Establishment of hepatitis B virus (HBV) infection model under novel human liver biological scaffold three-dimensional culture system
A bio-scaffold, hepatitis B virus technology, applied in the field of biological research, can solve the problems of poor repeatability in vivo, huge phenotypic differences, low HBV infection efficiency, etc., and achieve the effect of high replication level, high infection efficiency and long infection duration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
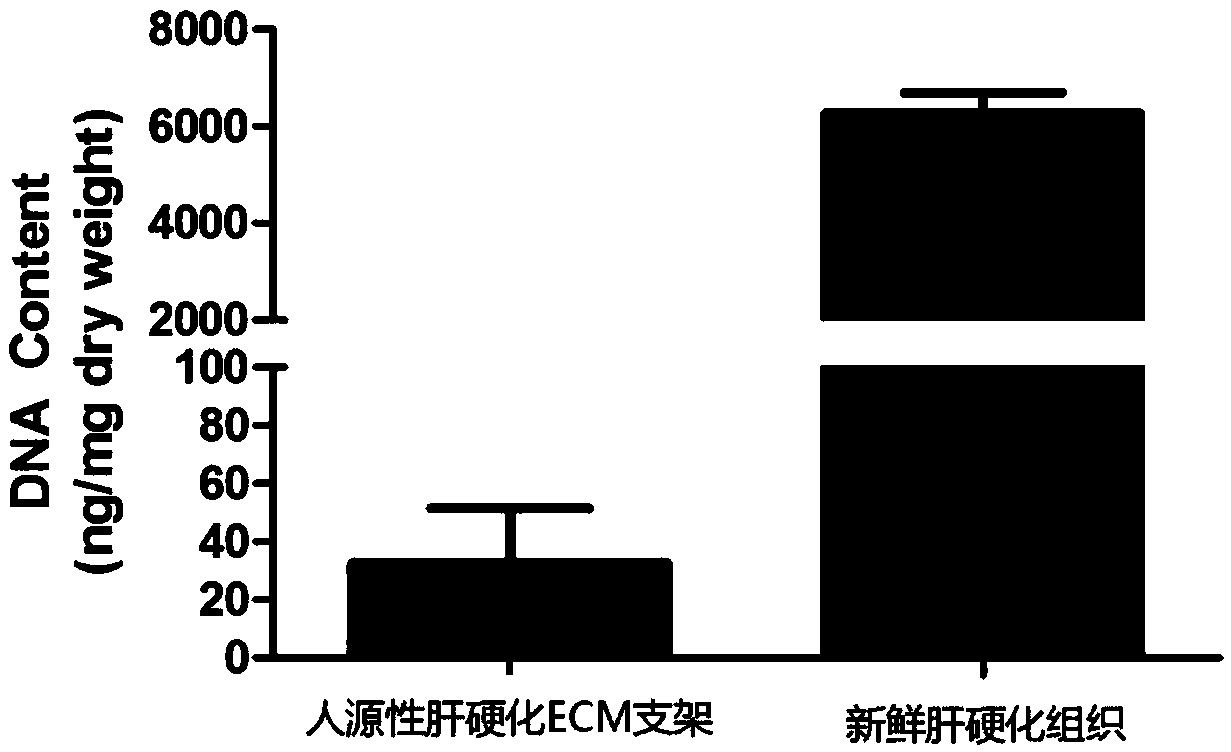
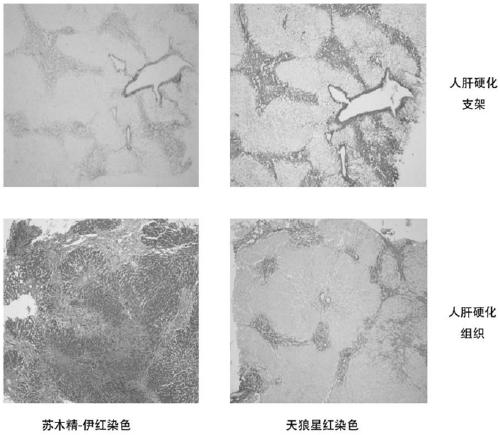
[0090] Example 1 Preparation and identification of human liver cirrhosis ECM bioscaffold
[0091] 1. Experimental method
[0092] (1) Obtaining human cirrhotic liver tissue
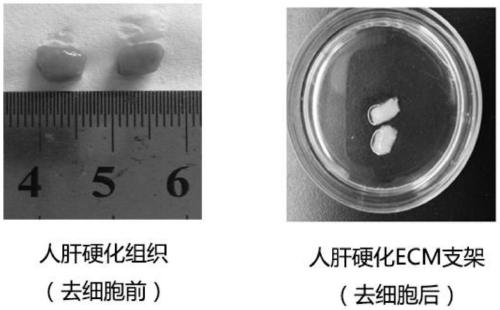
[0093] Collected cirrhotic liver tissues from patients undergoing liver transplantation in Children's Hospital Affiliated to Chongqing Medical University, approved by the Ethics Committee of Children's Hospital Affiliated to Chongqing Medical University, and signed an informed consent form with the patients and their families. Harden the liver tissue 2-3 times, remove the blood cells and blood clots in the vessels, wash with PBS, and cut the liver into eight segments according to the anatomical structure of the liver tissue according to the Couinaud segmentation method. and the falciform ligament to segment the liver, cut it and place it in a -80°C refrigerator for cryogenic freezing and preservation.
[0094] (2) Preparation of human-derived liver cirrhosis ECM bioscaffold
[0095] 1. Cutting and stor...
Embodiment 2
[0122] In this example, a normal human-derived liver ECM bioscaffold was prepared.
[0123] 1. Experimental method
[0124] (1) Obtaining normal human liver tissue
[0125] The normal tissues adjacent to the cancer after liver tumor resection and discarded liver transplantation donor tissues were collected from the Children's Hospital Affiliated to Chongqing Medical University. They were approved by the Ethics Committee of the Children's Hospital Affiliated to Chongqing Medical University. The informed consent was signed with the patients and their families. The normal liver tissues during the operation After the body is isolated, lavage with heparin solution twice to remove blood cells and blood clots in the vasculature, and cut into small pieces with a size of about (5~7)mm*(5~7)mm*(5~7)mm. Blocks were placed in a -80°C refrigerator for low-temperature freezing and storage.
[0126] (2) The preparation method of normal human-derived liver ECM bioscaffold, comprising the fo...
Embodiment 3
[0143] The establishment of embodiment 3 hepatitis B virus infection model
[0144] 1. Test method
[0145] 1 Stereoscopic cell culture
[0146] 1.1. Disinfection of human-derived liver cirrhosis ECM bioscaffolds and normal human liver ECM bioscaffolds
[0147] Place the qualified human-derived liver cirrhosis ECM bioscaffold prepared in Example 1 and the qualified normal human-derived liver ECM bioscaffold prepared in Example 2 in 0.1% peracetic acid solution, and shake gently on a shaker for 30 minutes before replacing New 0.1% peracetic acid, shake lightly for 15 minutes, wash with sterile PBS solution for 10 minutes twice. Finally, it was sterilized under ultraviolet light for 2 hours.
[0148] 1.2. Three-dimensional cell culture
[0149] 1.2.1. Three-dimensional culture of primary hepatocytes
[0150] Human primary human hepatocytes (Primary Human Hepatocyte, PHH) were cultured in hepatocyte culture medium (Sciencell Company, Cat. No. 5210). Before inoculation, the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com