viral load is reduced in a subject to a concentration of less than 6×10−5 HCV virions per milliliter of plasma in a time period of less than or equal to about 24 weeks. With the present invention, a hepatitis C viral production is suppressed with an effectiveness in a range of 0.7 to 0.997.
Owner:SCHERING CORP
Benzimidazole compounds and antiviral uses thereof
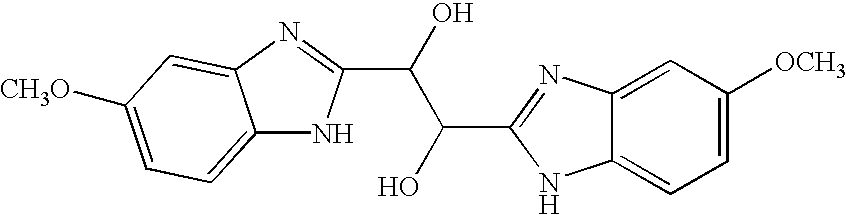
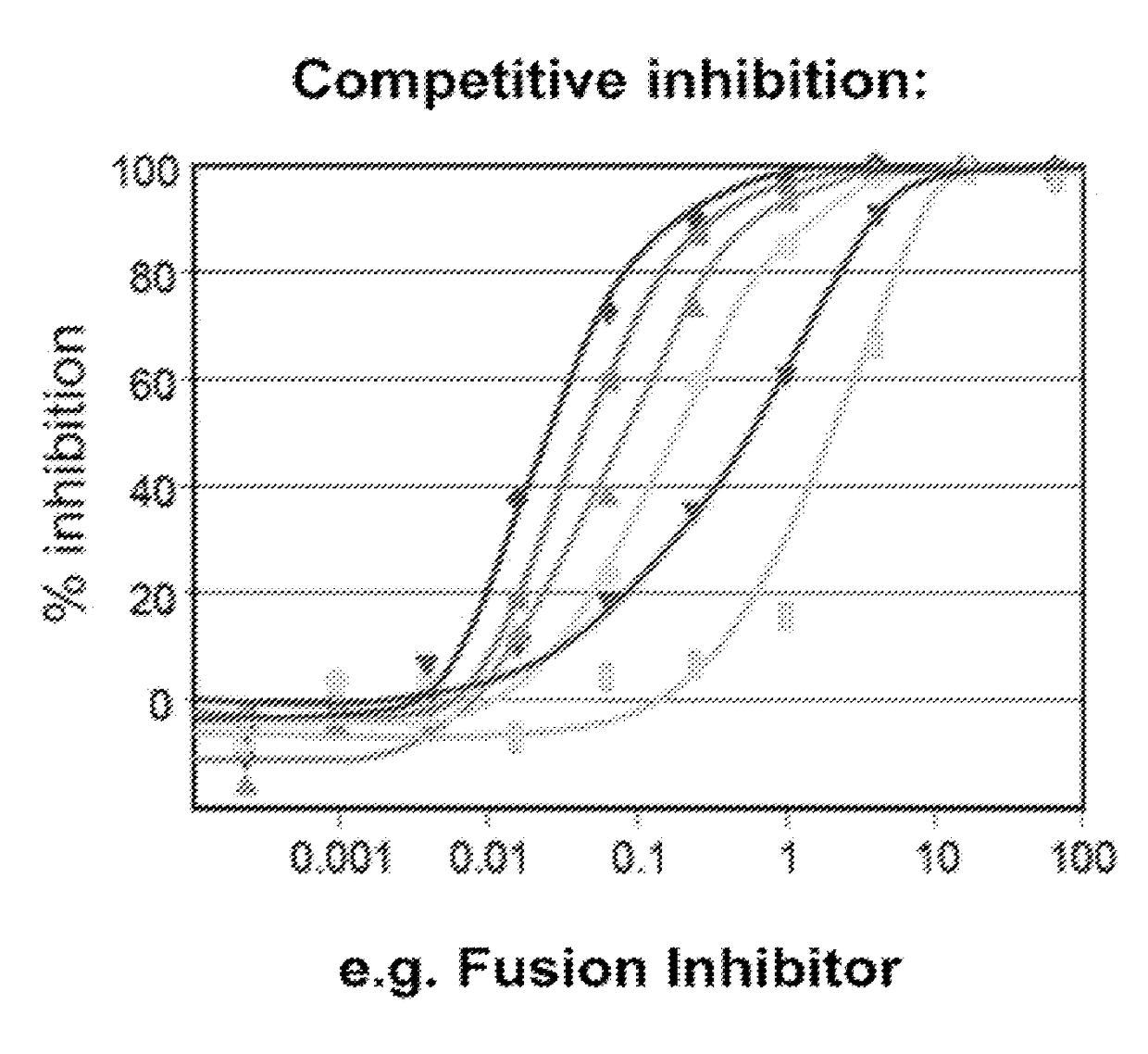
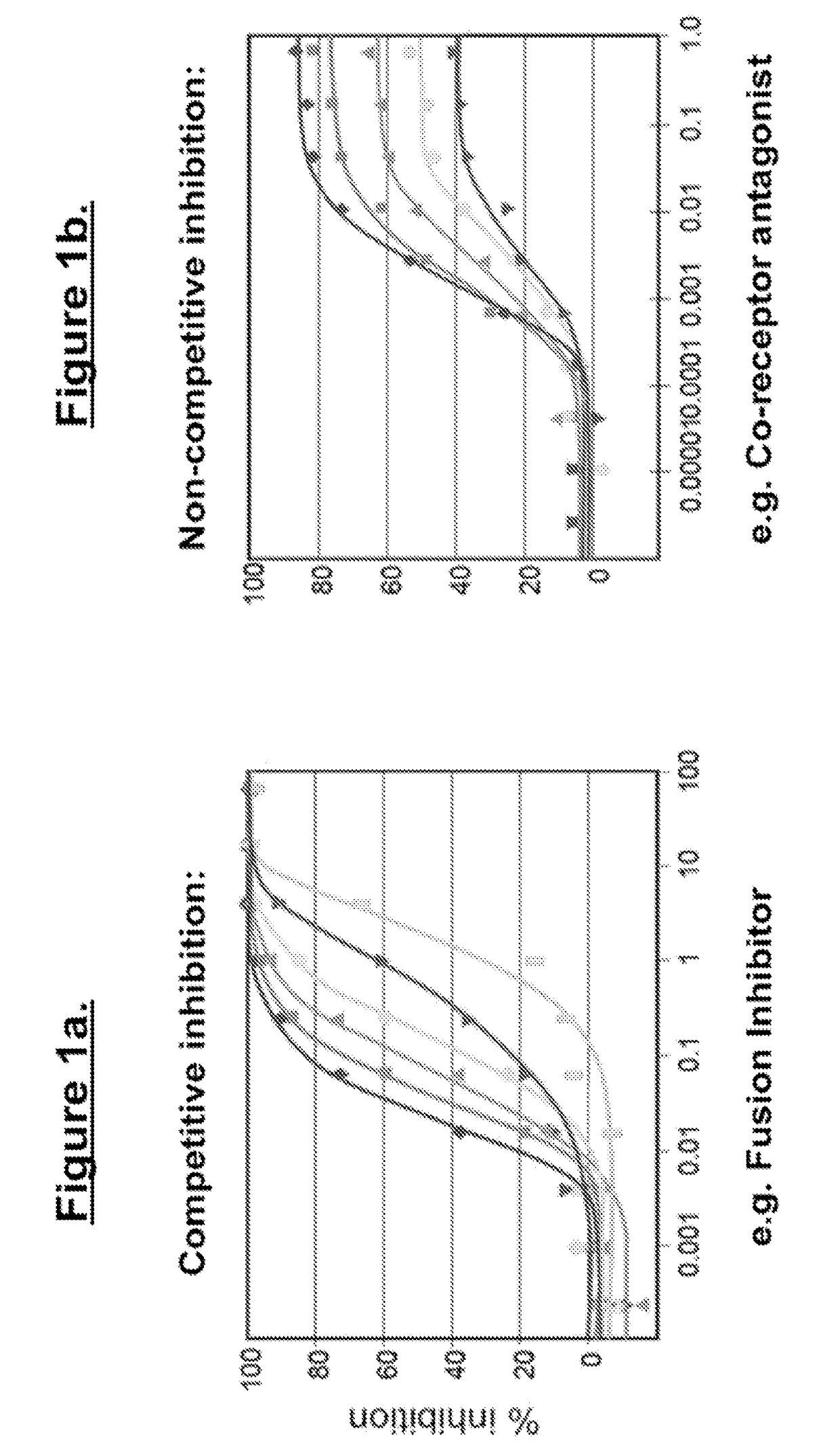
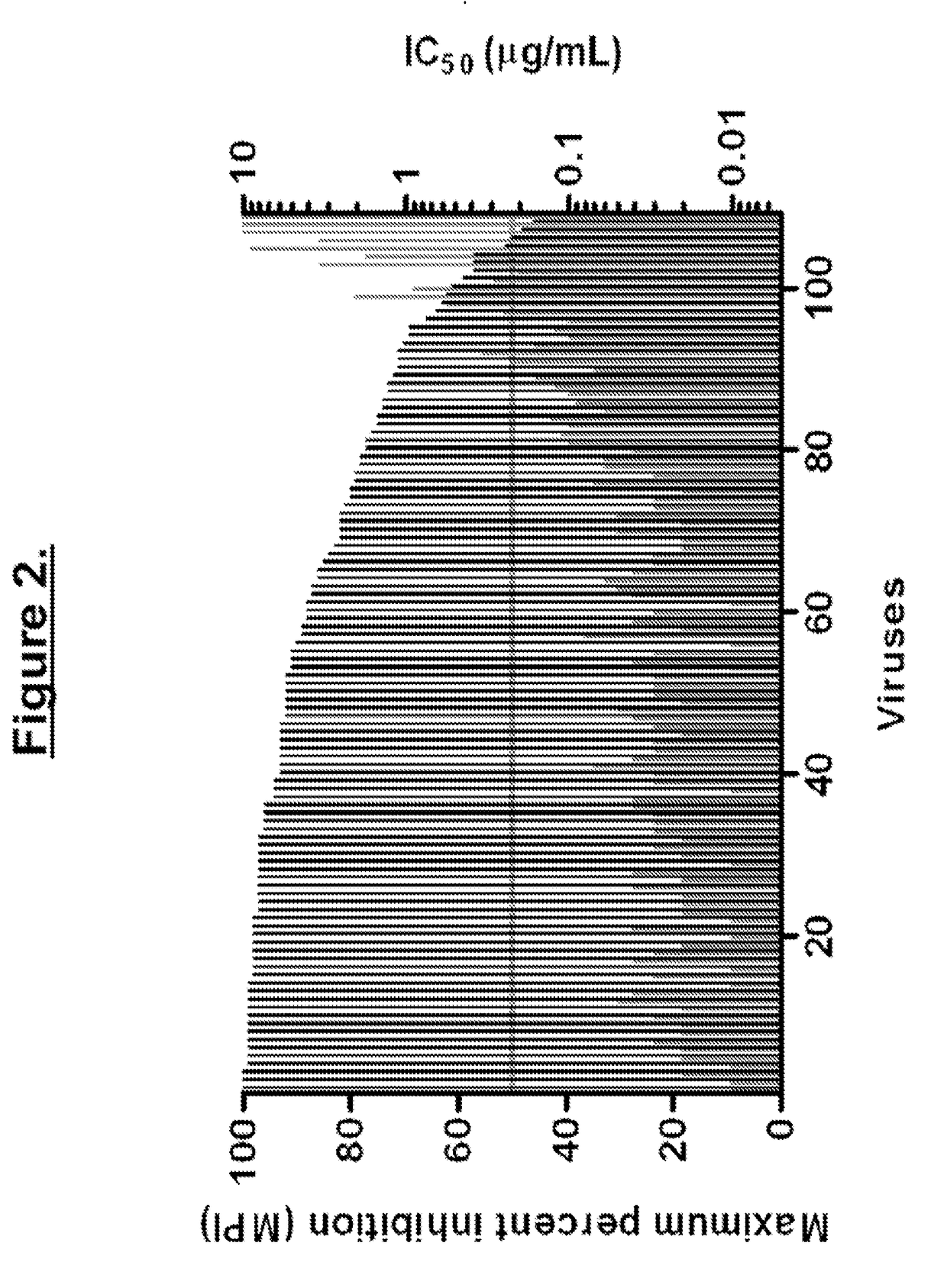
InactiveUS20030119754A1Reducing viral titer viralReduce viral loadBiocideSugar derivativesRespiratory infectionViral infection
Owner:TRIMERIS
Antibody gene transfer and recombinant AAV therefor
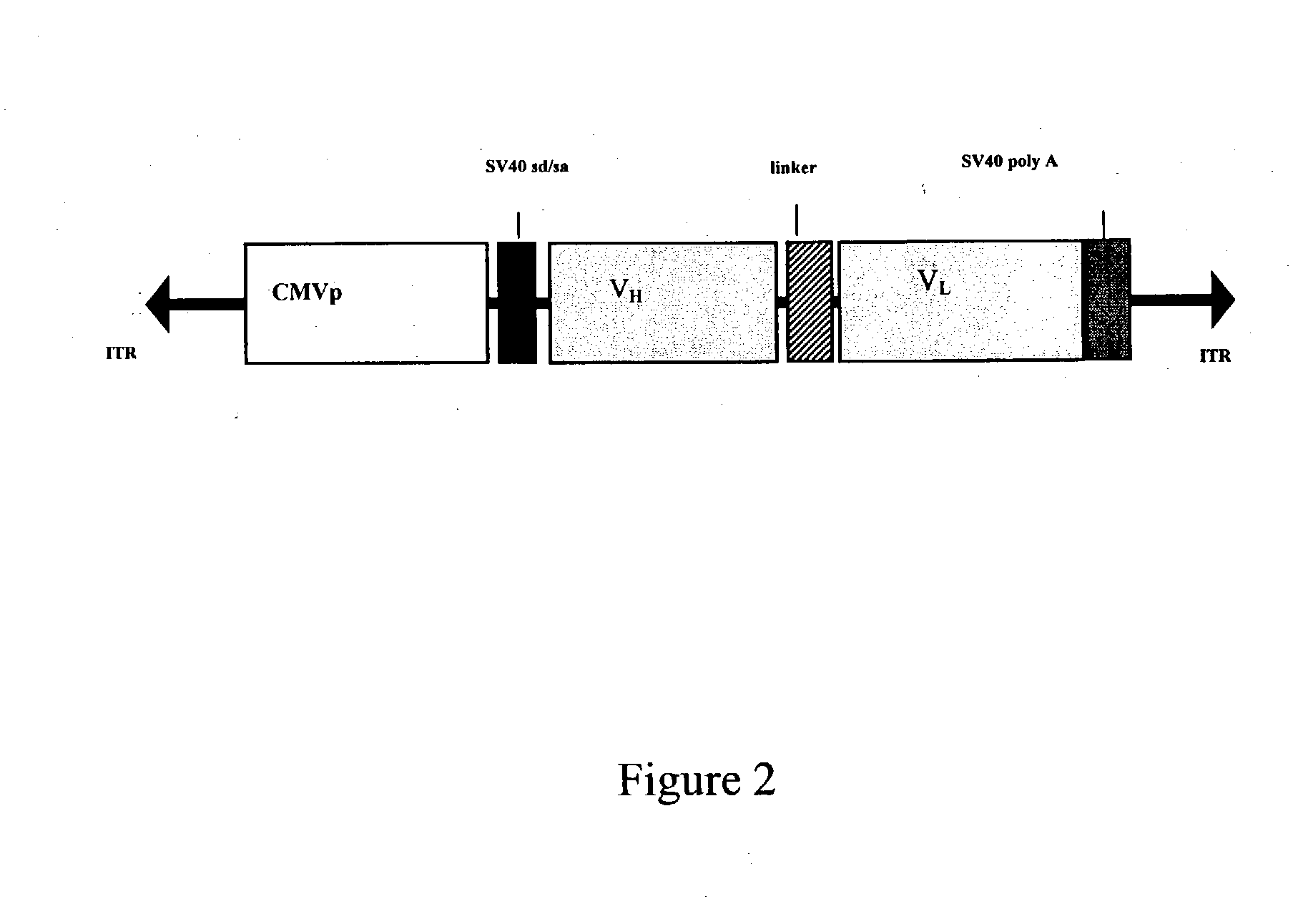
InactiveUS20030219733A1Inhibit progressIncrease in CD4-positiveAntibacterial agentsNervous disorderGene deliveryMammal
The present invention relates generally to the use of recombinant adeno-associated viruses (rAAV) for gene delivery and more specifically to the use of rAAV to deliver antibody genes to target cells in mammals. Administration of rAAV encoding antibodies that neutralize the HIV-1 virus is exemplified.
Owner:NATIONWIDE CHILDRENS HOSPITAL
Methods and compositions for treatment of HIV infection
InactiveUS20160095850A1Reduce viral loadBiocidePeptide/protein ingredientsImmunodeficiency virusCD4 antigen
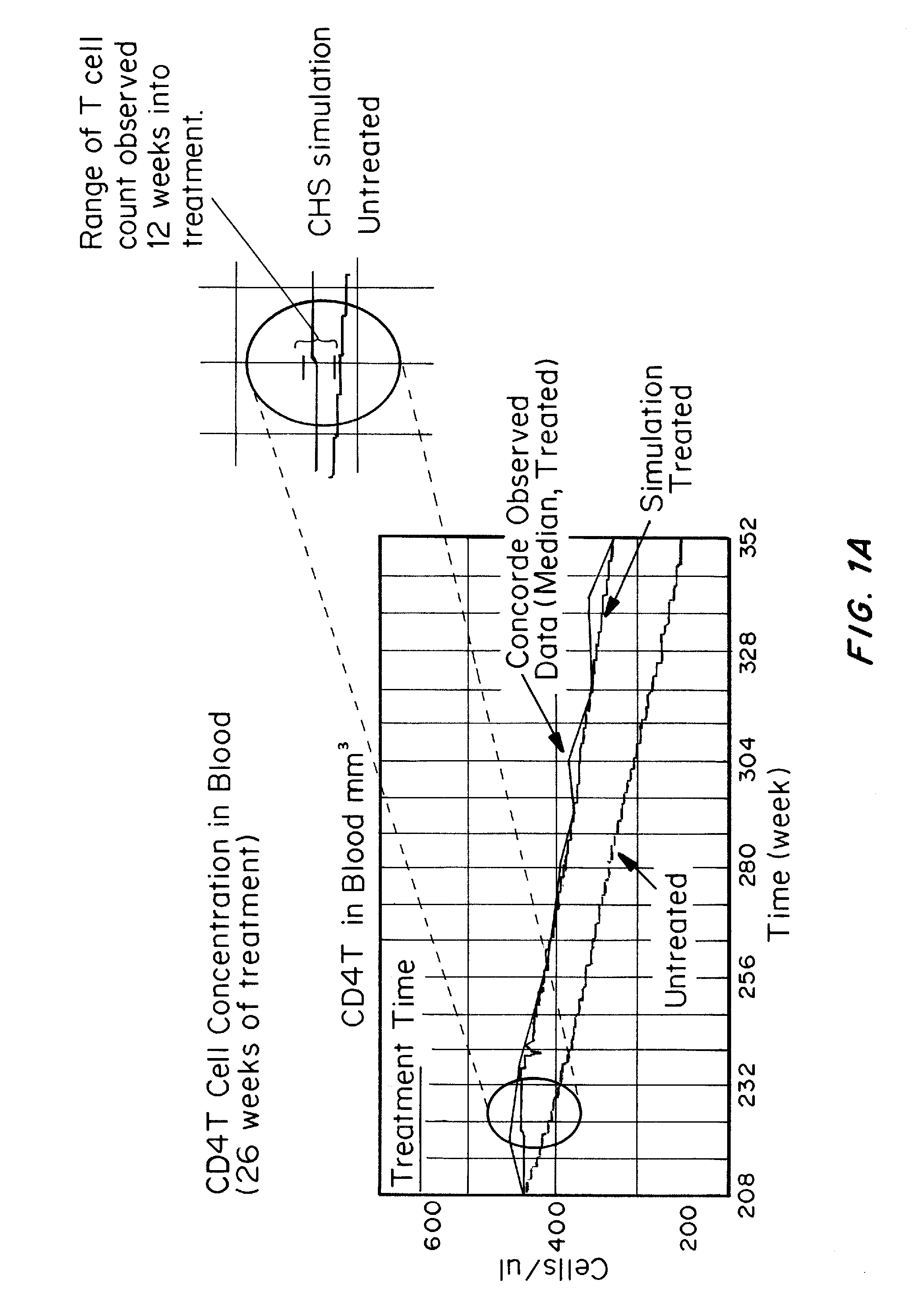
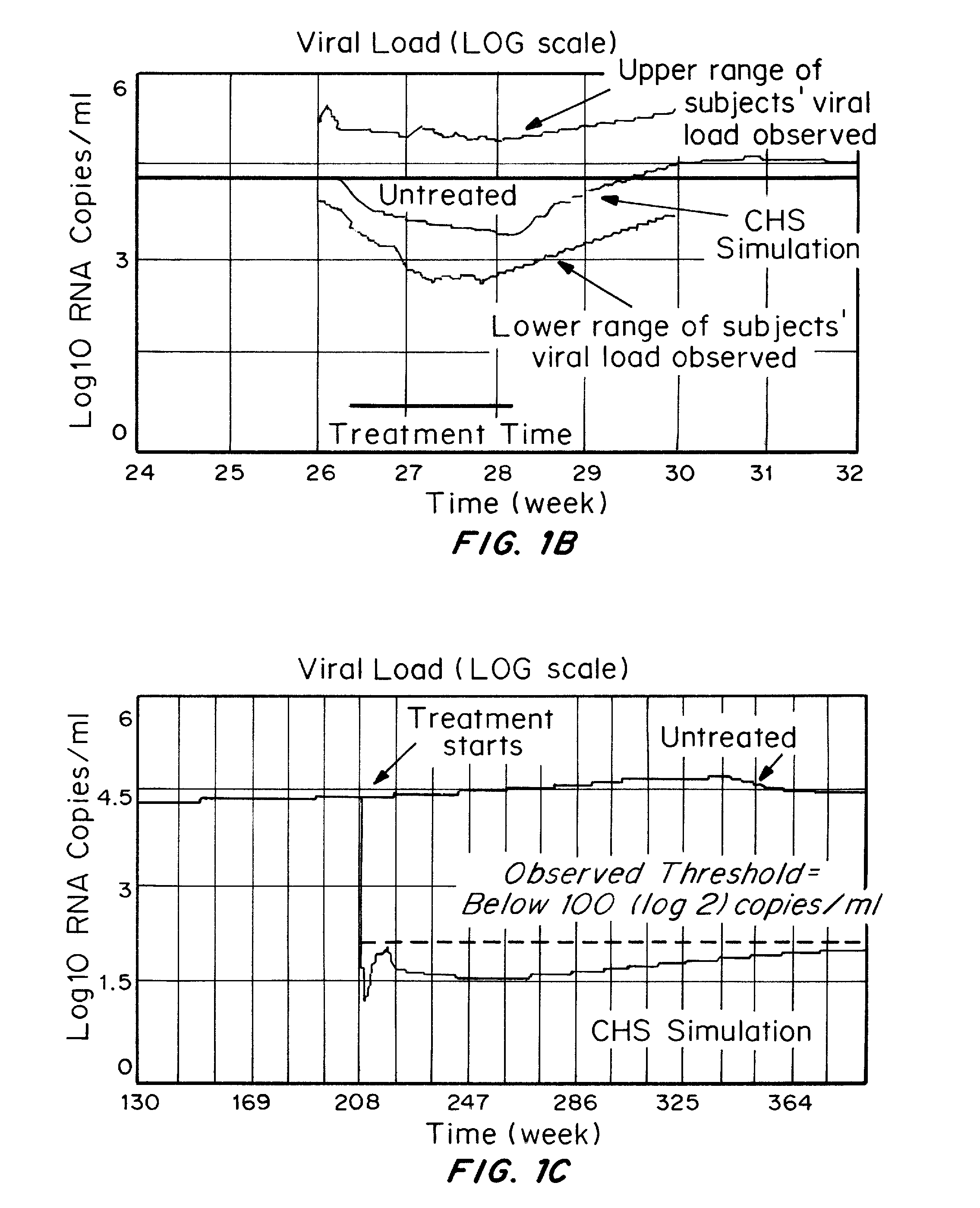
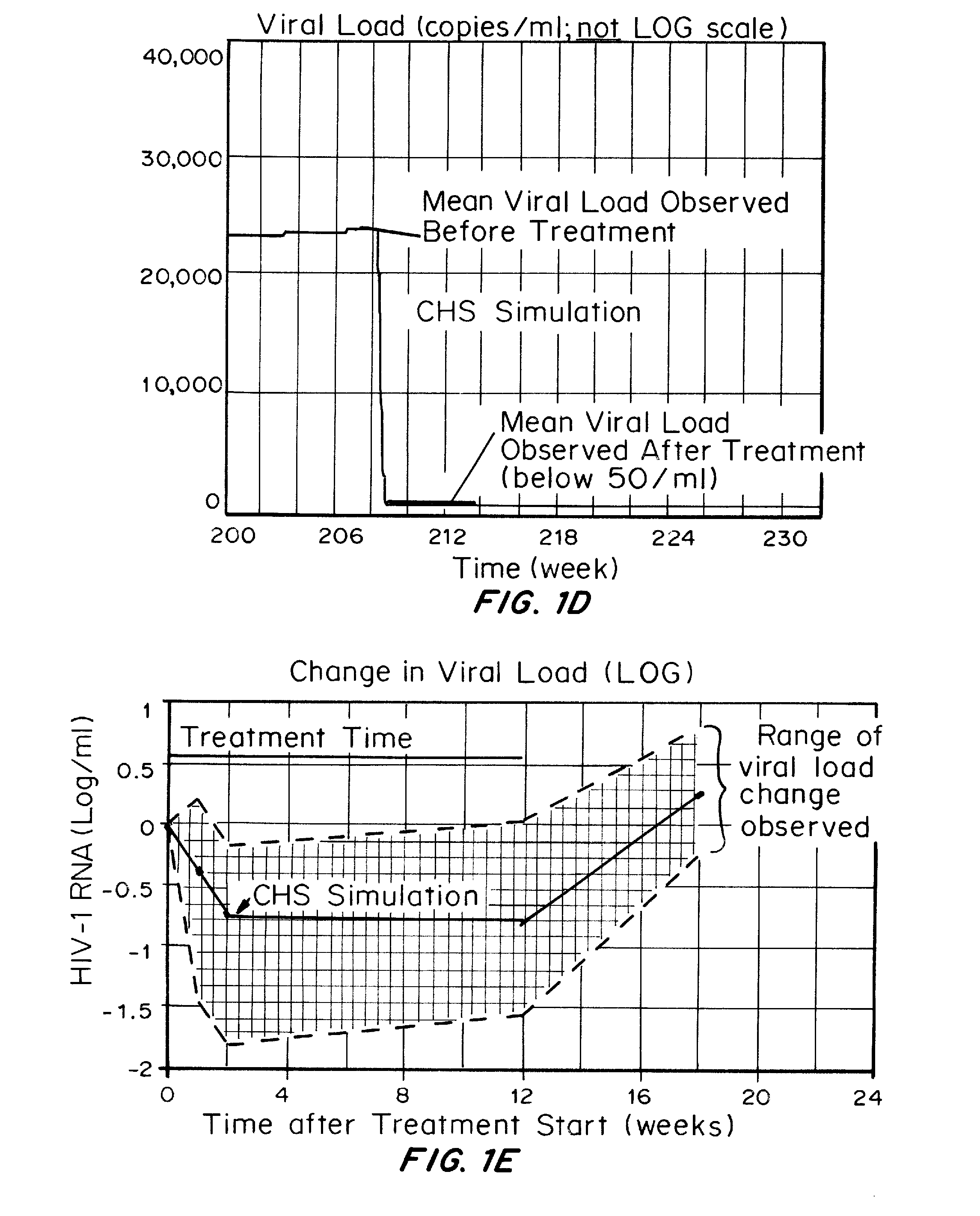
Methods and compositions for treatment of human immunodeficiency virus (HIV) infections have been developed which dampen immune activation with a bias more on the CD4 T cells relative to the CD8 T cell response, inhibit HIV replication, reactivate latent HIV, and inhibit infection of cells by HIV. Pushing latent HIV into active infections with hindrance of cell infection by the reactivated HIV can substantially reduce the number of cells infected with HIV and the viral load of HIV, which is not achieved using just the combination of ART and compounds which activate latent HIV. The methods involve administering to an HIV-infected subject three or more compounds which collectively dampen immune activation with a bias more on the CD4 T cells relative to the CD8 T cell response, inhibit HIV replication, reactivate latent HIV, and inhibiting infection of CD4 T cells by HIV.
Owner:COOPER HUMAN SYST
Vaccines and methods for prevention and treatment of drug-resistant hiv-1 and hepatitis b virus
ActiveUS20090317418A1Reduce viral loadLower viral loadSsRNA viruses positive-sensePeptide/protein ingredientsAntiviral drugResistant virus
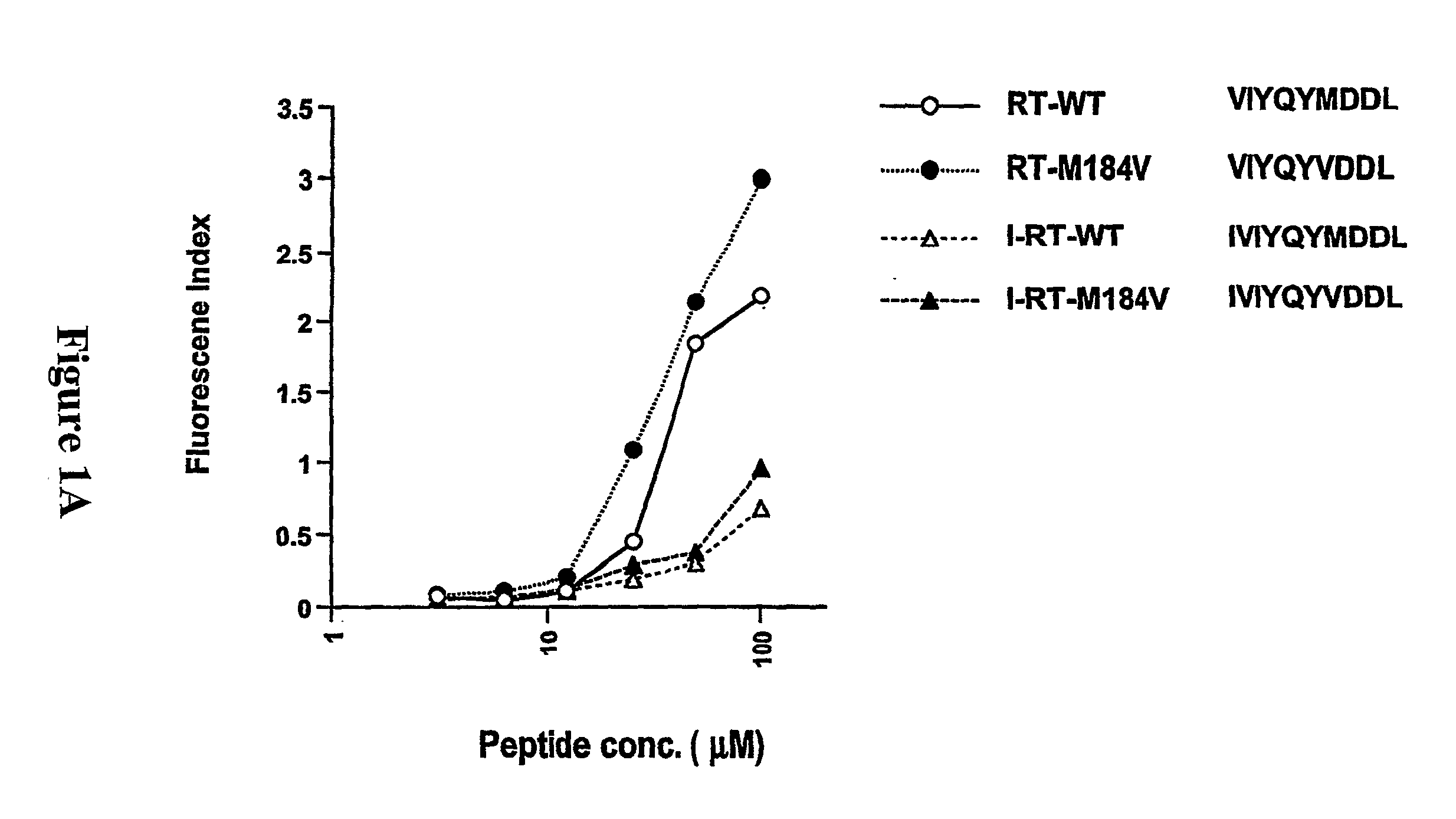
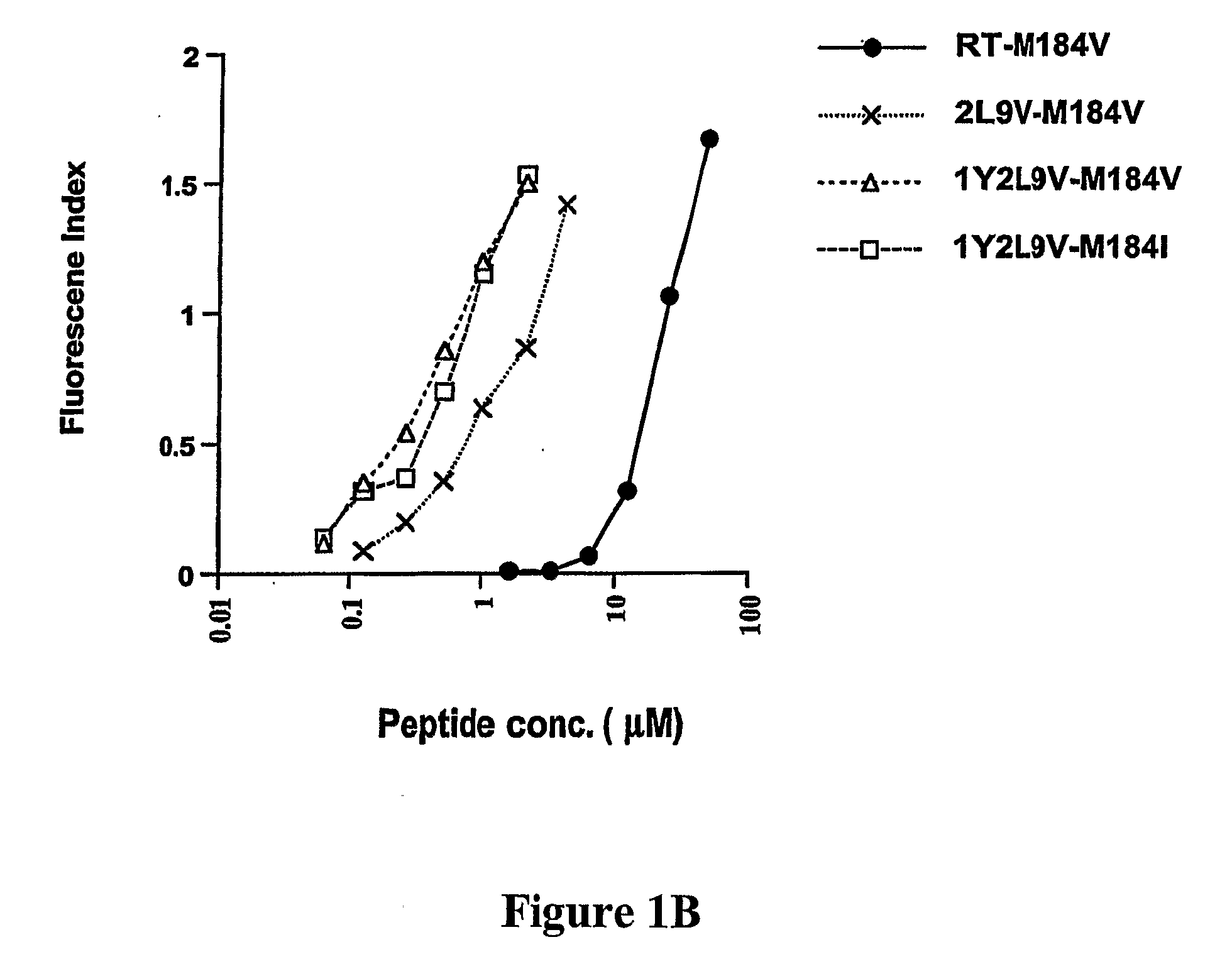
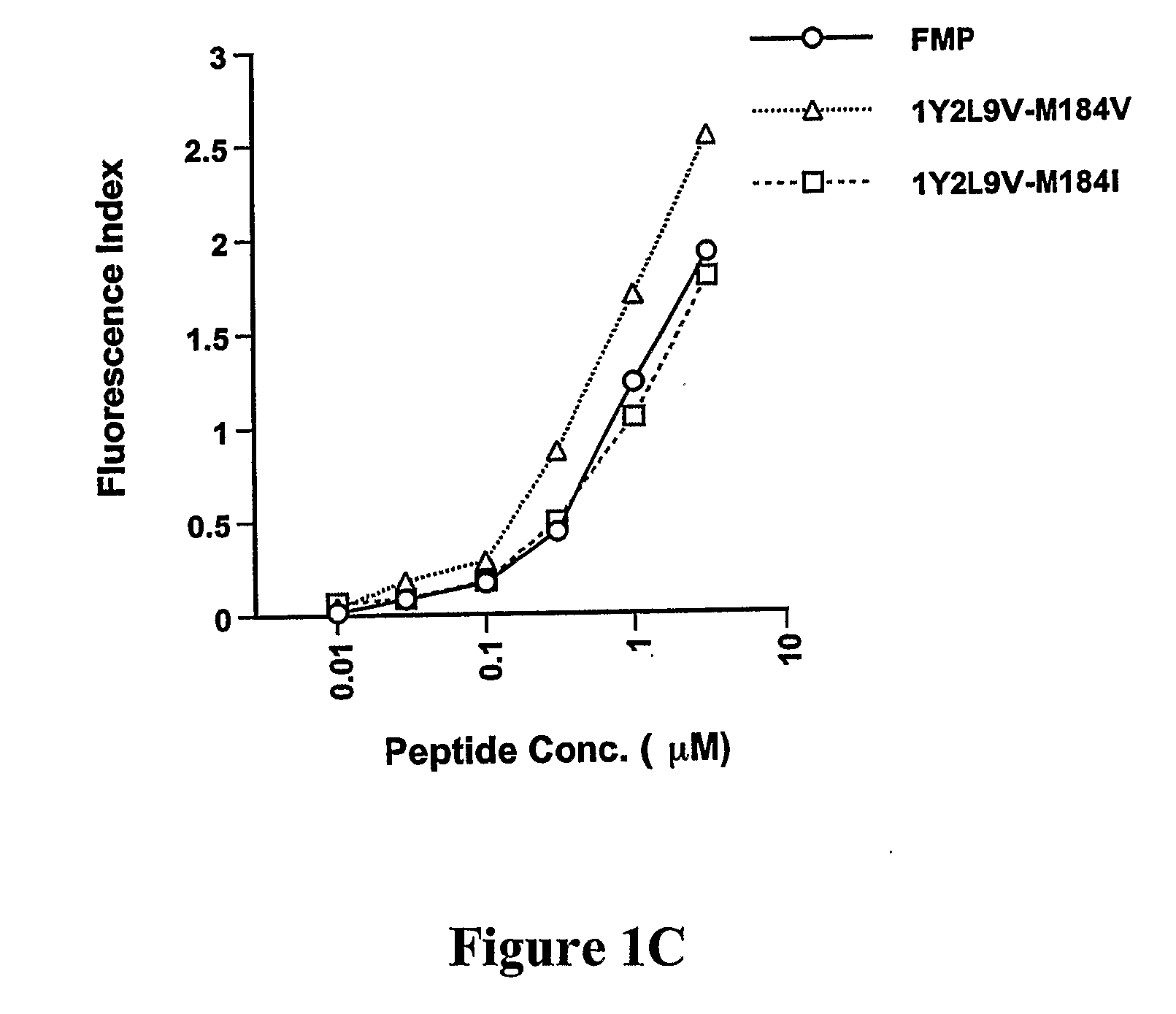
The present invention provides methods for lowering a viral load of a virus resistant to an antiviral drug by inducing cytotoxic T lymphocytes (CTL) to recognize a predetermined mutated epitope within a viral protein of the drug-resistant virus. CTLs are induced by immunizing a host with a peptide comprising the predetermined mutation. The immunostimulating peptide may be further improved by epitope-enhancement for inducing specific CTLs. The antiviral protection against drug-resistant virus shown by compositions of the present invention and mediated by human HLA-restricted CTL has not been previously achieved.
Owner:HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US SEC THE DEPT OF
Heavy Chain And Single Domain Antibodies
InactiveUS20080206233A1Good health benefitsProvide health benefitBiocideBacteriaImmunoglobulin heavy chainSingle-Chain Antibodies
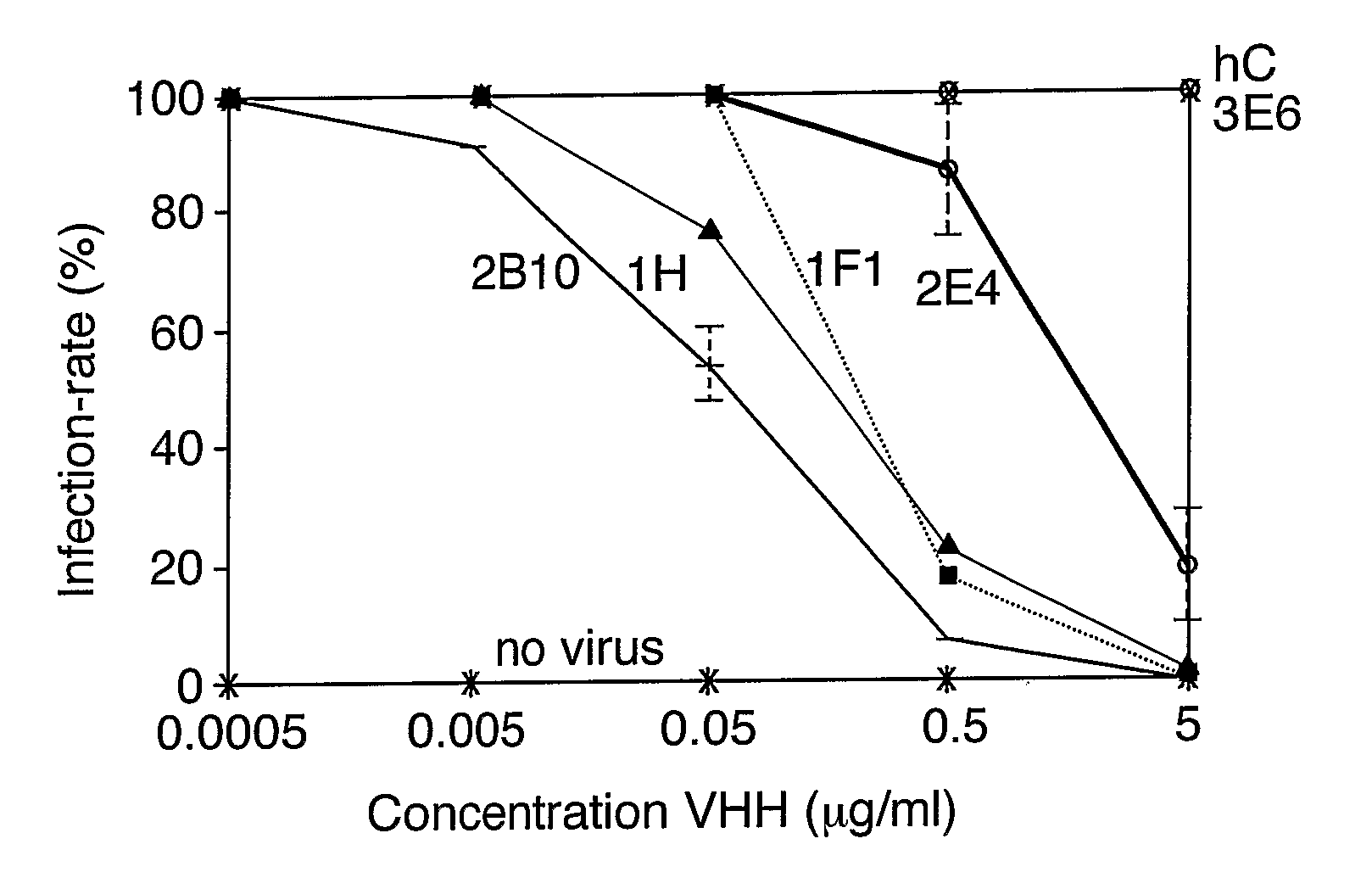
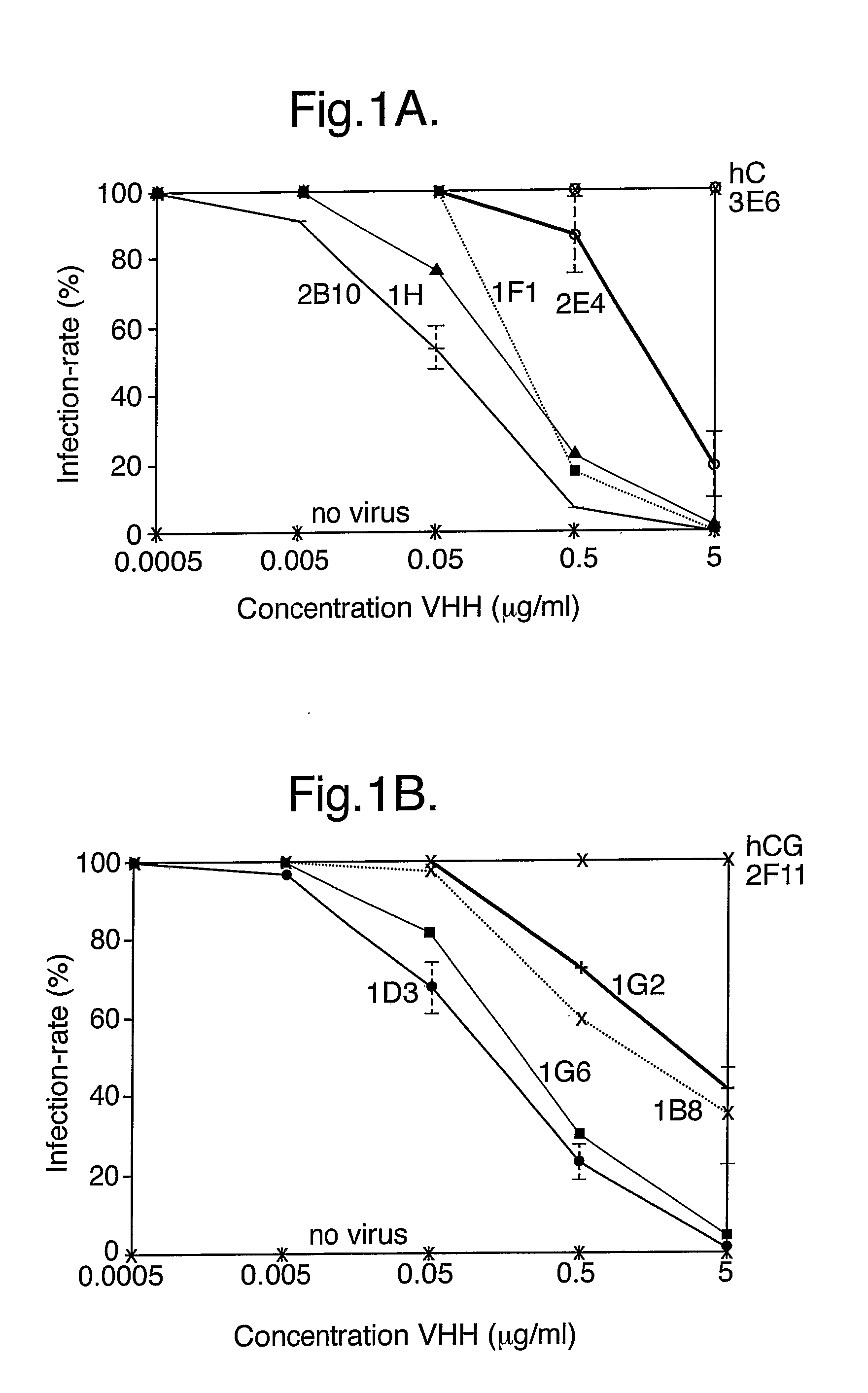
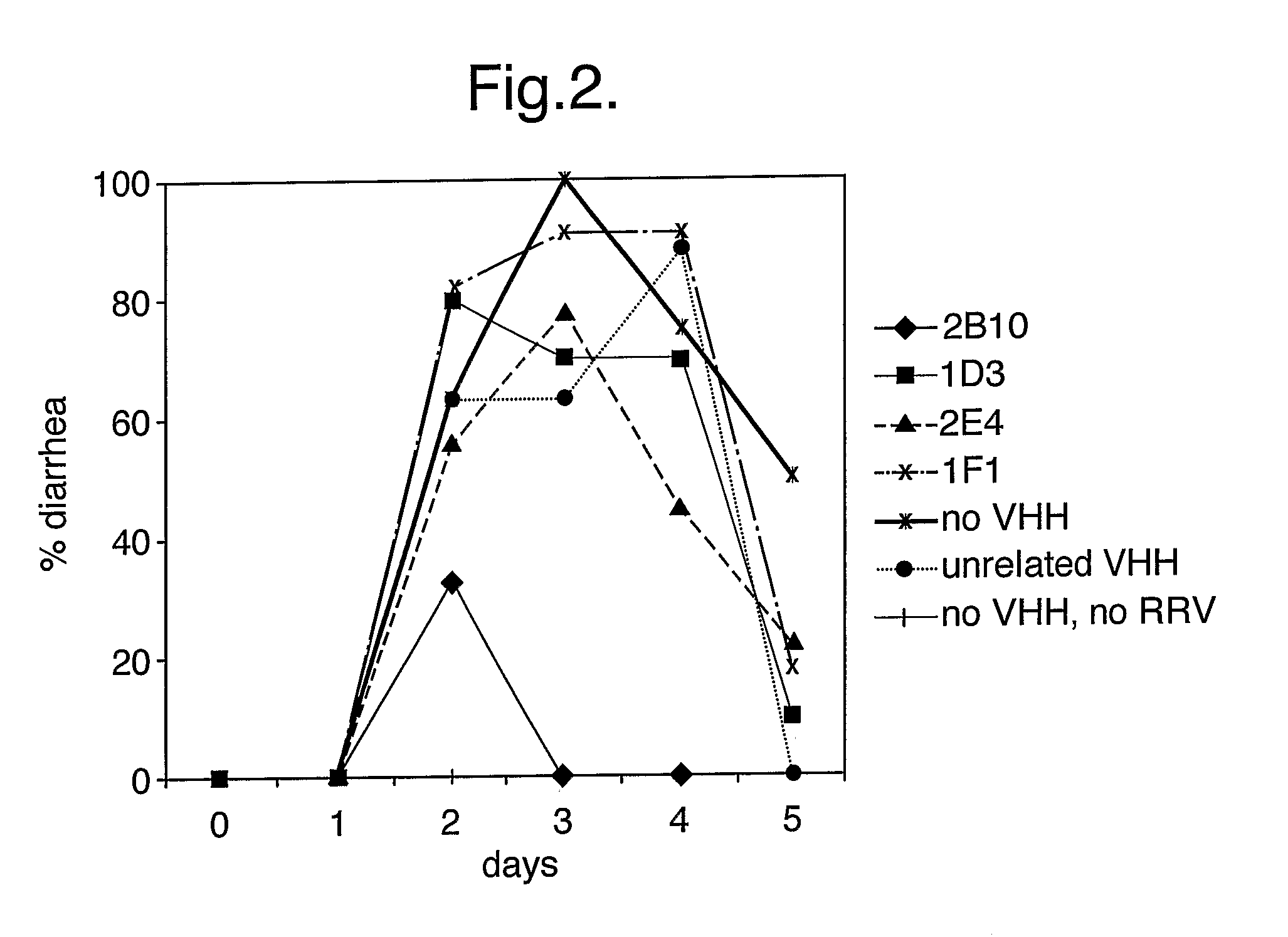
The present invention relates to heavy chain immunoglobulins or fragments thereof of the VHH or VNAR type, or domain antibodies (dAbs) of the heavy or light chains of immunoglobulins or fragments thereof, suitable for use in the management of infections, in particular of the gastrointestinal tract. The present invention also relates to a delivery system comprising these heavy chain immunoglobulins or functional fragments thereof of the VHH or VNAR type, or domain antibodies (dAbs) of the heavy or light chains of immunoglobulins or fragments thereof, and hosts comprising expression vectors encoding for these heavy chain immunoglobulins or functional fragments thereof of the VHH or VNAR type, or domain antibodies (dAbs) of the heavy or light chains of immunoglobulins or fragments thereof. The invention also relates to food products and pharmaceutical preparations comprising the delivery system, and methods for the preparation of food products according to the invention.
Owner:VHSQUARED
Jcv neutralizing antibodies
ActiveUS20150056188A1Reduce viral loadIncrease awarenessImmunoglobulins against virusesAntibody ingredientsAntibody SuppressionAntiendomysial antibodies
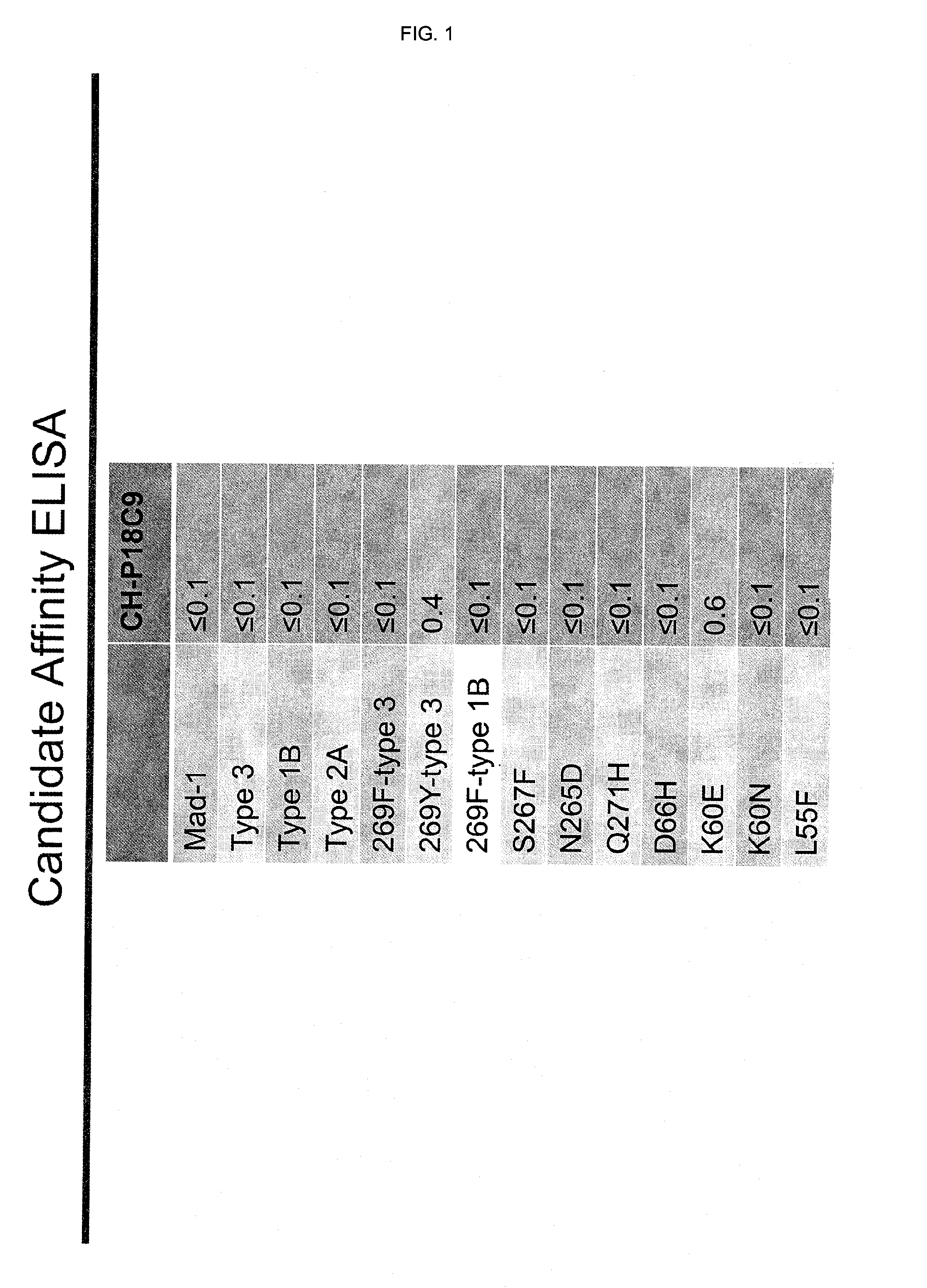
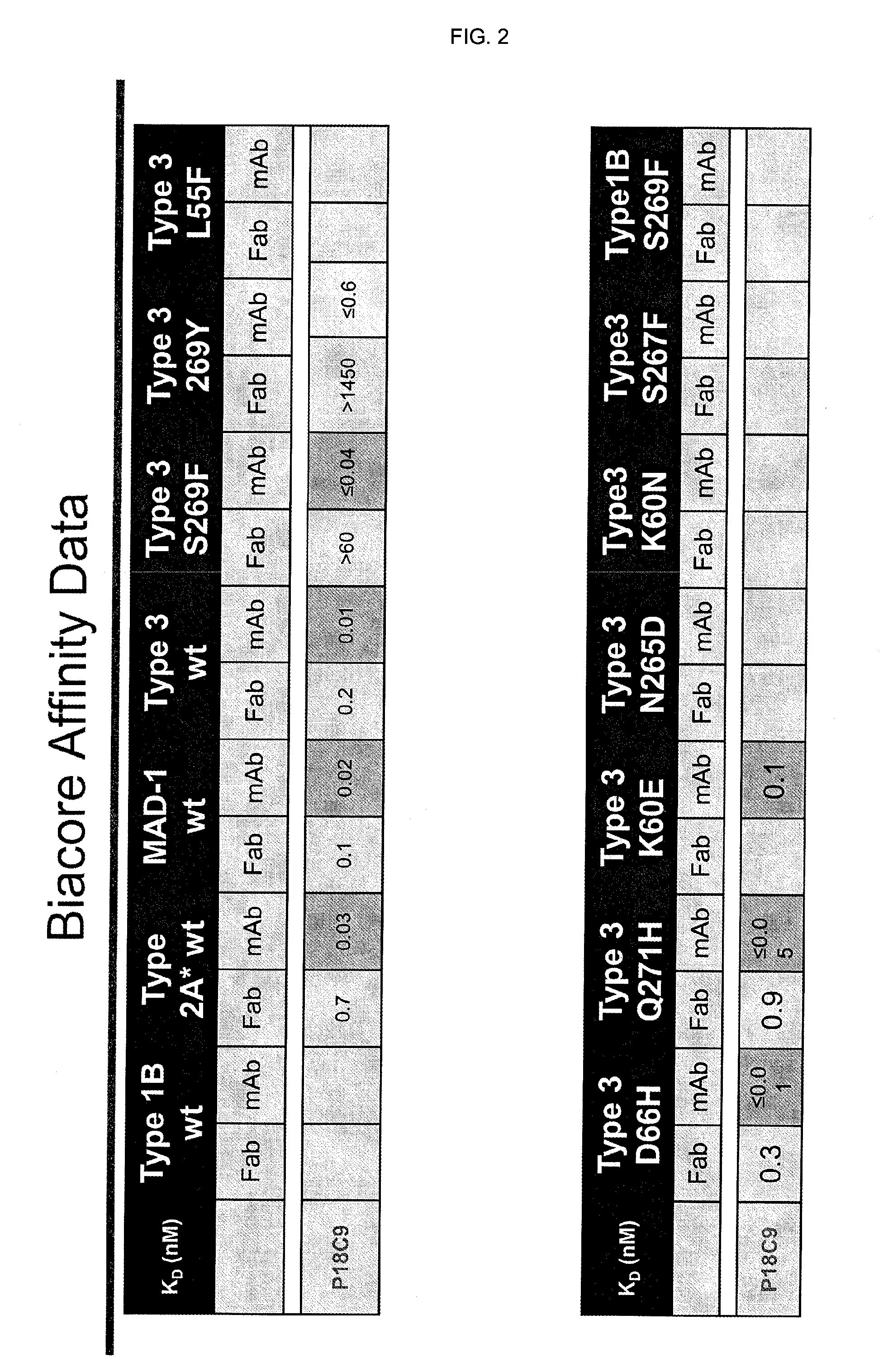
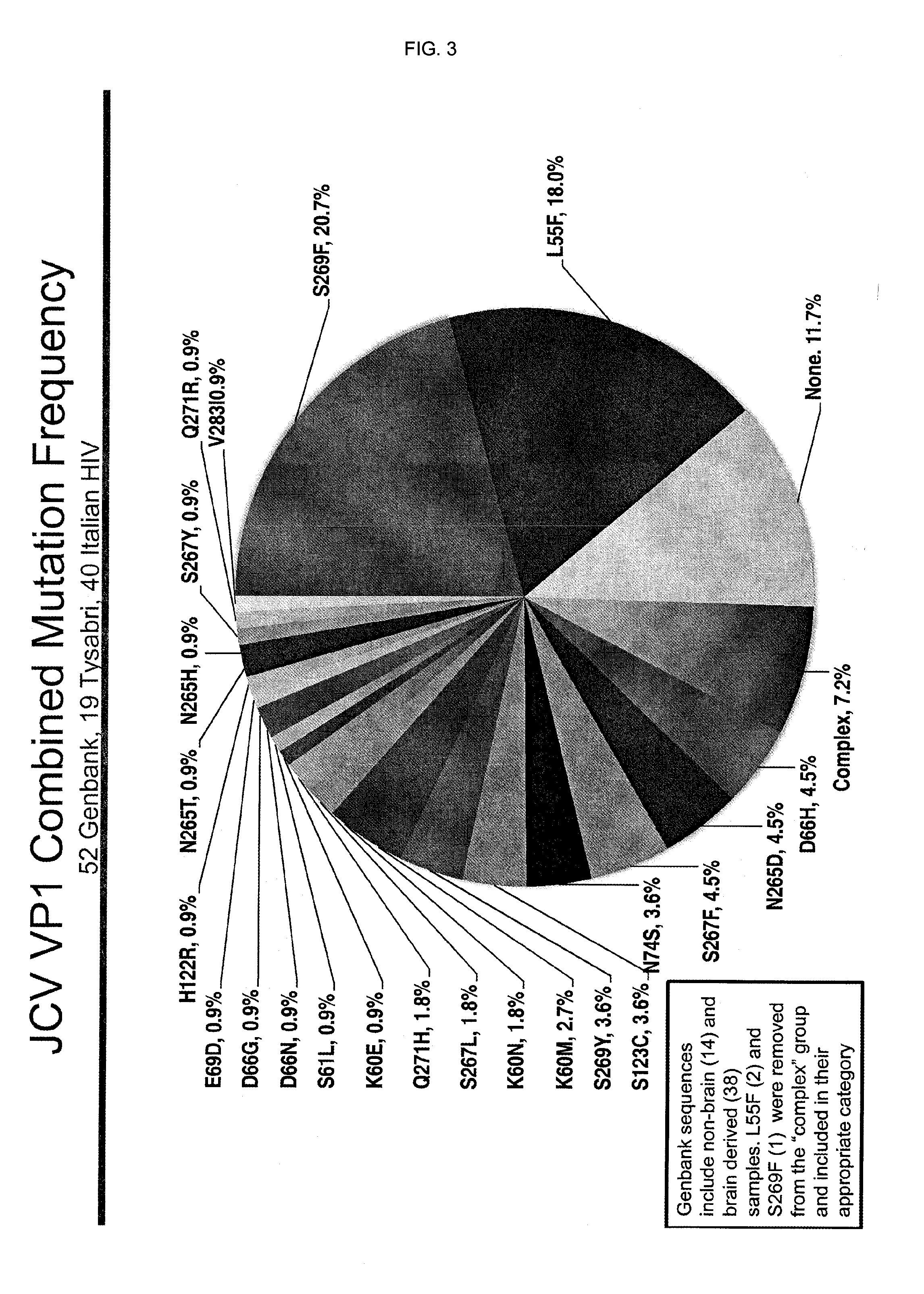
In one aspect, the disclosure provides neutralizing antibodies against JCV and methods for the treatment of PML. In some embodiments, aspects of the invention relate to an isolated JC-virus neutralizing monoclonal antibody against JCV capsid protein VPI (JCV-VP1). In some embodiments, the antibody suppresses infectivity of the JC-virus. In some embodiments, the antibody binds the sialic acid binding pocket of JCV-VPI. In some embodiments, the antibody binds JCV-VP 1 comprising one or more of the following mutations: S269F, S269Y, S267F, N265D, Q271 H, D66H, K60E, K60N and L55F.
Owner:BIOGEN MA INC
Halides in the treatment of pathogenic infection
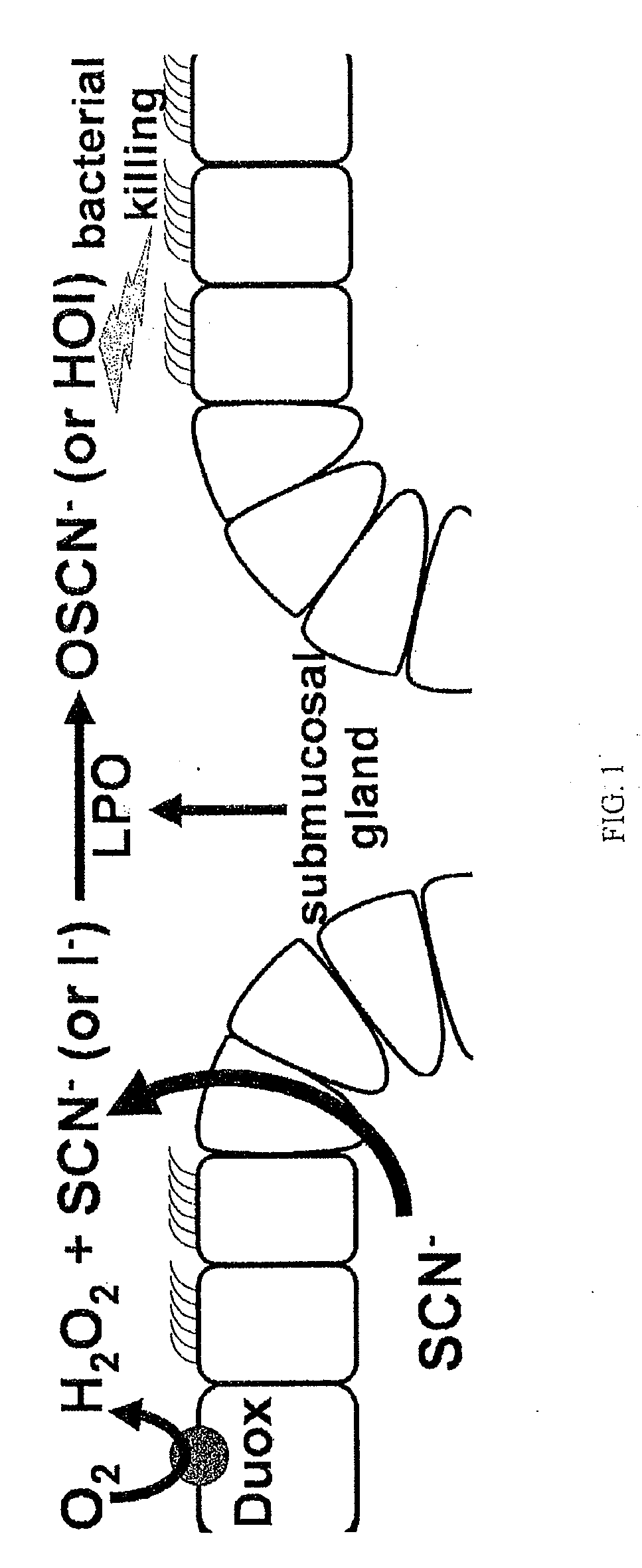
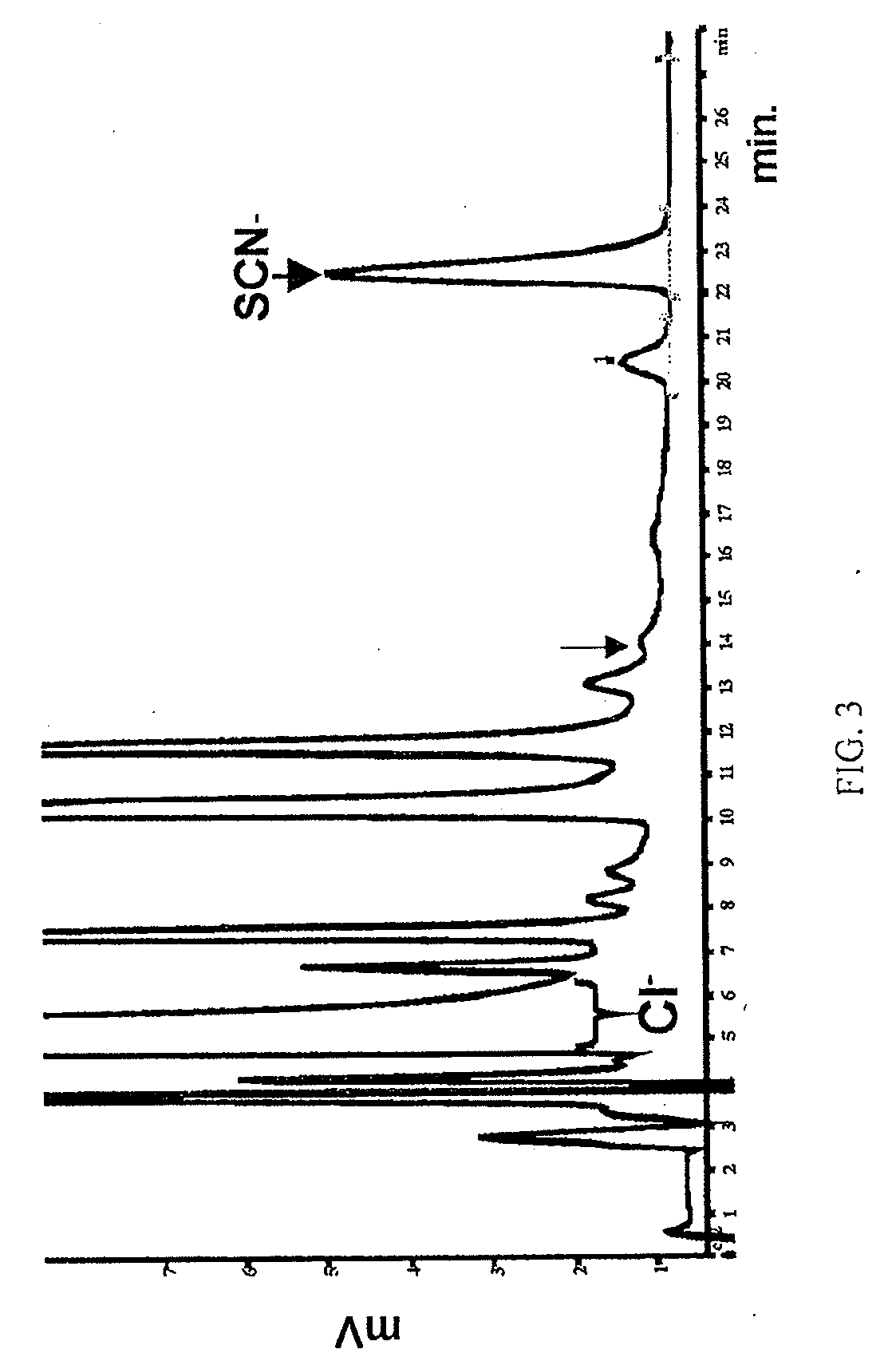
InactiveUS20090246146A1Limiting durationReduce severityBiocideDispersion deliveryBacteroidesMicroorganism
The present invention relates to the use of halides and halide salts for the treatment of microbial infections, including those caused by bacteria, fungi and viruses. The present invention takes advantage of endogenous immune function and augments this system using a non-toxic and inexpensive reagent that can be delivered to mucosal surfaces, for example, orally, topically, opthalmically and via inhalation.
Owner:UNIV OF IOWA RES FOUND
Use of rhubard and rhubard-polysaccharide in anti-HIV
InactiveCN1778310APromote growthIncreased viral loadOrganic active ingredientsAntiviralsImmunodeficiency virusHuman immunodeficiency
Owner:SHENZHEN WU JIN QIU RES CENT OF MODERN ENG & TECH OF CHINESE MEDICINE
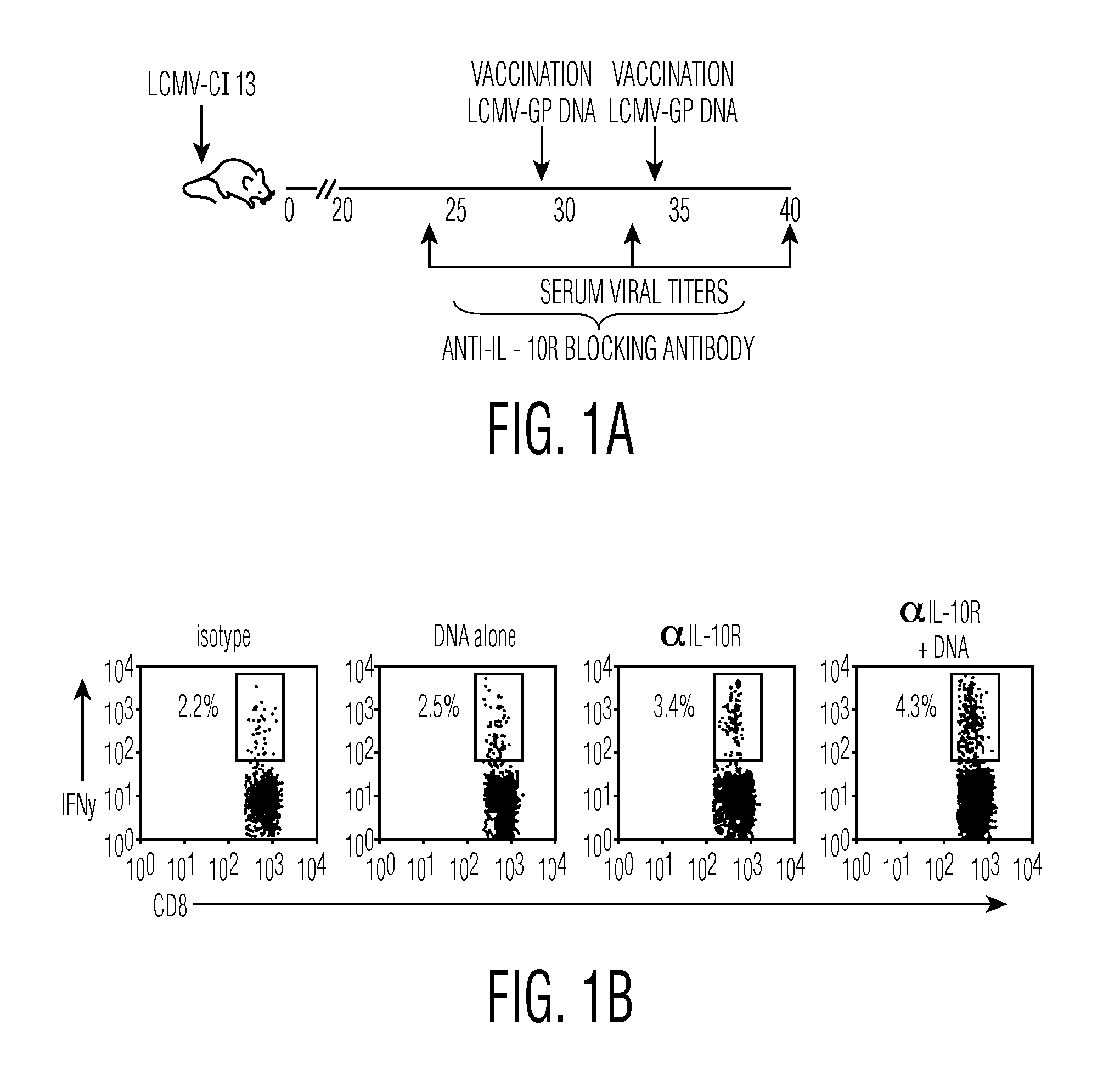
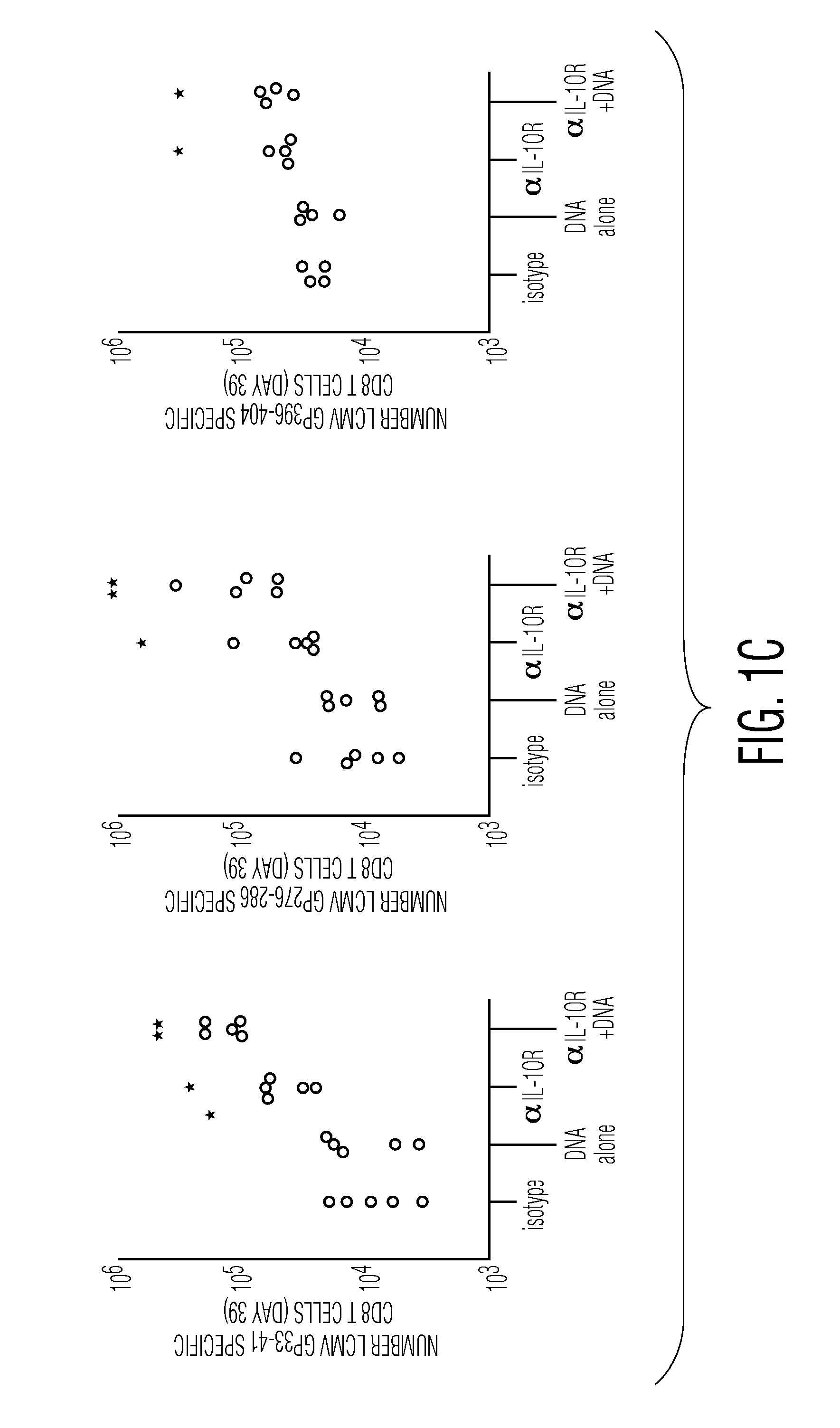
Combination Therapy to Treat Persistent Viral Infections
InactiveUS20110008332A1Reduce viral loadReduce loadAntibody mimetics/scaffoldsSnake antigen ingredientsCombined Modality TherapyAntagonist
The present invention pres ides combination therapies to treat persistent viral infections. In particular, combinations of vaccines and IL-10 or IL-10 receptor (IL-10R) antagonists to clear such infections are provided.
Owner:THE SCRIPPS RES INST
Method for treatment of HIV infection
InactiveUS20060134646A1Readily observable inflammatory responseEliminate side effectsPeptide/protein ingredientsViral antigen ingredientsVacciniaAnti-hiv drugs
A method for treatment of HIV infection includes administering at least one anti-HIV drug, such as a reverse transcriptase inhibitor, to a patient in need of such treatment and administering an extract from inflammatory tissue inoculated with vaccinia virus to the patient following the administration of the at least one anti-HIV drug. The extract maintains suppressive action on HIV replication, even if the administration of the anti-HIV drug is terminated.
Owner:NIPPON ZOKI PHARM CO LTD
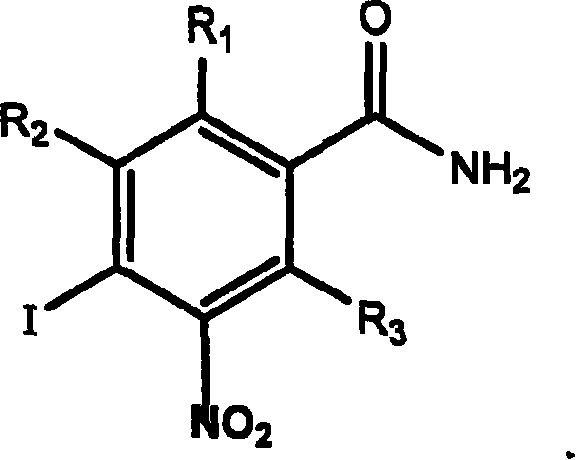
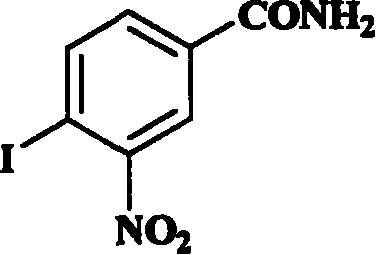
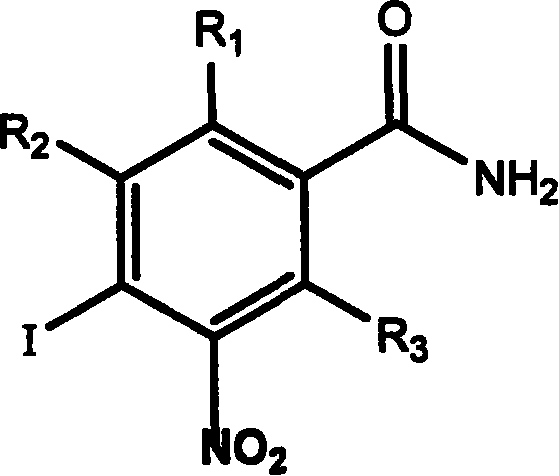
Application of aromatic nitro compound in preparing medicine for treating virus hepatitis
InactiveCN101190211APrevent proliferationNo drug resistanceOrganic active ingredientsDigestive systemNitro compoundDisease
The invention relates to a novel application of aromatic nitro compound in the pharmaceutical field, in particular to the application of aromatic nitro compound in treating viral hepatitis. The aromatic nitro compound can be used alone or can be combined with other commonly used anti- viral hepatitis drugs to effectively restrain the replication and secretion of hepatitis virus. The aromatic nitro compound can remarkably restrain the replication and secretion of hepatitis virus in the bodies of mammals. The aromatic nitro compound is especially suitable for treating the diseases caused by hepatitis B virus and hepatitis C. The invention provides the concrete method for treating the patients with hepatitis B and hepatitis C.
Owner:上海富海科星咨询管理有限公司
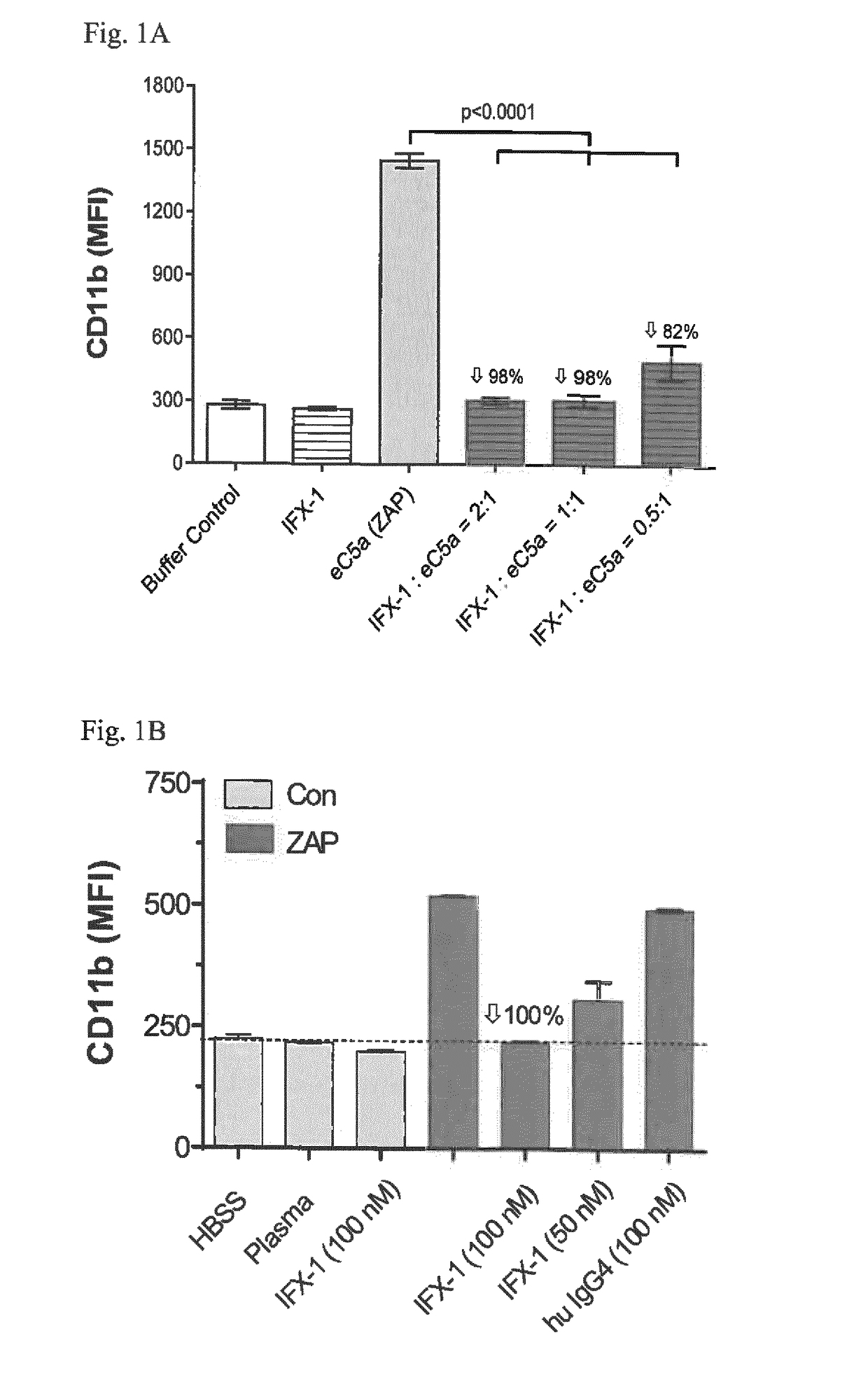
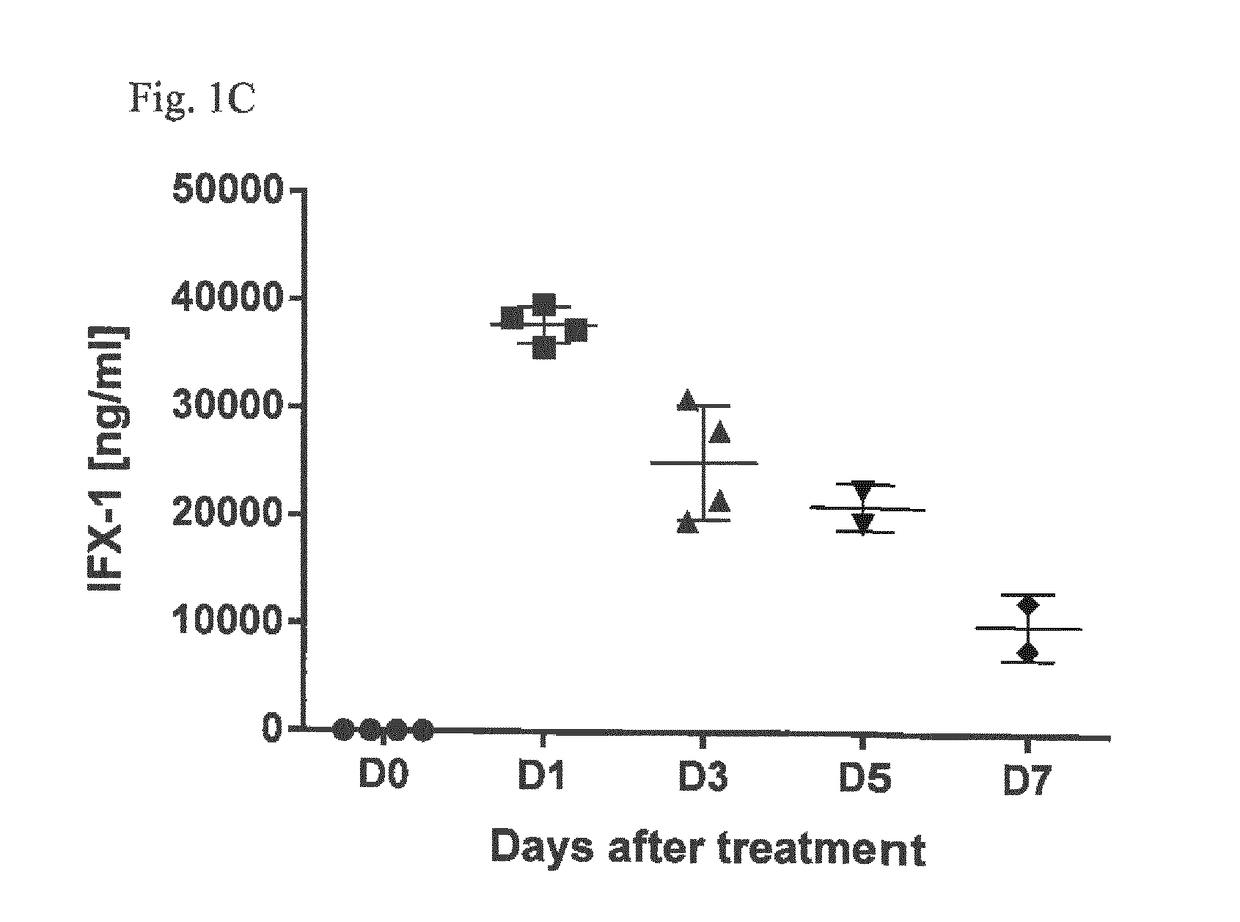
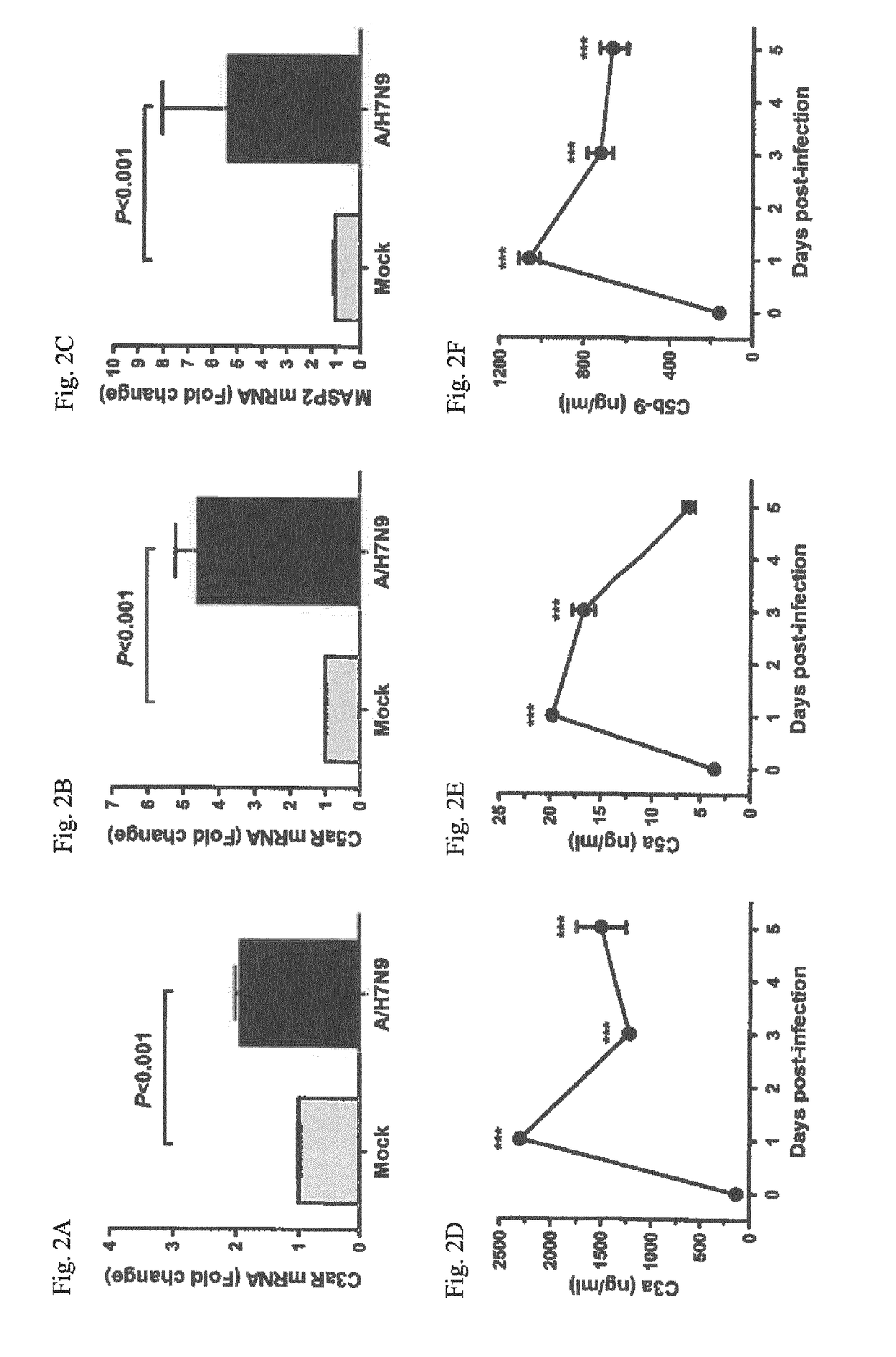
Inhibitors of c5a for the treatment of viral pneumonia
ActiveUS20170137499A1Reduce viral loadLessen lung damageImmunoglobulins against animals/humansAntiviralsC5a-inhibitorsPneumonitis
The present invention relates to inhibitors of C5a for use in the treatment of pneumonia, especially viral pneumonia. The invention also relates to the use of inhibitors of C5a in the preparation of a pharmaceutical composition for the treatment of pneumonia, especially viral pneumonia. The inventors further relates to methods for the treatment of pneumonia, especially viral pneumonia, comprising the step of administering a therapeutic amount of an inhibitor of C5a to a subject in need thereof.
Owner:INFLARX
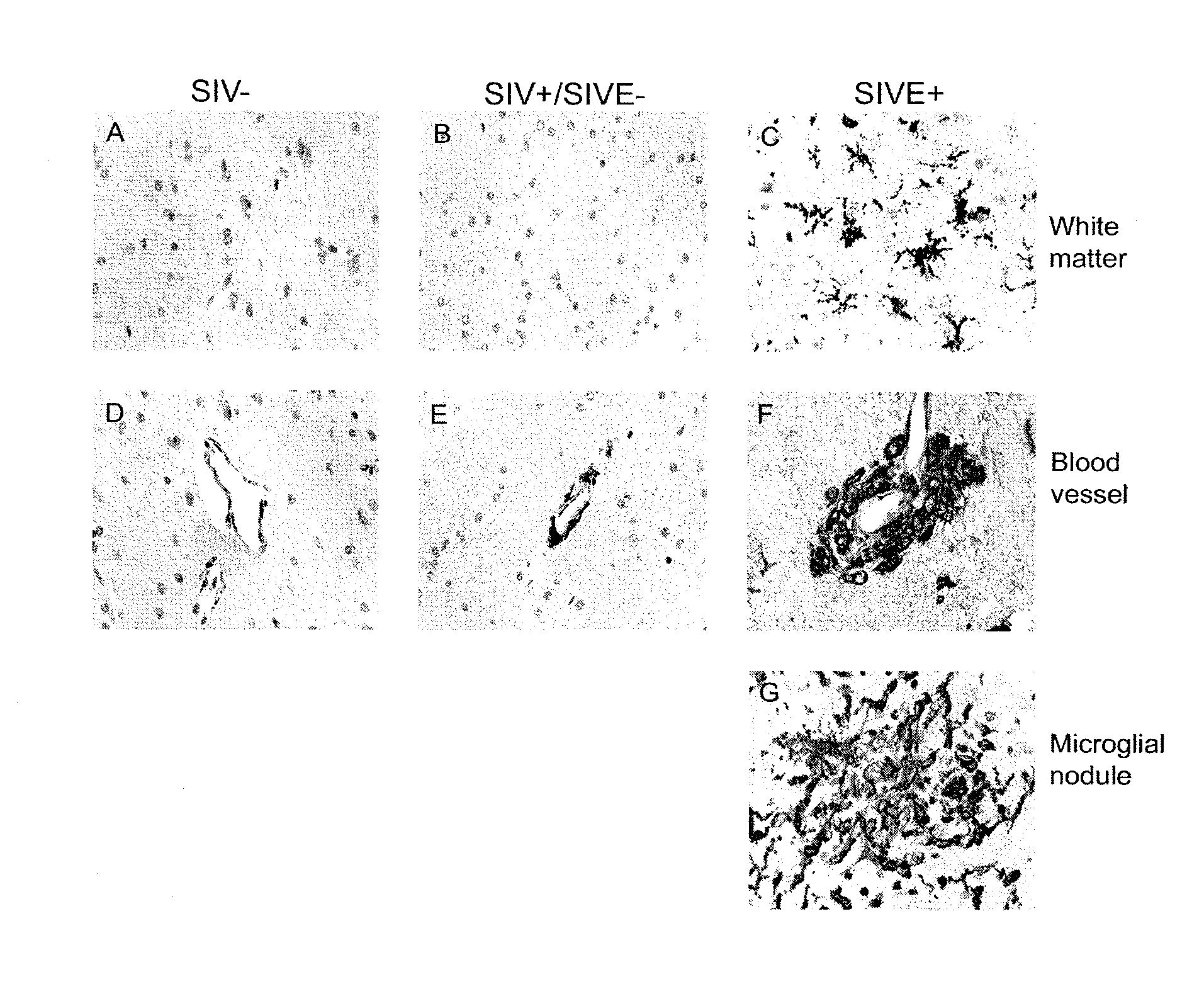
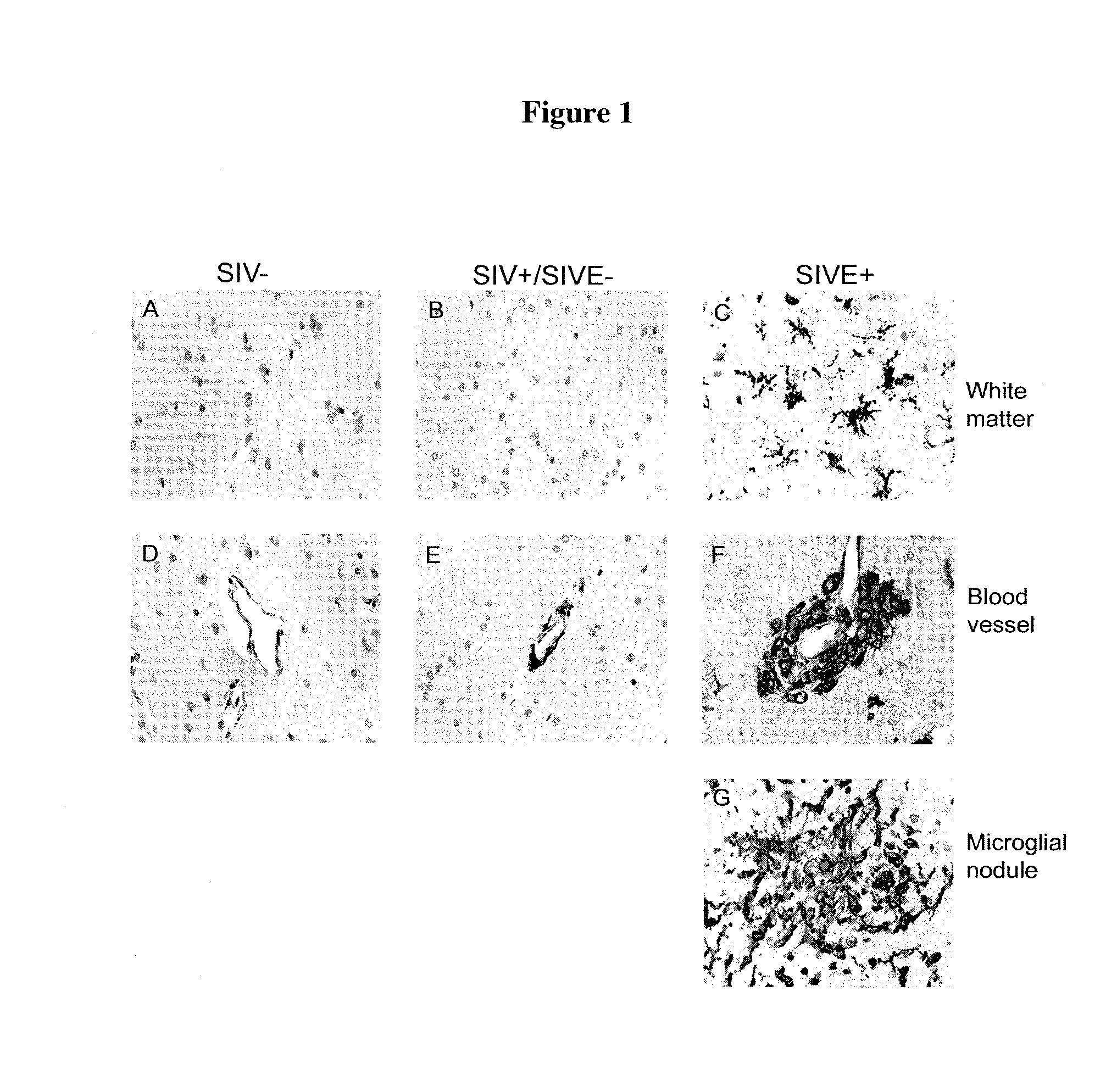
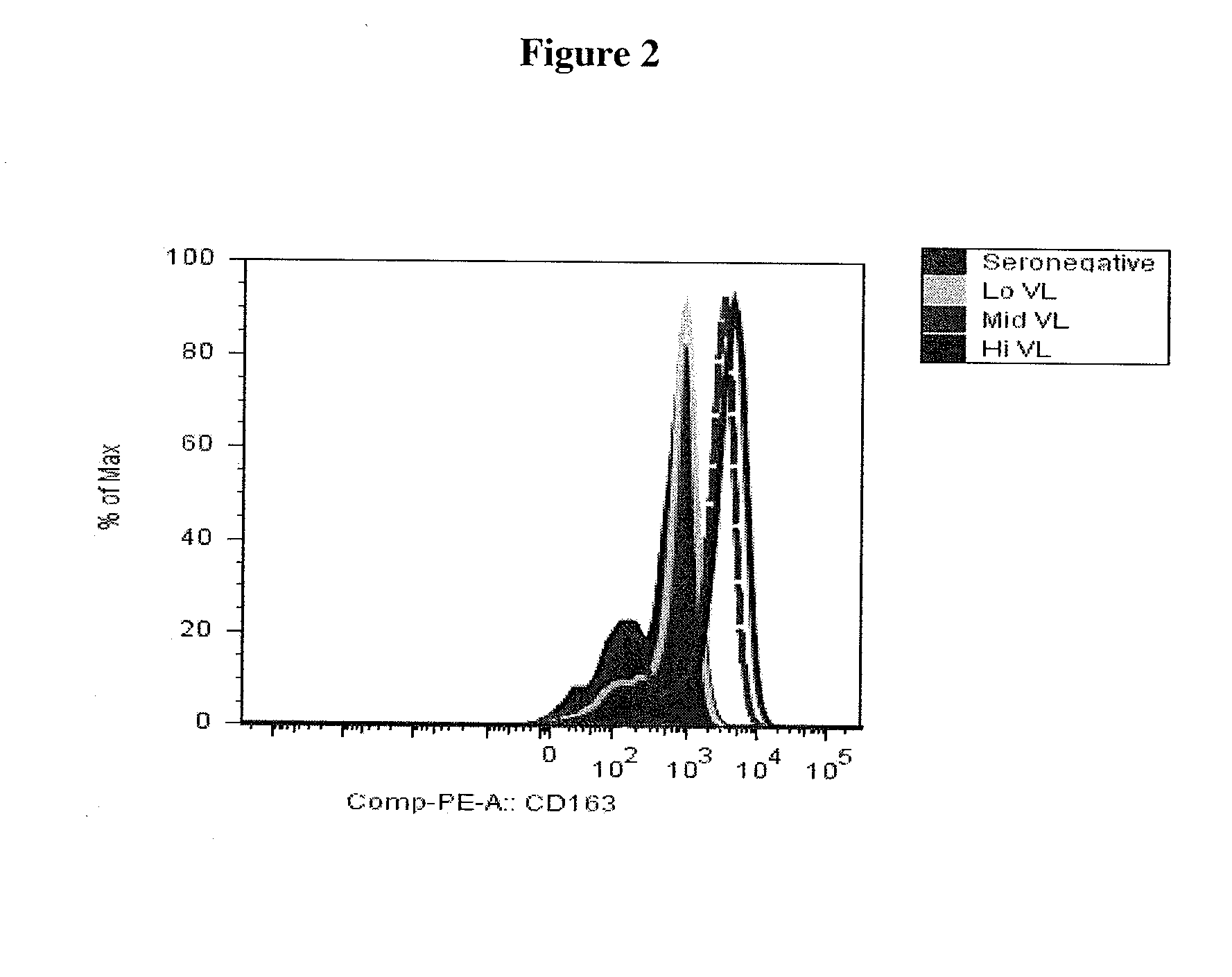
Blood monocyte cd163 expression as a biomarker in hiv-1 infection and neuroaids
ActiveUS20100291595A1Reduce viral loadElevated cell countBioreactor/fermenter combinationsPeptide librariesPeripheral blood mononuclear cellCD16
The invention provides a method for detecting CD163+ / CD16+ cell population in peripheral blood mononuclear cells in a biological sample from a subject infected with HIV-1 which comprises contacting the biological sample with an anti-CD163 antibody, so that levels of CD163+ / CD16+ peripheral blood mononuclear cells in the biological sample can be quantified. The method of the present invention is particularly useful for monitoring the course of HIV-1 infection and / or HIV Encephalopathy
Owner:TEMPLE UNIVERSITY
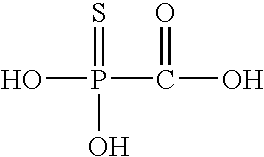
Compositions and methods for the treatment of viral infections
InactiveUS20080045482A1Reduce viral loadInhibits viral replicationBiocideInorganic phosphorous active ingredientsViral replicationViral infection
The present invention provides compositions and methods for inhibiting the replication of influenza virus by administering thiophosphonoformic acid alone or in combination with a neuraminidase inhibitor, for example, oseltamivir.
Owner:ADVENTRX PHARMA INC
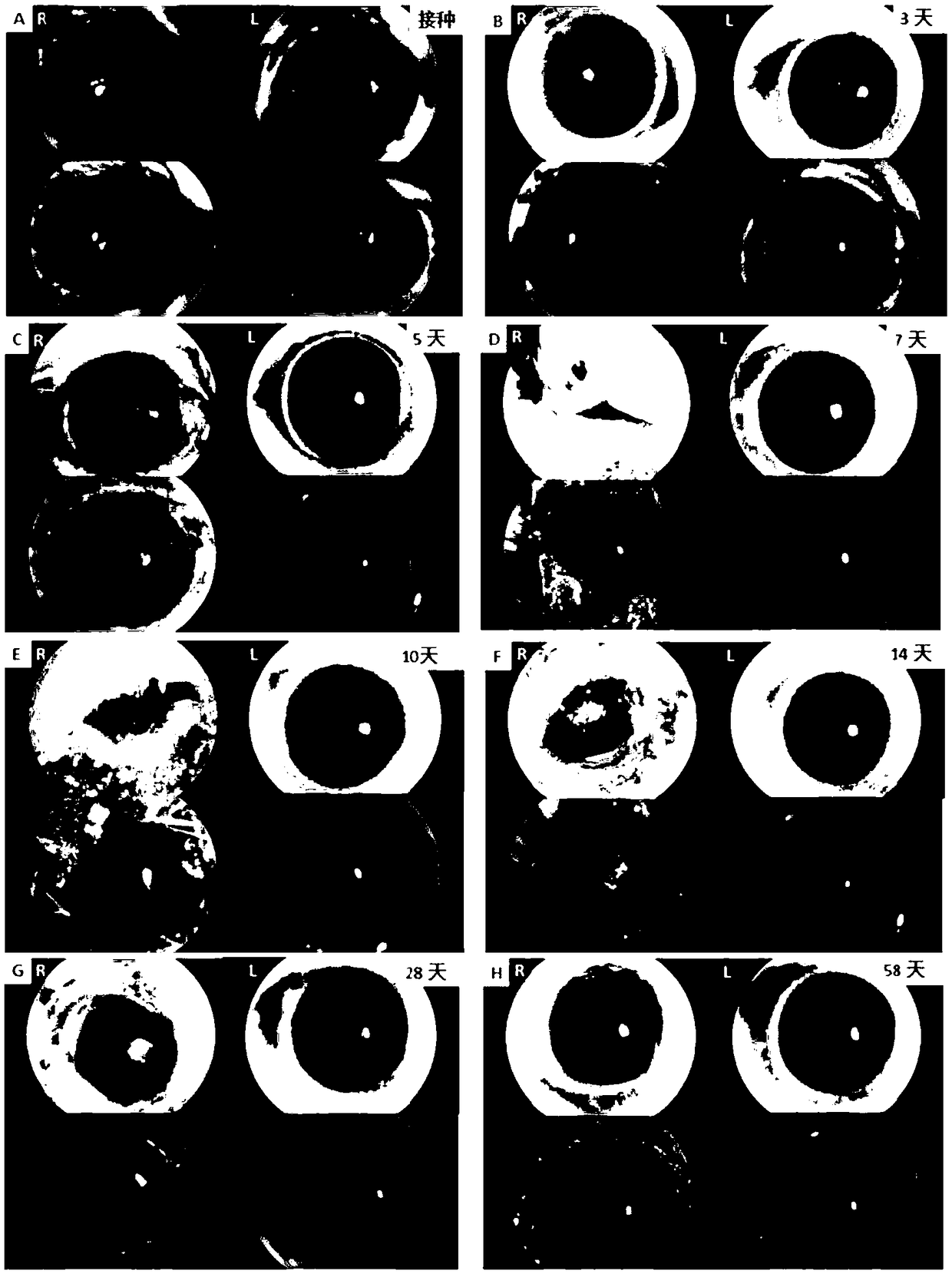
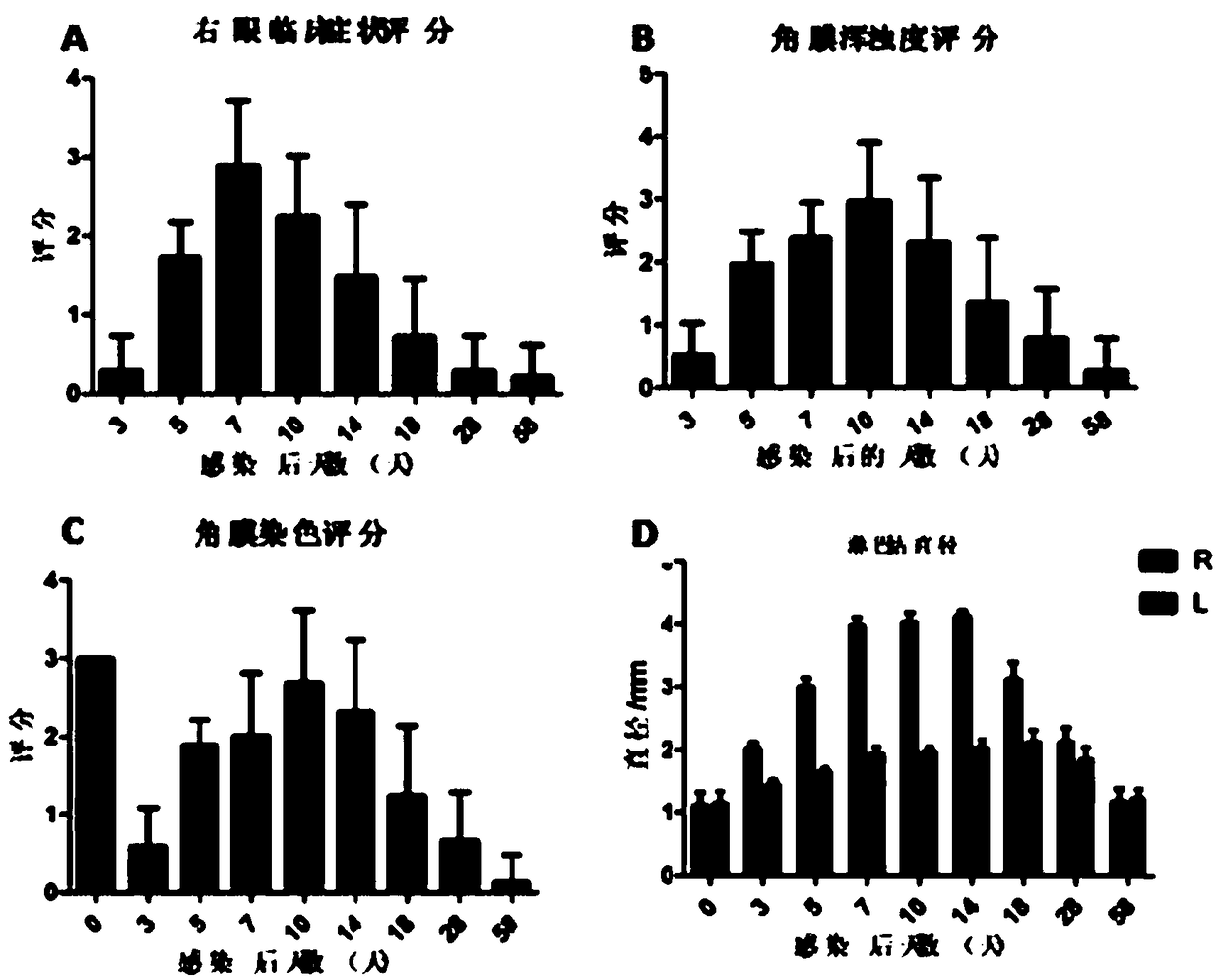
Building method for mouse herpes simplex keratitis model and application
InactiveCN109330732AModeling is fast and efficientReduce mortalityCompounds screening/testingAntibody medical ingredientsMedicineMortality rate
The invention discloses a building method for a mouse herpes simplex keratitis model and application. The method comprises the steps that the corneal epithelium in a circle is scrapped off by using acorneal trephine and an iris repositor, the herpes simplex virus SV-1 is inoculated on one eye of an infected mouse, and the mouse herpes simplex keratitis model is built. The depth and area of the corneal defect prepared by using the trephine method are fixed, the built model is more stable and unified, and large differences brought by the model can be avoided. The operation is precise, the influences caused by man-made operation differences can be avoided, and repetition is easy; the building method is suitable for various research directions and various model animals and easy to popularize;the virus is inoculated on the single eye, so that the association with clinic is closer; the corneal defect is large and the viral load is low, so that the death rate is easily controlled within anappropriate range; the animal model is high in repeatability and stability; the building and assessment method is simple in operation, low in requirements for technologies and devices and high in success rate.
Owner:FIRST AFFILIATED HOSPITAL OF KUNMING MEDICAL UNIV
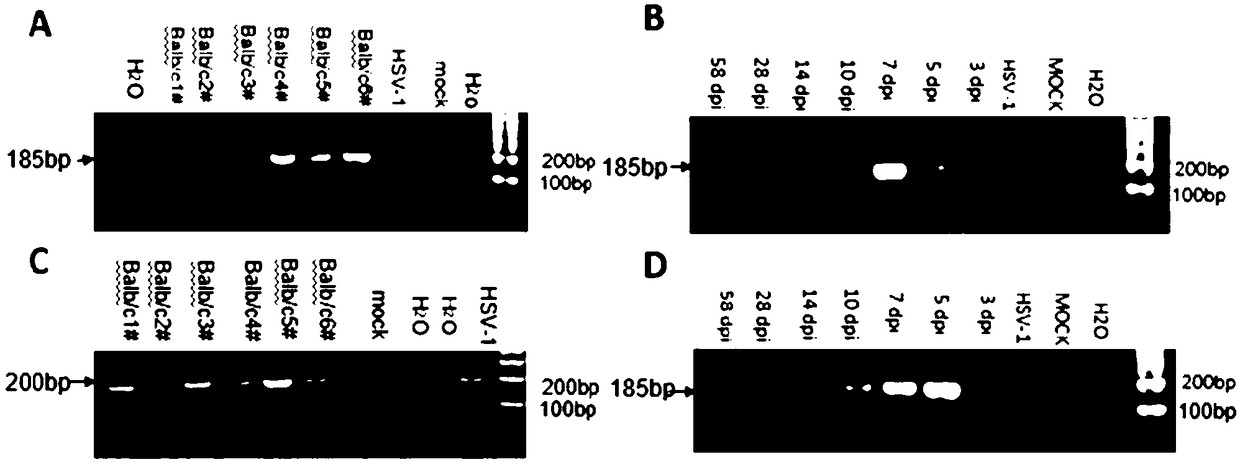
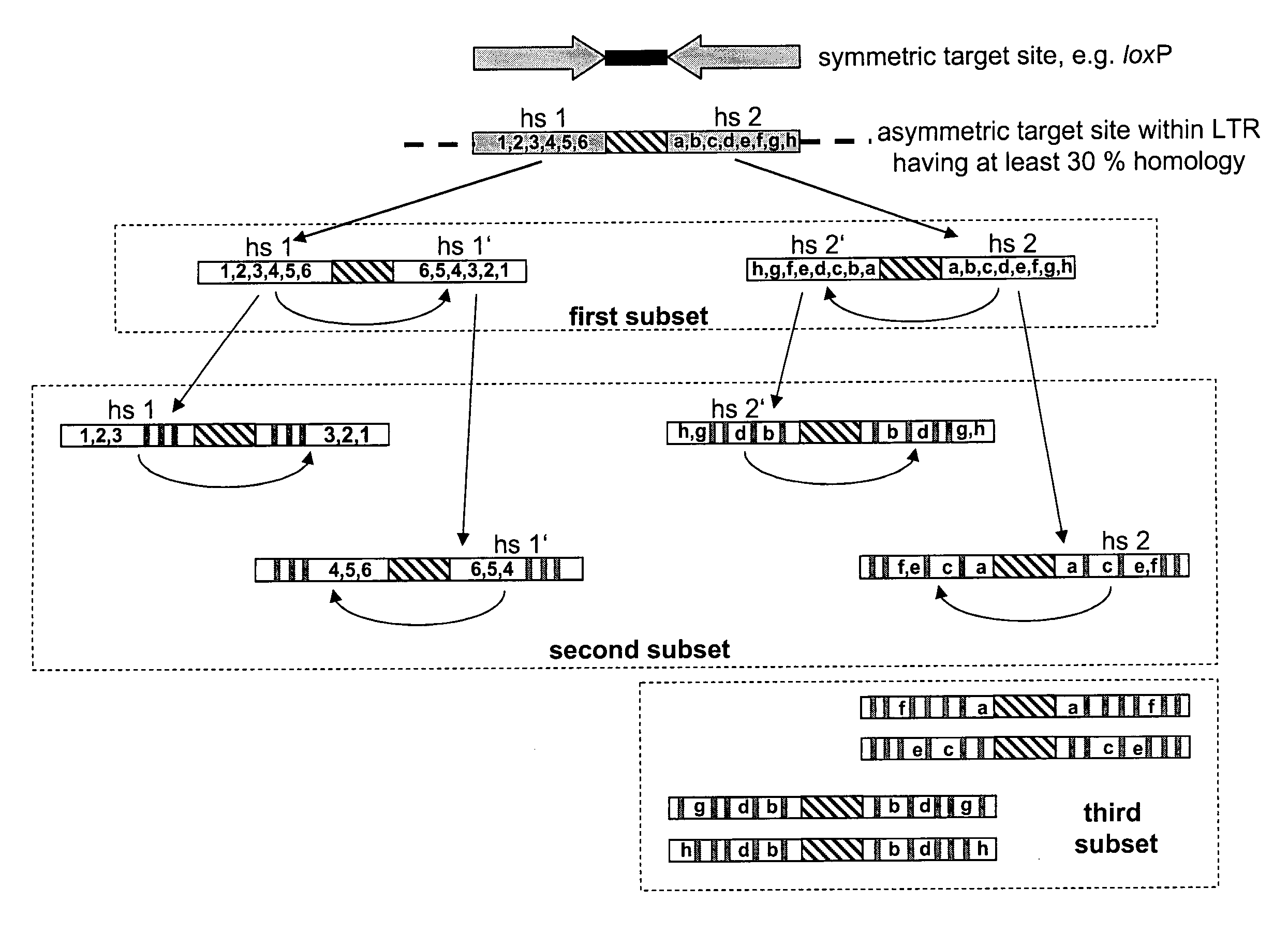
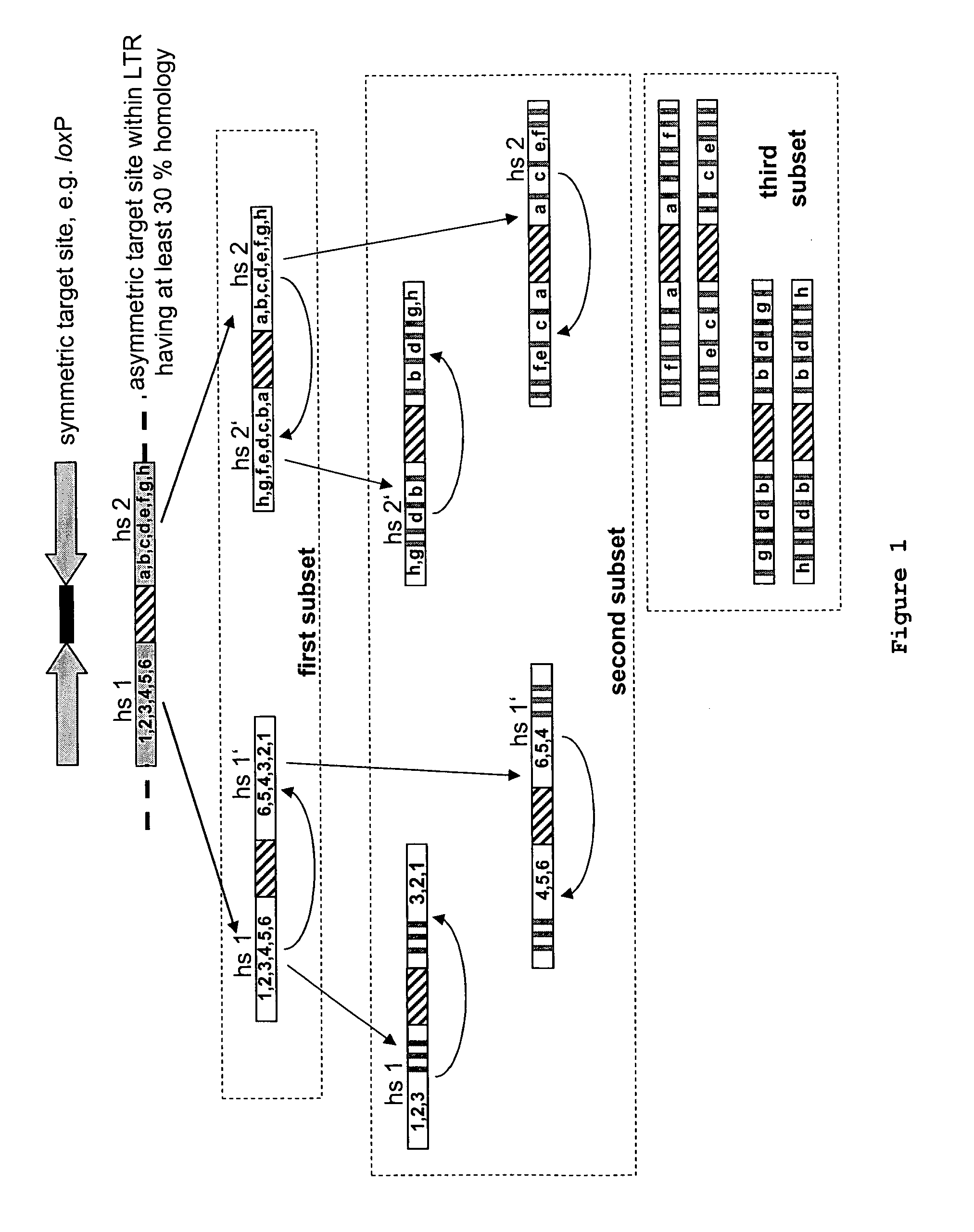
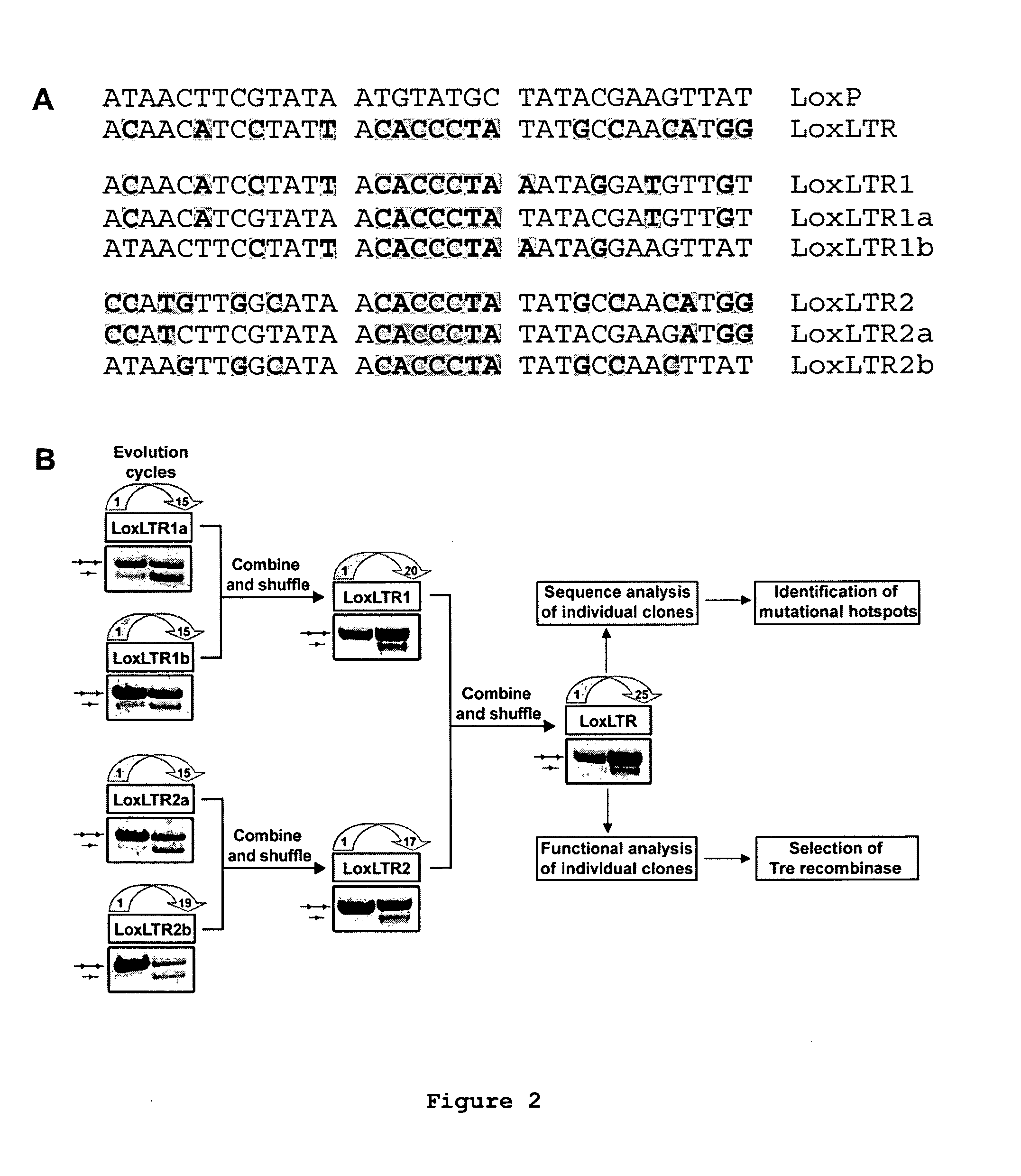
Use of tailored recombinases for the treatment of retroviral infections
ActiveUS20100172881A1Reduce viral loadTime consumingOrganic active ingredientsAnimal cellsProviral dnaRetroviral provirus
The present invention is directed to a method for preparing an expression vector encoding a tailored recombinase, wherein said tailored recombinase recombines asymmetric target sites within the LTR of proviral DNA of a retrovirus inserted into the genome of a host cell and is useful as means for excising the provirus from the genome of the host cell. The present invention further relates to an in vitro-method of optimising the treatment of a retroviral infection of a subject and to the use of tailored recombinases for the preparation of pharmaceutical compositions for reducing the viral load in a subjected infected by a retrovirus.
Owner:DRESDEN UNIVERSITY OF TECHNOLOGY +2
Combination Therapy for Treating Hepatitis C Virus Infection
InactiveUS20070258946A1Reduce morbidityReduce viral loadPeptide/protein ingredientsAntiviralsMortality rateInterferon receptor
The present invention provides methods of treating hepatitis C virus (HCV) infection; methods of reducing the incidence of complications associated with HCV and cirrhosis of the liver; and methods of reducing viral load, or reducing the time to viral clearance, or reducing morbidity or mortality in the clinical outcomes, in patients suffering from HCV infection. The methods generally involve administering to the individual i) a Type I interferon receptor agonist or a Type III interferon receptor agonist; ii) an immunomodulatory agent; and iii) an inhibitor of an HCV enzyme.
Owner:THREE RIVERS PHARMA LLC
Methods of treating hepatitis c virus infection
InactiveUS20110243894A1Reduce morbidityReduce viral loadBiocidePeptide/protein ingredientsSteatosisMortality rate
The present invention provides methods of treating hepatitis C virus (HCV) infection; methods of reducing the incidence of complications associated with HCV and cirrhosis of the liver; and methods of reducing viral load, or reducing the time to viral clearance, or reducing morbidity or mortality in the clinical outcomes, in patients suffering from HCV infection. Also provided are methods of treating liver steatosis and liver fibrosis.
Owner:THE J DAVID GLADSTONE INST A TESTAMENTARY TRUST ESTABLISHED UNDER THE WILL OF J DAVID GLADS
Macrophage derived chemokine (MDC) as an anti-viral agent for the treatment and prevention of lentivirus infection
InactiveUS6548631B1Reduce viral loadPeptide/protein ingredientsGenetic material ingredientsMacrophage-Derived ChemokineImmunodeficiency virus
The present invention relates to therapeutic compositions of macrophage derived chemokine (MDC) and methods for treating and preventing infection by a lentivirus, particularly an immunodeficiency virus, particularly HIV, using MDC proteins, nucleic acids and / or derivatives or analogues thereof. The present invention further relates to methods for detection and prognosis of lenivirus infection, particularly HIV infection using MDC as a prognostic indicator. The present invention further provides MDC proteins, nucleic acids encoding such proteins, that have amino-terminal sequences that differ from other MDC isolates. Recombinant host cells and methods of production are also provided.
Owner:AKZO NOBEL NV +1
Plant composition for the treatment or prevention of viral blood-borne diseases such as diseases caused by the human immunodeficiency virus (HIV) or hepatitis c
InactiveUS20120076849A1Less secondary effectReduce viral loadBiocideDispersion deliveryTanninDisease
A composition based on plants for treating or preventing viral diseases of the blood, such as the diseases caused by the virus of human immunodeficiency (HIV) or hepatitis C. The composition comprises flower of sulfur, a plant containing catechin and a tannin agent, and a pharmaceutically acceptable carrier. The plant is selected from Agrimonia Eupatoria and Uncaria gambir.
Owner:EL KETTANY SLEIMEN +1
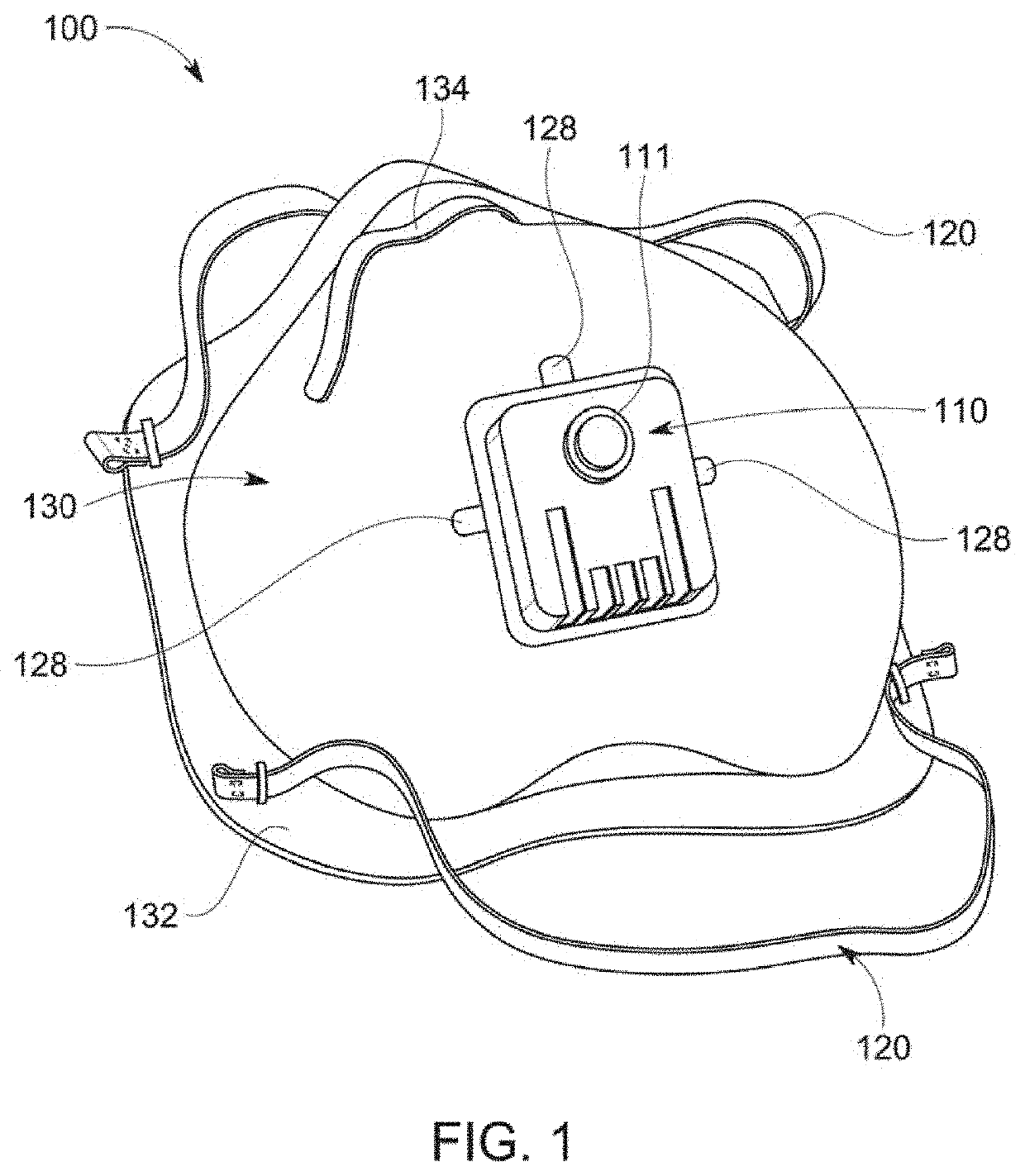
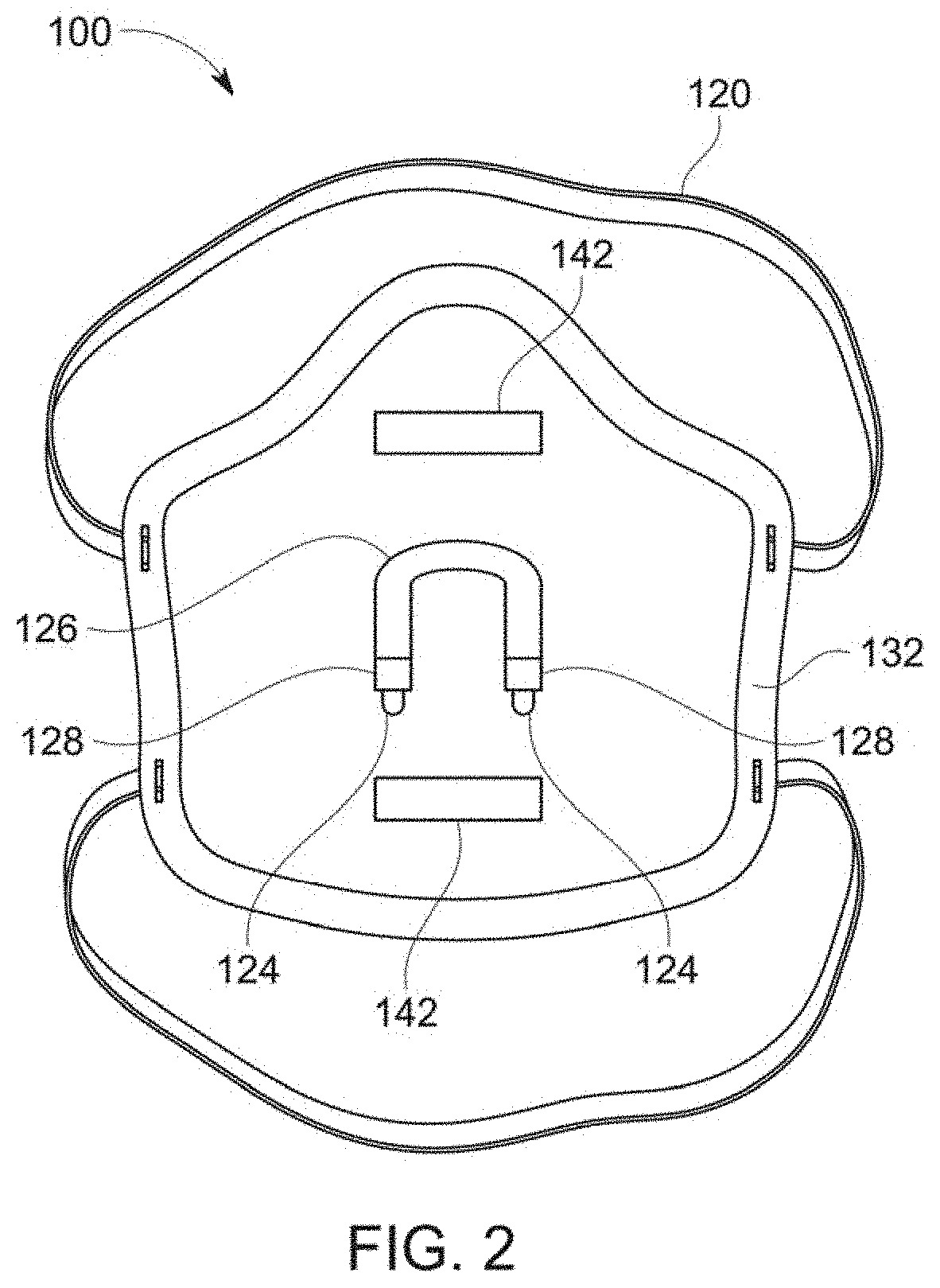
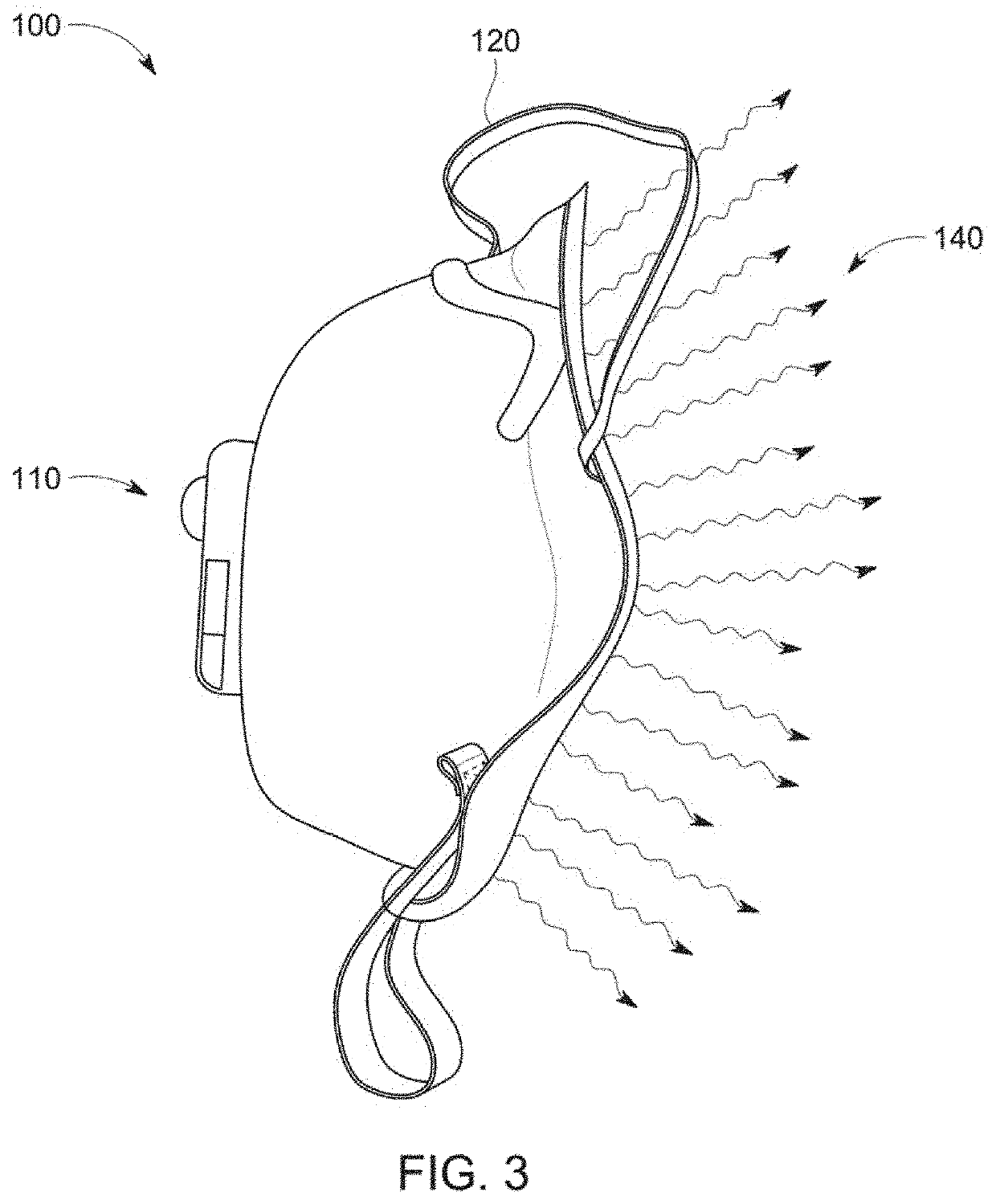
Anti-microbial, disinfection chamber respiratory face mask/shield
ActiveUS20210298391A1Reduce viral loadHigh recovery rateBreathing filtersBreathing masksRhinorrheaRespiratory pathogen
A self-sterilizing, face mask, respirator, open or enclosed face shield employs germicidal, far UV-C light safe for direct human exposure, mitigating risk of acquisition or transmission of respiratory pathogens, such as COVID 19. Device attachments or special lenses direct far UV-C light into the respiratory chamber and / or reservoir areas of infection including nasal, oropharyngeal or ocular areas to reduce or eliminate pathogens, serving also as a treatment modality. In a preferred embodiment the face shield has a removable front section, which can be exchanged or replaced with sections that include various functional attributes while maintaining germicidal protections and avoiding need to remove PPE during these activities including consumption of liquids, oral medications or food, as well as providing antimicrobial absorbent sneeze or cough guard or rhinorrhea secretion absorption, or a communication device to address communication deficiencies caused by respiratory protection barriers.
Owner:KEENE SHARON A +1
Treatment And Functional Cure Of HIV Infection By Monoclonal Antibodies To CD4 Mediating Competitive HIV Entry Inhibition
ActiveUS20170369576A1Reduce viral loadReduce loadImmunoglobulins against cell receptors/antigens/surface-determinantsAntiviralsAntibodyDisease cure
The present disclosure is directed to compositions and methods for the prevention, treatment, and / or functional cure of HIV infection. One aspect of the present disclosure relates to monoclonal antibodies directed against CD4, compositions thereof, and methods employing such compositions for the prevention, treatment, and functional cure of HIV infection.
Owner:UBI US HLDG LLC
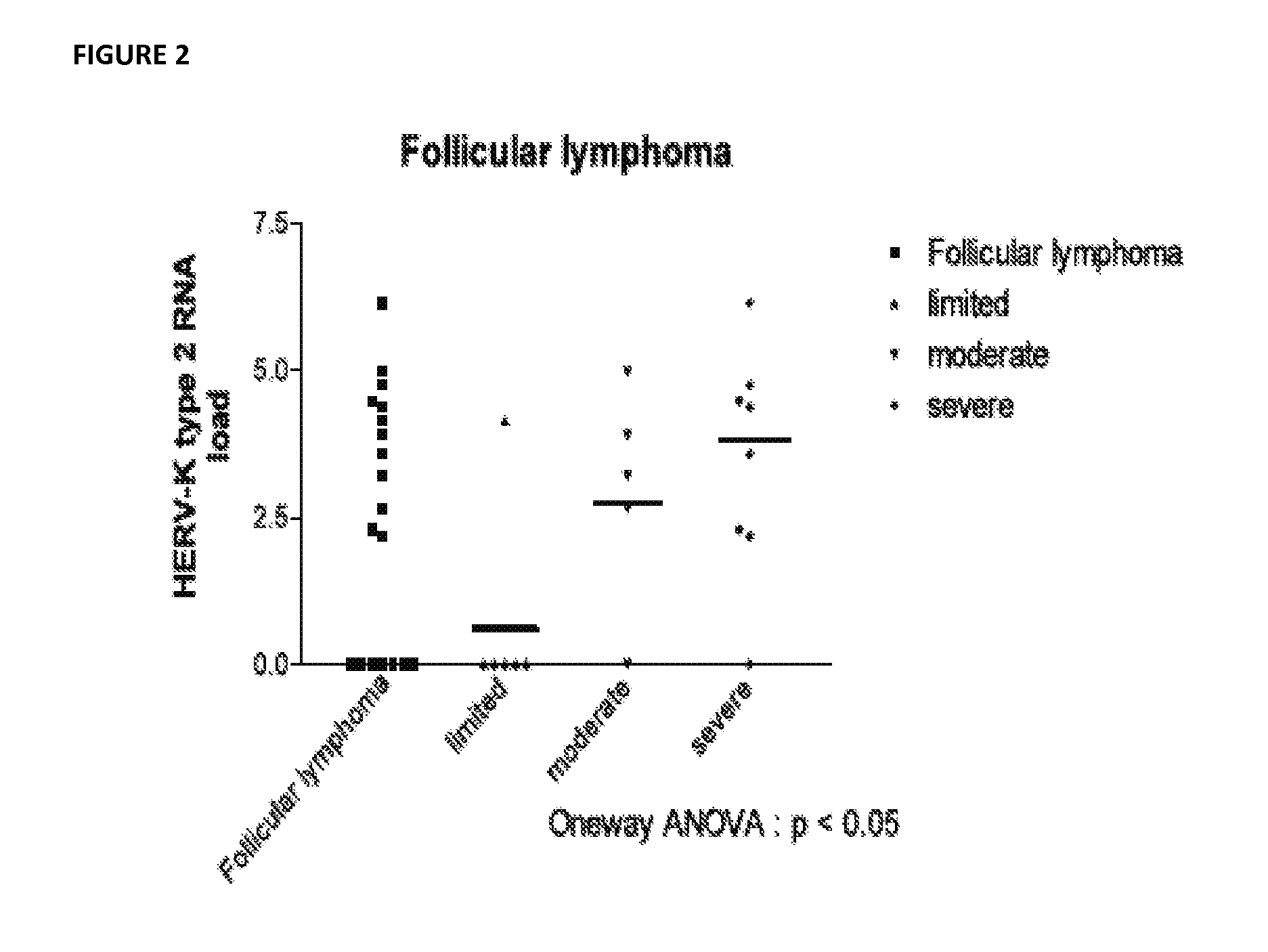
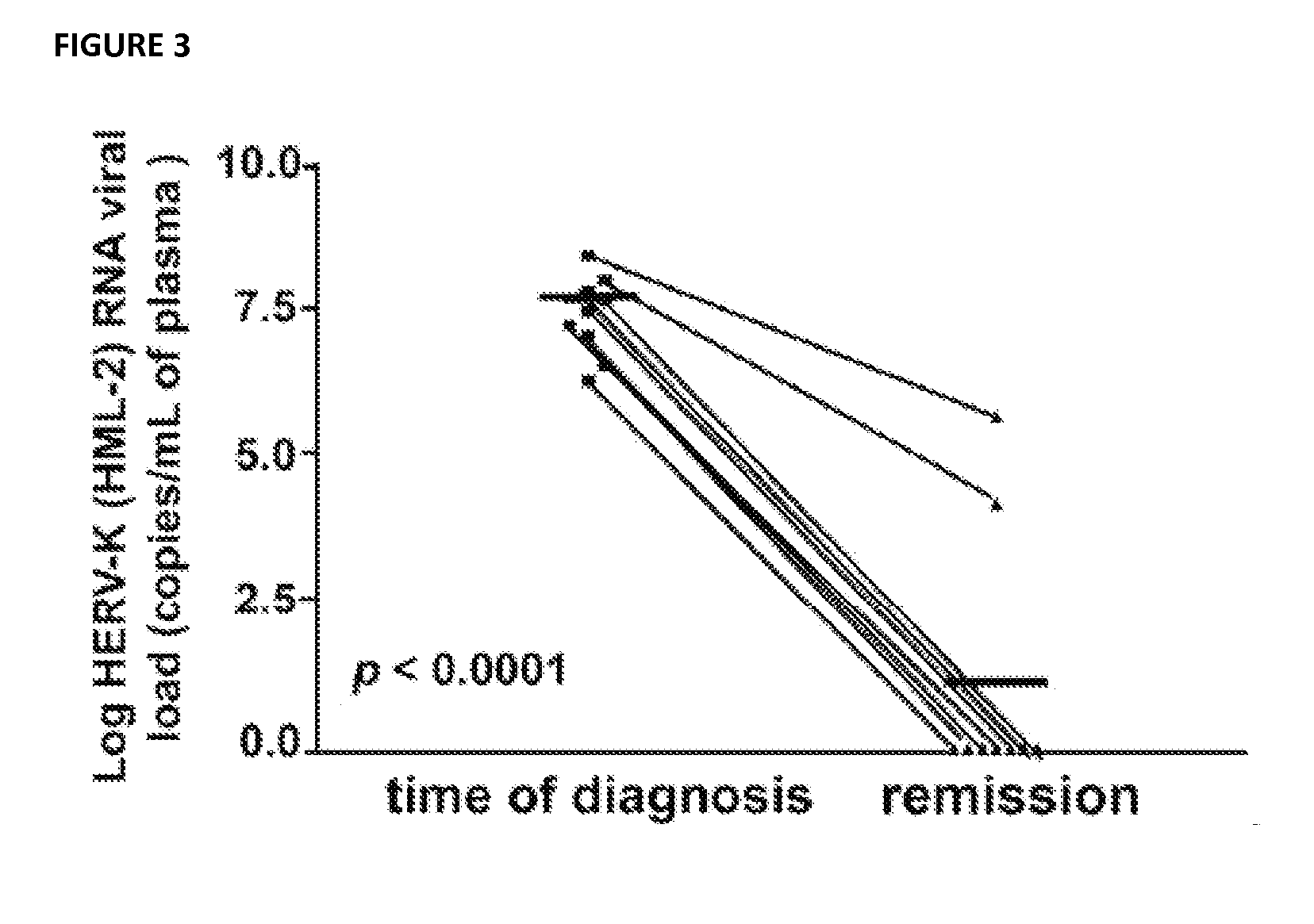
Antiviral treatment of lymphoma and cancer
InactiveUS20120135950A1Reduce tumor burdenReduce viral loadBiocideMicrobiological testing/measurementAntiviral treatmentCancer
Compositions and methods to treat lymphoma and cancer are disclosed. In particular, the method teaches treatment of lymphoma and cancer using anti-HERV-K(HML-2) therapies. Further taught are compositions and methods for characterizing patient samples to, for example, select or identify therapeutic options or assess the impact of therapies.
Owner:RGT UNIV OF MICHIGAN
Small-molecule inhibitors of dengue virus proteases
ActiveUS20150141521A1Reduce viral loadShorten the progressBiocideOrganic chemistryWest Nile virus RNAProtease
The present invention concerns methods and compositions involving small molecule inhibitors for the treatment or prophylaxis of flavivirus infection, such as dengue virus and West Nile virus.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Establishment of hepatitis B virus (HBV) infection model under novel human liver biological scaffold three-dimensional culture system
ActiveCN109679891AHigh replication levelHigh infection efficiencyHepatocytesMicrobiological testing/measurementHepatitis B virusIn vivo
The invention belongs to the field of biological research and specifically relates to the establishment of a hepatitis B virus (HBV) infection model under a novel human liver biological scaffold three-dimensional culture system. According to the invention, the hepatitis B virus infection model is established through wide and deep research; the model has the main characteristics that the infectionefficiency is high, a viral load used in an infection process is much lower than that of other models, but the replication level of a virus in cells is high. Furthermore, the infection lasting time ofthe model is long, and specifically, the in vitro infection of common primary liver cells only can last for about 10 days, but the infection time of the model involved in the invention can last for nearly 20 days. In addition, the expression of the liver cells in the model involved in the invention is close to an in vivo real state and the model can be used for screening hepatitis B resisting medicines.
Owner:CHILDRENS HOSPITAL OF CHONGQING MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com