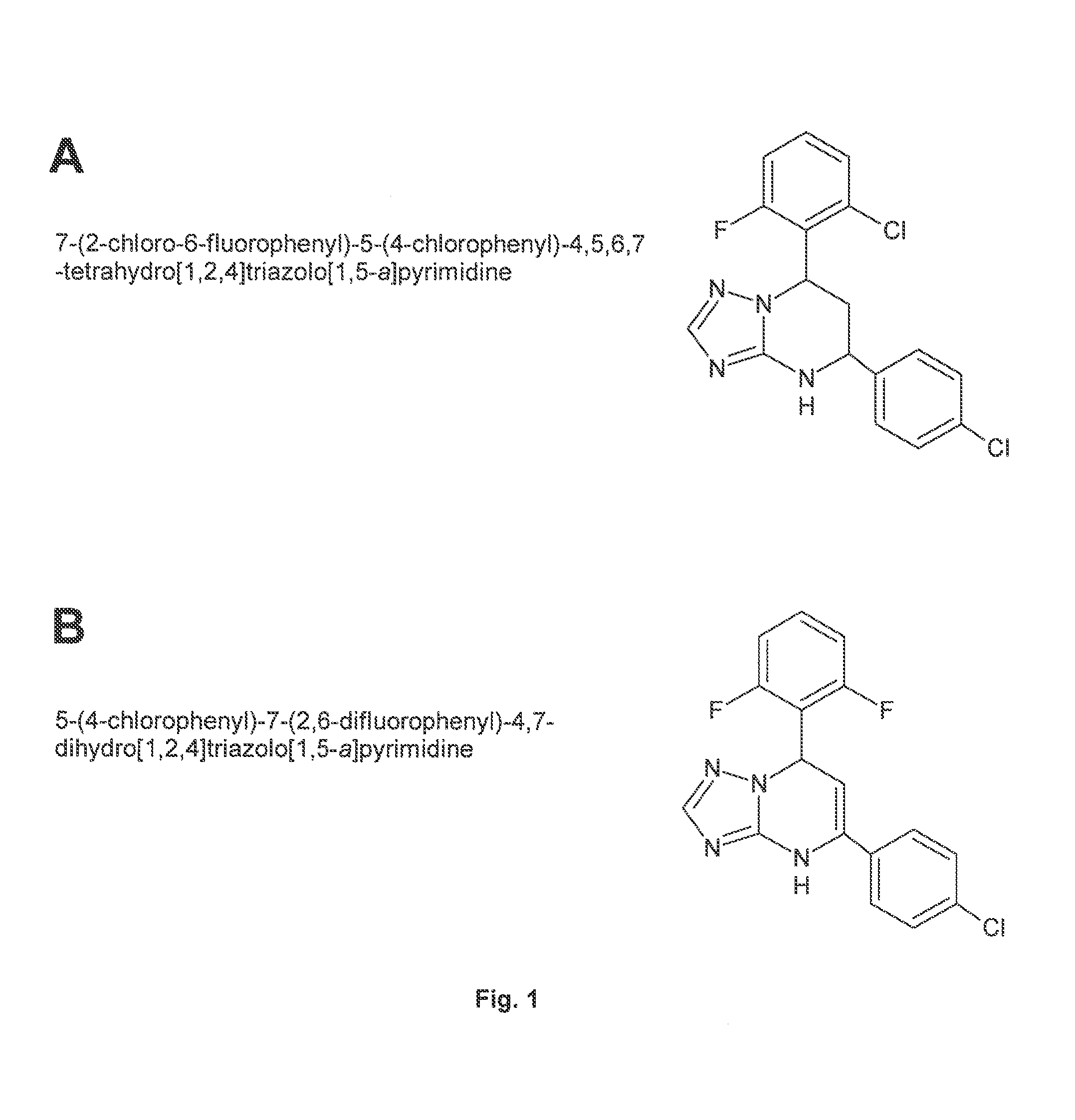
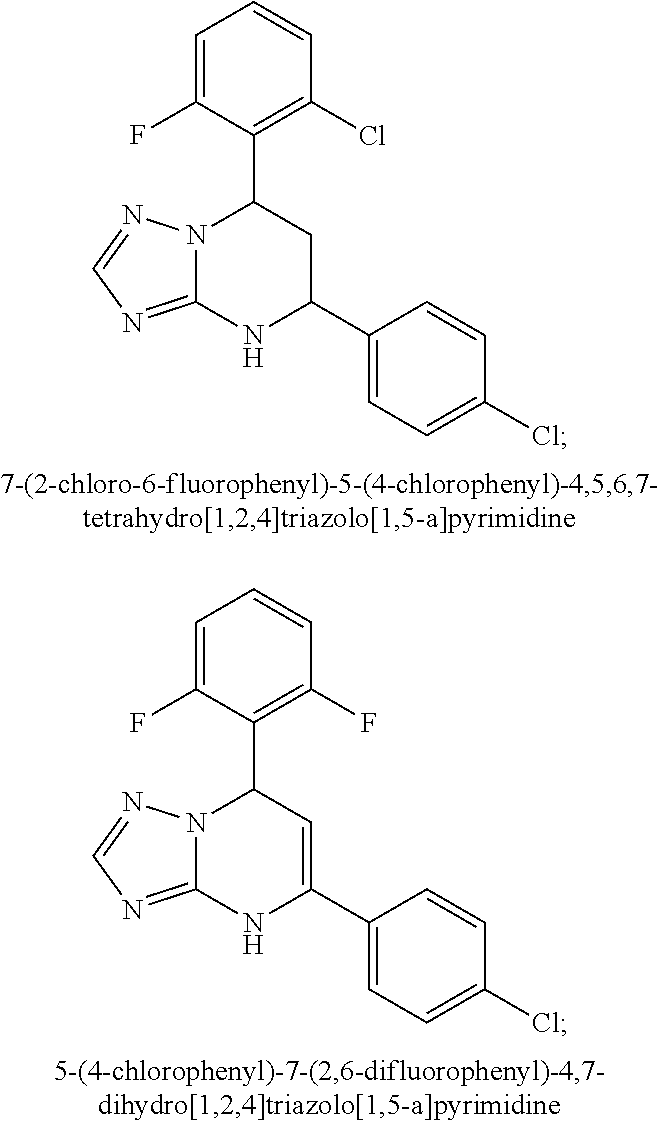
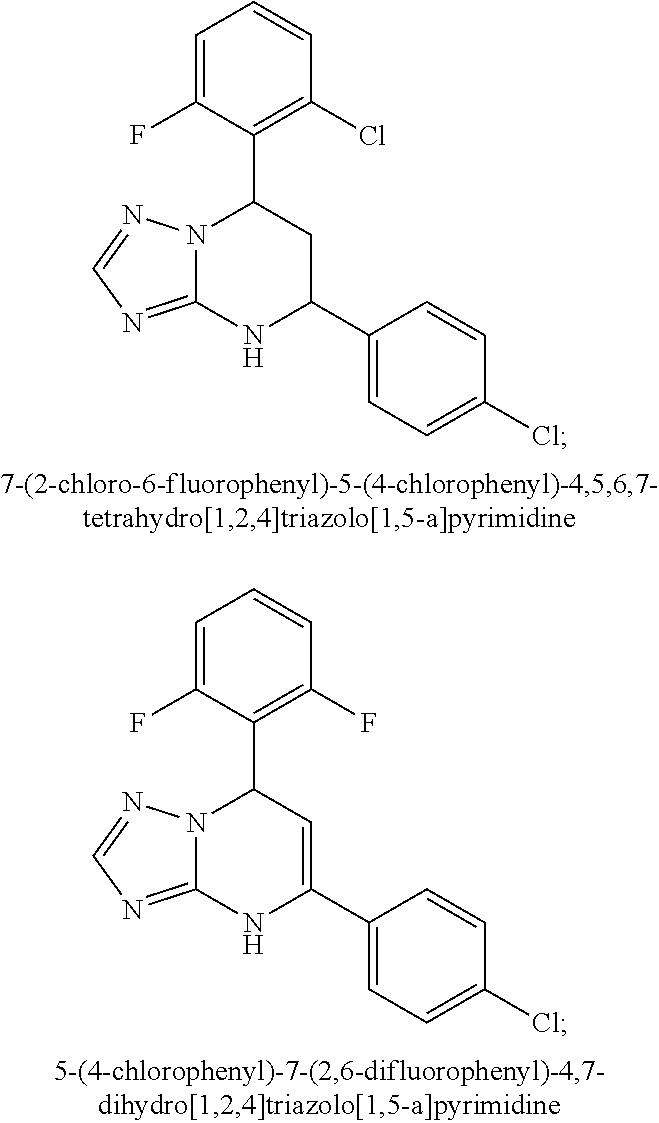
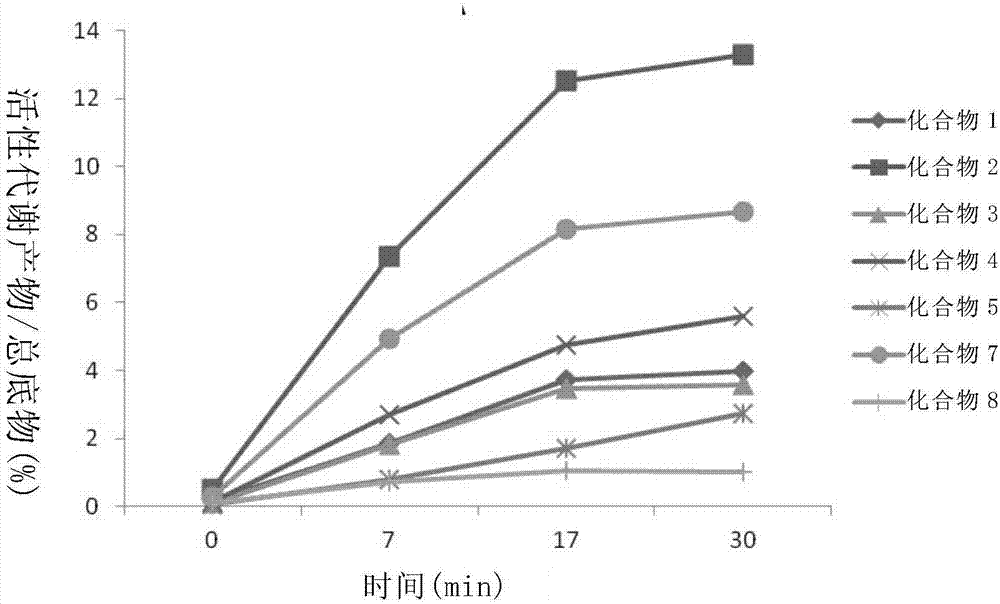
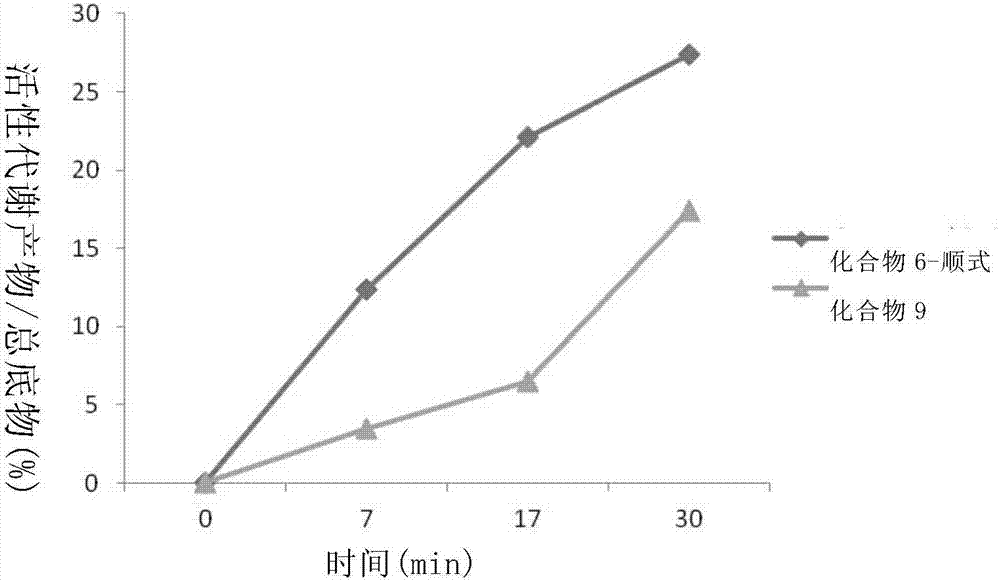
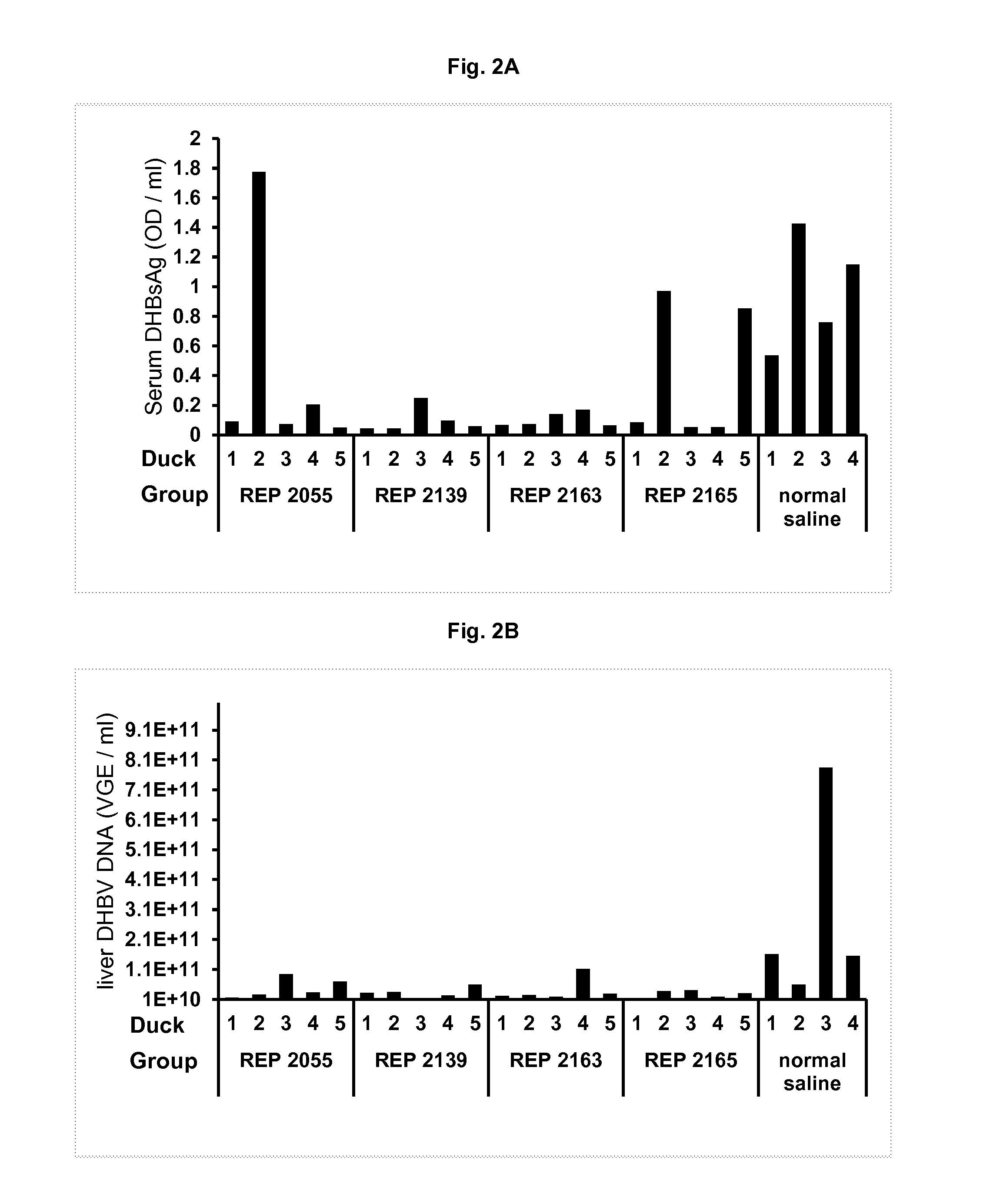
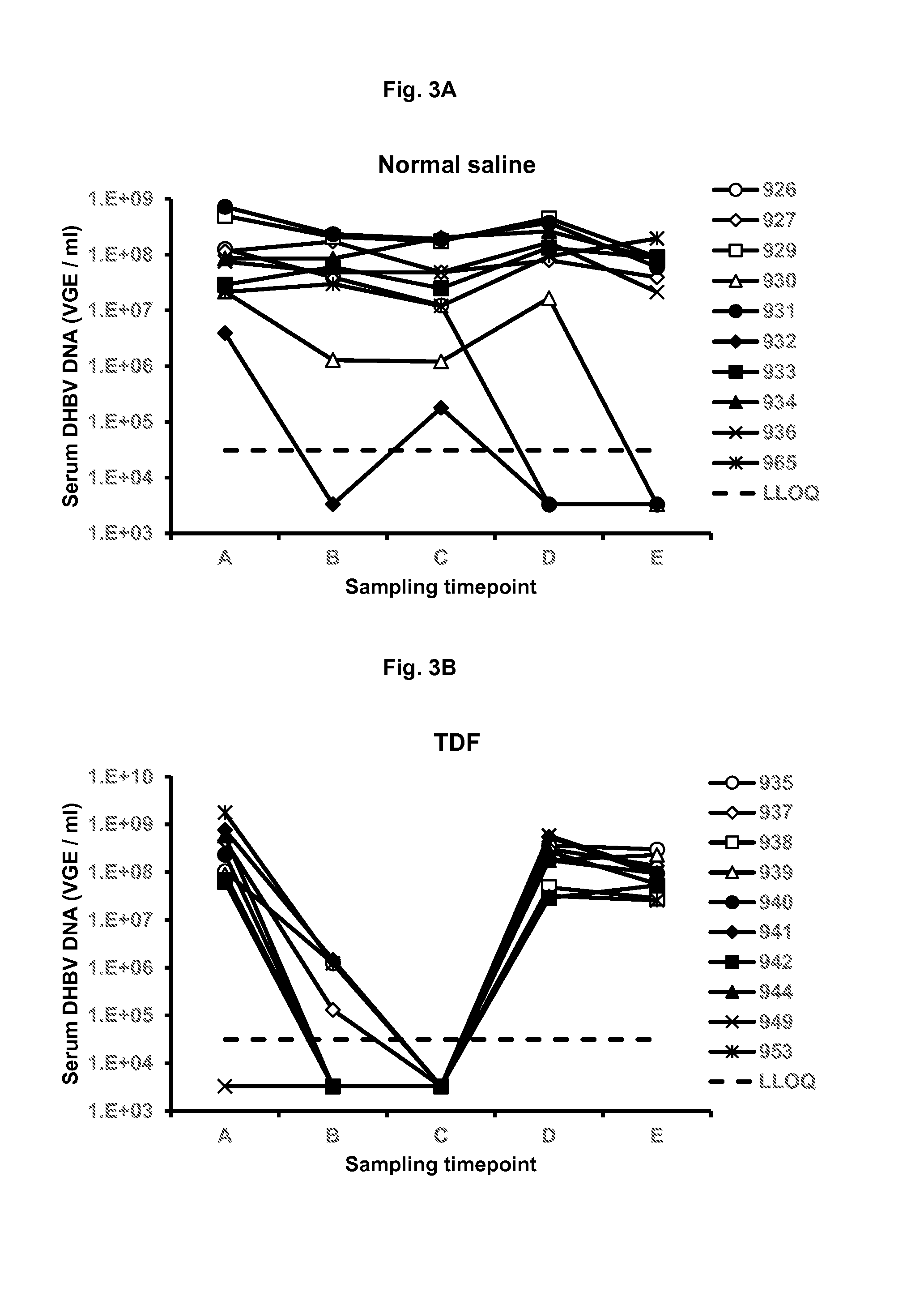
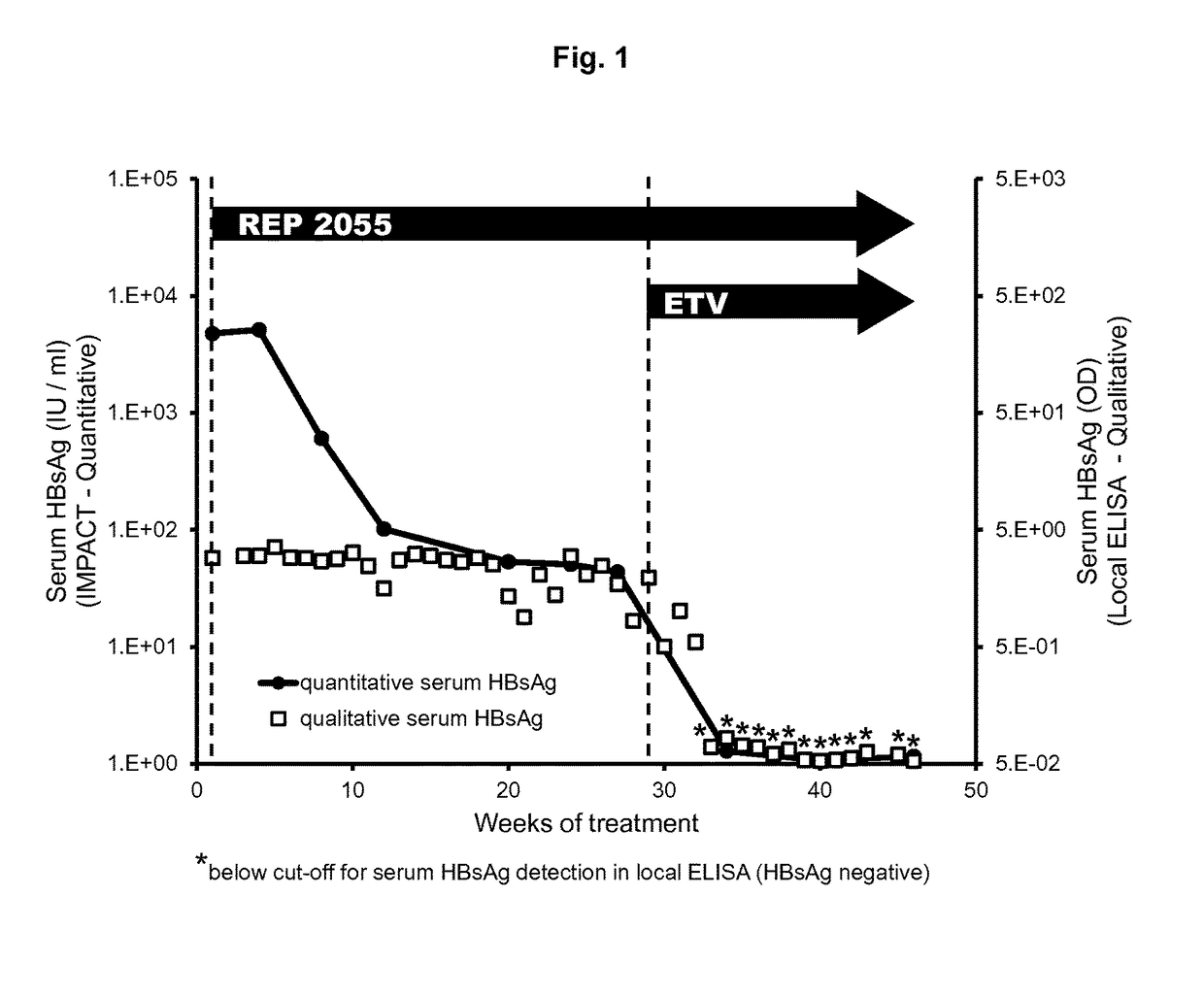
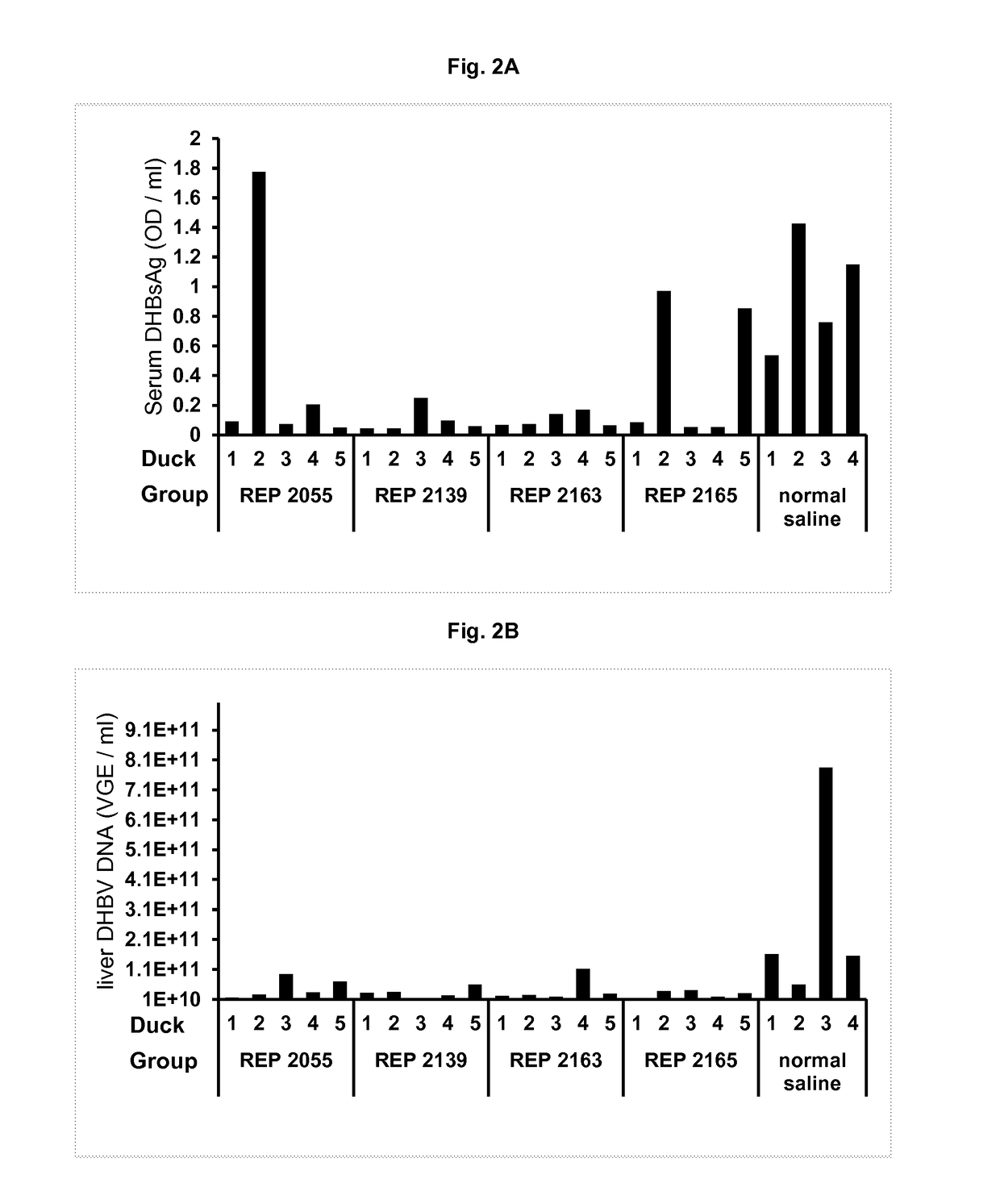
Patents
Literature
30 results about "Hepatitis D" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
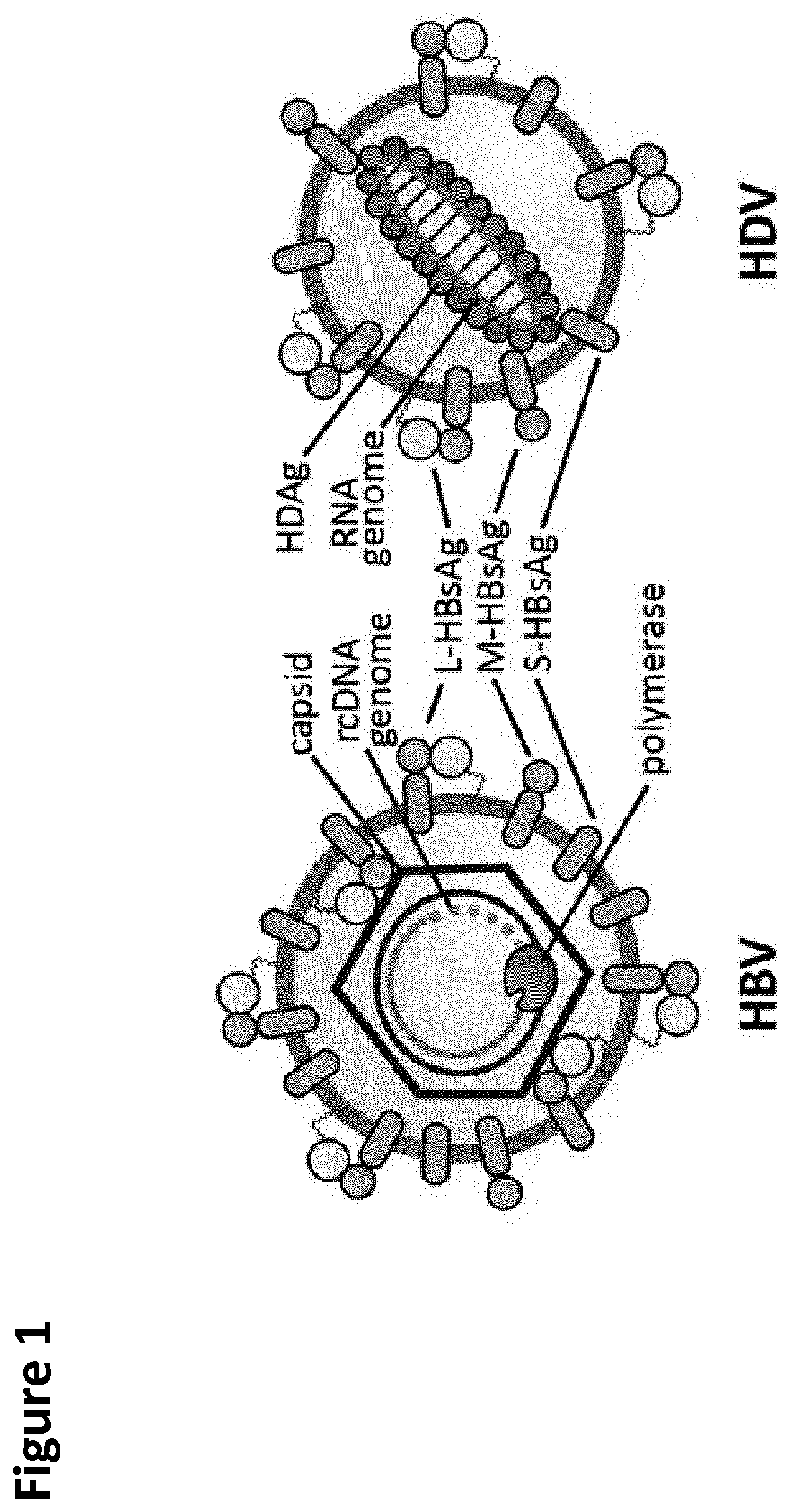
Hepatitis D (hepatitis delta) is a disease caused by the hepatitis D virus (HDV), a small spherical enveloped virusoid. This is one of five known hepatitis viruses: A, B, C, D, and E. HDV is considered to be a subviral satellite because it can propagate only in the presence of the hepatitis B virus (HBV). Transmission of HDV can occur either via simultaneous infection with HBV (coinfection) or superimposed on chronic hepatitis B or hepatitis B carrier state (superinfection).
Compositions and methods for treatment of viral diseases
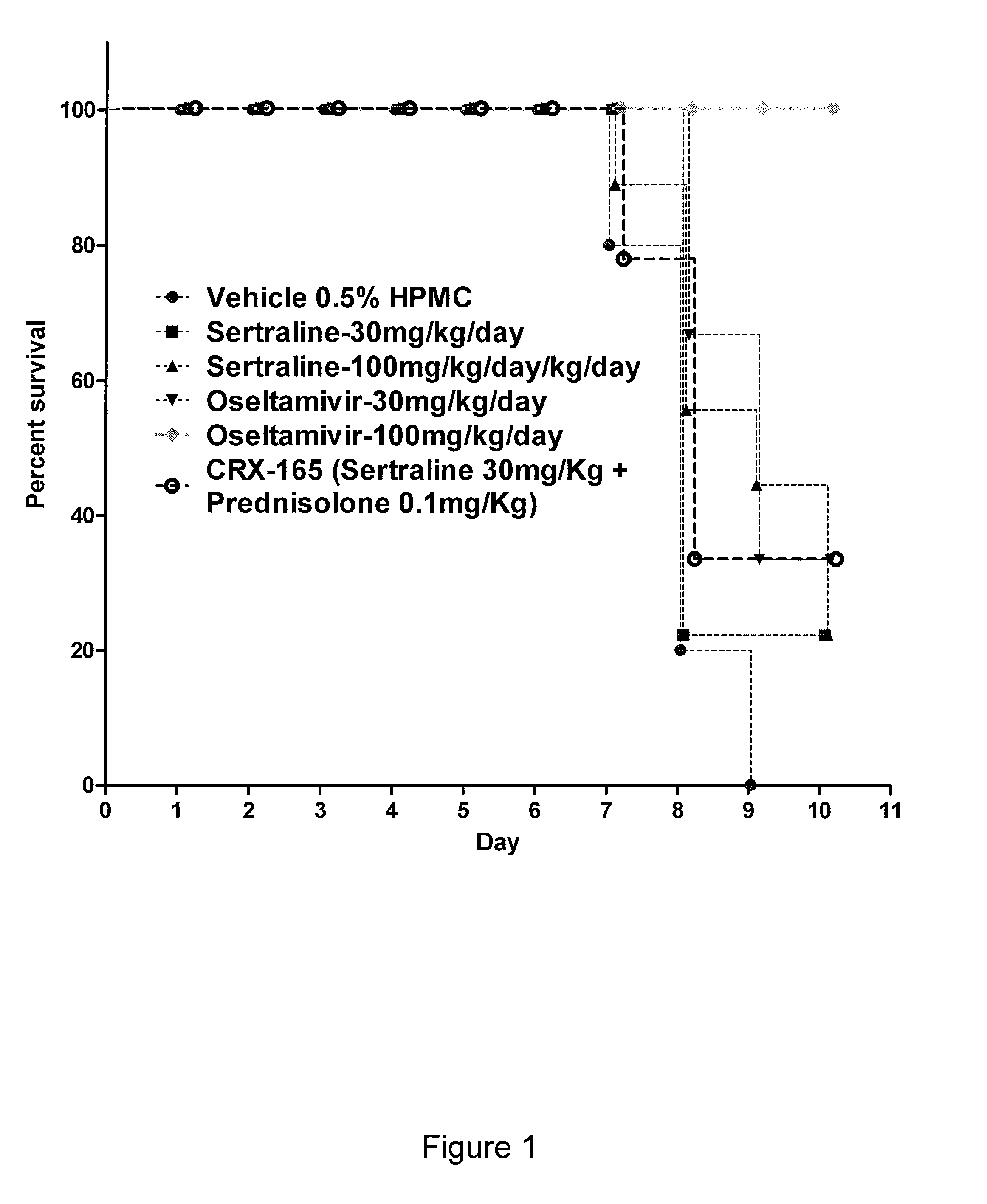
InactiveUS20080161324A1Slow and stop replicationReduce loadBiocideMicrobiological testing/measurementSingle-Stranded RNADisease
The present invention features compositions, methods, and kits useful in the treatment of viral diseases. In certain embodiments, the viral disease is caused by a single stranded RNA virus, a flaviviridae virus, or a hepatic virus. In particular embodiments, the viral disease is viral hepatitis (e.g., hepatitis A, hepatitis B, hepatitis C, hepatitis D, hepatitis E). Also featured are screening methods for identification of novel compounds that may be used to treat a viral disease.
Owner:EXCRX SINGAPORE PTE +1
Compositions and methods for treatment of viral diseases
InactiveUS20100009970A1Slow and stop replicationReduce loadBiocideNervous disorderSingle-Stranded RNADisease
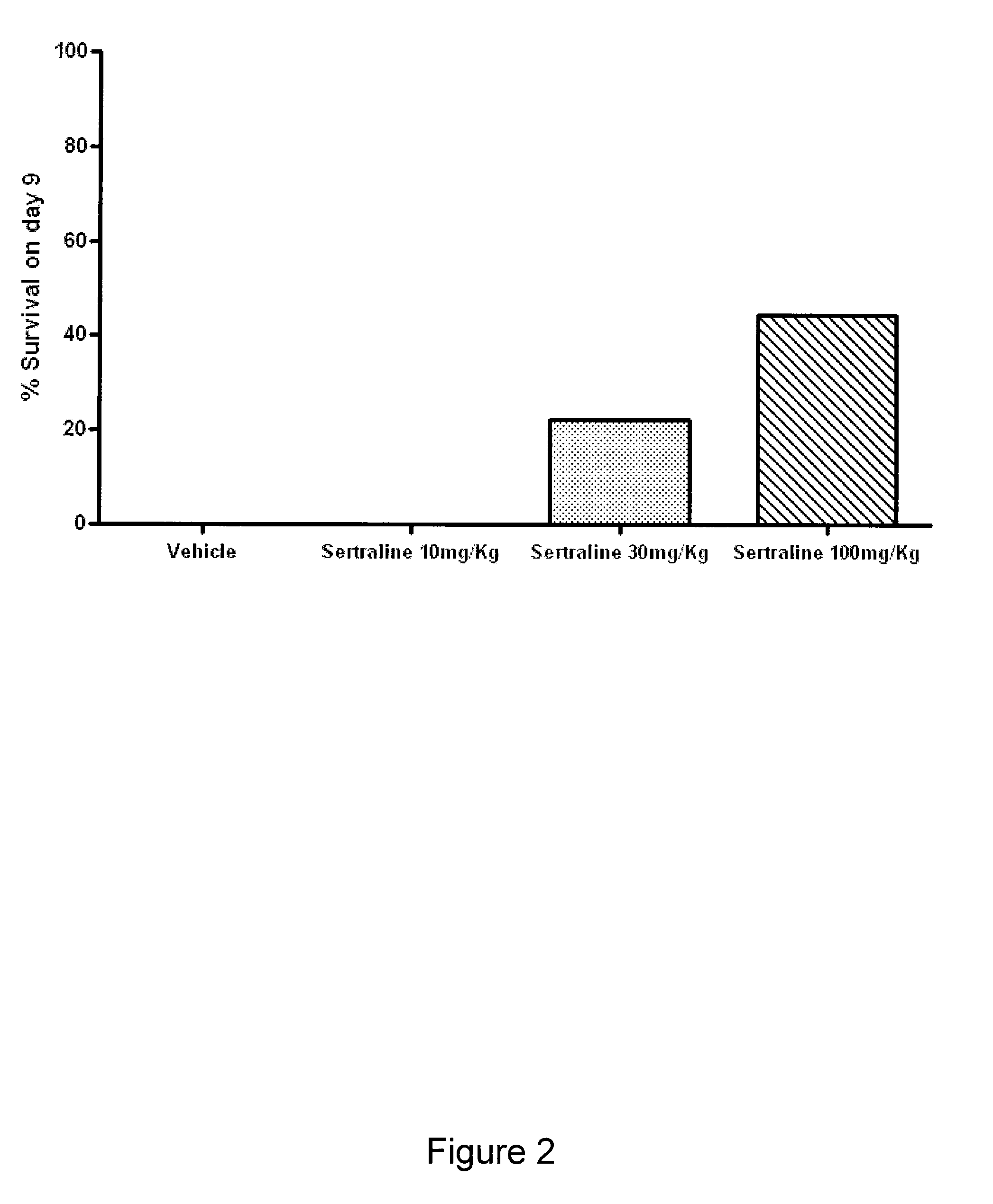
The present invention features compositions, methods, and kits useful in the treatment of viral diseases. In certain embodiments, the viral disease is caused by a single stranded RNA virus, a flaviviridae virus, or a hepatic virus. In particular embodiments, the viral disease is viral hepatitis (e.g., hepatitis A, hepatitis B, hepatitis C, hepatitis D, hepatitis E) and the agent or combination of agents includes sertraline, a sertraline analog, UK-416244, or a UK-416244 analog. Also featured are screening methods for identification of novel compounds that may be used to treat a viral disease.
Owner:EXCRX SINGAPORE PTE +1
Methods for the treatment of hepatitis b and hepatitis d infections
ActiveUS20140065102A1BiocideOrganic active ingredientsHepatitis D InfectionHepatitis B Surface Antigens
It is disclosed a method for the treatment of HBV infection or HBV / HDV co-infection, the method comprising administering to a subject in need of treatment a first pharmaceutically acceptable agent that removes the hepatitis B surface antigen from the blood and a second pharmaceutically acceptable agent which stimulates immune function.
Owner:REPLICOR INC
Method of treating hepatitis delta virus infection
InactiveUS7511027B2Lower Level RequirementsCut surfaceBiocidePeptide/protein ingredientsAntigenDeltaretrovirus Infections
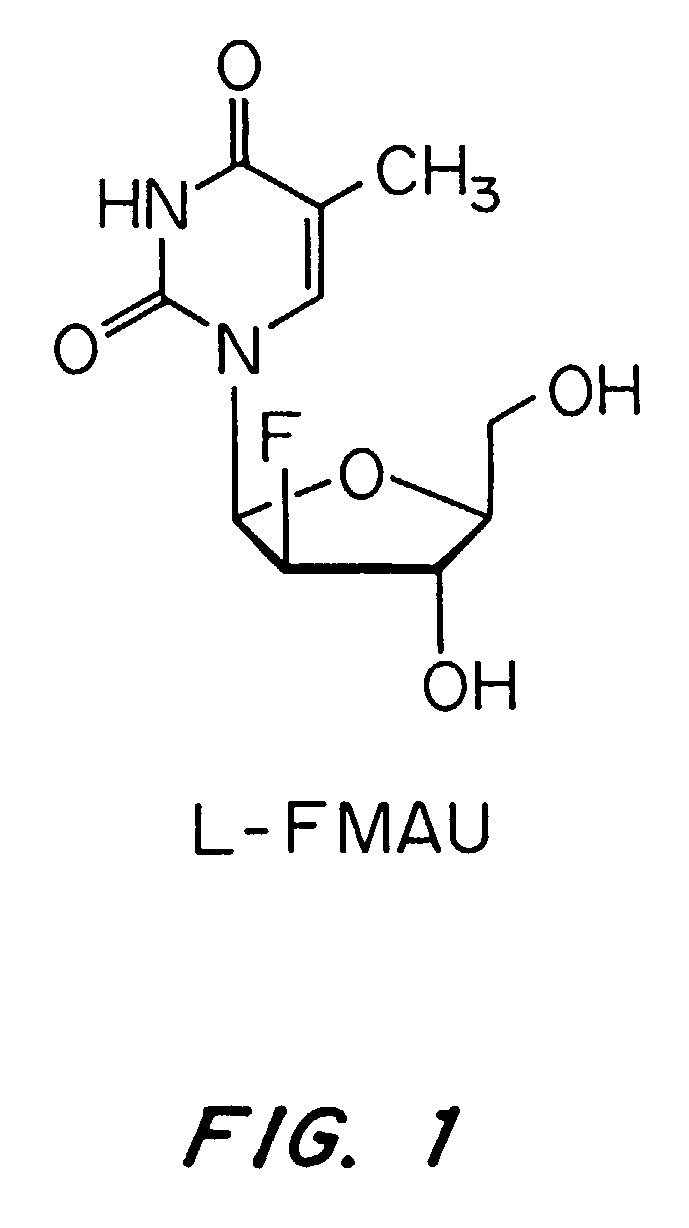
A method for the treatment for hepatitis delta infection in a host, that includes administering an effective amount of a nucleoside or a nucleoside analog that suppresses the expression of the hepatitis B surface or preS1 antigen in the host 100-fold or more relative to pretreatment values in vivo; or to not more than 1 microgram per milliliter in vivo. In a preferred embodiment, the nucleoside is L-FMAU, or a pharmaceutically acceptable salt or prodrug thereof.
Owner:GEORGETOWN UNIV +1
Methods for the treatment of hepatitis b and hepatitis d infections
It is disclosed a method for the treatment of hepatitis B (HBV) infection or HBV / hepatitis D (HDV) co-infection, the method comprising administering to a subject in need of treatment a first pharmaceutically acceptable agent that removes the hepatitis B surface antigen from the blood and a second pharmaceutically acceptable agent which stimulates immune function.
Owner:REPLICOR INC
Inactivated Poliomyelitis Vaccine Derived From Sabin Strain Of Polio Virus
InactiveUS20080193478A1Effective immunizationEffective and stableSsRNA viruses positive-senseViral antigen ingredientsAntigenHuman immunodeficiency
An inactivated Polio Vaccine derived from Sabin strain for safe and effective immunization against Poliomyelitis is provided. A process of preparation for such vaccine and formulations thereof are also provided. Administration of the vaccine of the present invention along with other antigens provides immunization not only against polio infection but also against other pathogens causing Hepatitis C. Hepatitis D. Hepatitis E. Meningitis A. Meningitis B. Meningitis C. Meningitis W. Meningitis Y. Pnemococcal (23 valent or more). Smallpox, Typhoid, Bacille Calmette Guerin, Tuberculosis. Human Immunodeficiency Virus. Anthrax or the like, to which children or adults not immunized earlier are susceptible, particularly to which children are susceptible.
Owner:PANACEA BIOTEC
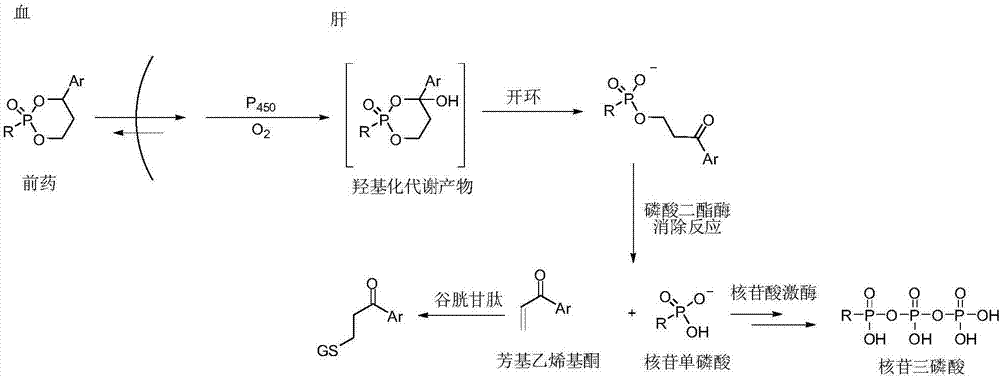
Liver specific delivery-based antiviral prodrug nucleoside cyclophosphate compound and use thereof
ActiveCN107540710AOrganic active ingredientsGroup 5/15 element organic compoundsDiseaseHuman immunodeficiency
The invention provides a liver specific delivery (LSD)-based antiviral prodrug nucleoside cyclophosphate compound and a use thereof and concretely, provides a compound shown in the formula II, its isomer, pharmaceutically acceptable salt, hydrate and solvate, and a corresponding pharmaceutical composition. The invention also provides a use of the compound or a composition of the compound and otherantiviral drugs in resistance to hepatitis B viruses (HBV), hepatitis D viruses (HDV) and human immunodeficiency viruses (HIV) and treatment on diseases caused by the above viruses.
Owner:ZHEJIANG PALOALTO PHARMA TECH CO LTD
Detection type gene chip for detecting various peptitis
InactiveCN1392268AImprove detection efficiencyReduce dosageMicrobiological testing/measurementGenotypeInfective disorder
The present invention provides a detection gene chip for diagnosing serveral kinds of hepatitis and new primer with high detection rate for using the gene chip in various subtype amplification. The gene chip includes detection quality controlling system and disease diagnosing system. The chip can be used to detect the virus ites of hepatitis B and hepatitis C and detect hepatitis D, hepatitis E, hepatitis G and TTV simultaneously. It has short detection period, high diagnosis accuracy and low diagnosis cost and may be used in epidemiological investigation and blood detection for identifying hepatitis gene type.
Owner:赵伟 +2
Methods for the treatment of hepatitis b and hepatitis d infections
It is disclosed a method for the treatment of hepatitis B (HBV) infection or HBV / hepatitis D (HDV) co-infection, the method comprising administering to a subject in need of treatment a first pharmaceutically acceptable agent that removes the hepatitis B surface antigen from the blood and a second pharmaceutically acceptable agent which stimulates immune function.
Owner:REPLICOR INC
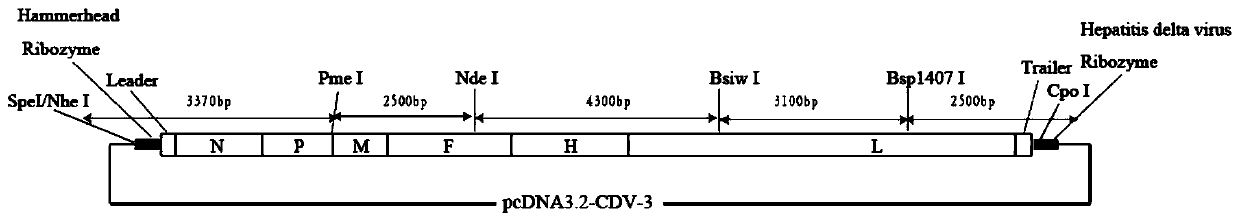
Canine distemper virus CDV-3 strain infectious cDNA cloning and constructing method and application thereof
ActiveCN110951778AFast growthStable and efficient rescueSsRNA viruses negative-senseVirus peptidesCanine distemper virus CDVCdna cloning
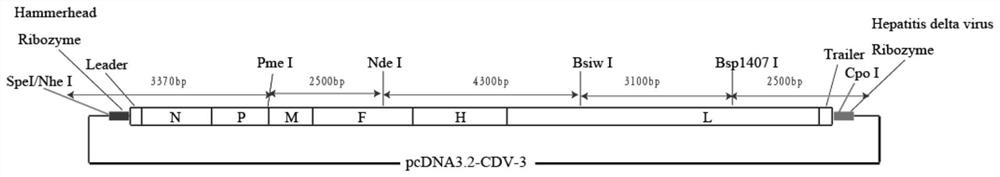
The invention discloses a canine distemper virus CDV-3 strain infectious cDNA cloning and constructing method and application thereof. A canine distemper virus CDV-3 strain is used as a template, theoverall length of the CDV-3 is divided into 5 fragments, and RT-PCR amplification is performed. Through enzyme-cutting splicing, the 5 fragments are sequentially inserted into a eukaryotic vector, besides, a hammerhead ribozyme sequence and a hepatitis D ribozyme sequence are respectively added to an F1 head end and an F5 tail end, and canine distemper virus CDV-3 strain infectious cDNA clone is obtained; then three auxiliary plasmids expressing CDV-3N, P and L proteins are constructed; the canine distemper virus CDV-3 infectious cDNA clone and the three auxiliary plasmids are used for performing cotransfection on 293T cells so that saved recombinant CDV-3 viruses (rCDV-3) are obtained. Through research, the highest titer of the obtained rCDV-3 virus can reach 107.667TCID50 / mL, which is 10times higher than that of wtCDV-3. After Vero cells are infected with the rCDV-3, within 36h after infection, the highest virus titer can be achieved; but after the Vero cells are infected with the wtCDV-3, within 72h after infection, the virus content can reach the highest value. A base is established for developing a canine distemper virus novel vaccine and studying the infective mechanism through the canine distemper virus CDV-3 strain infectious cDNA cloning and constructing method disclosed by the invention.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS
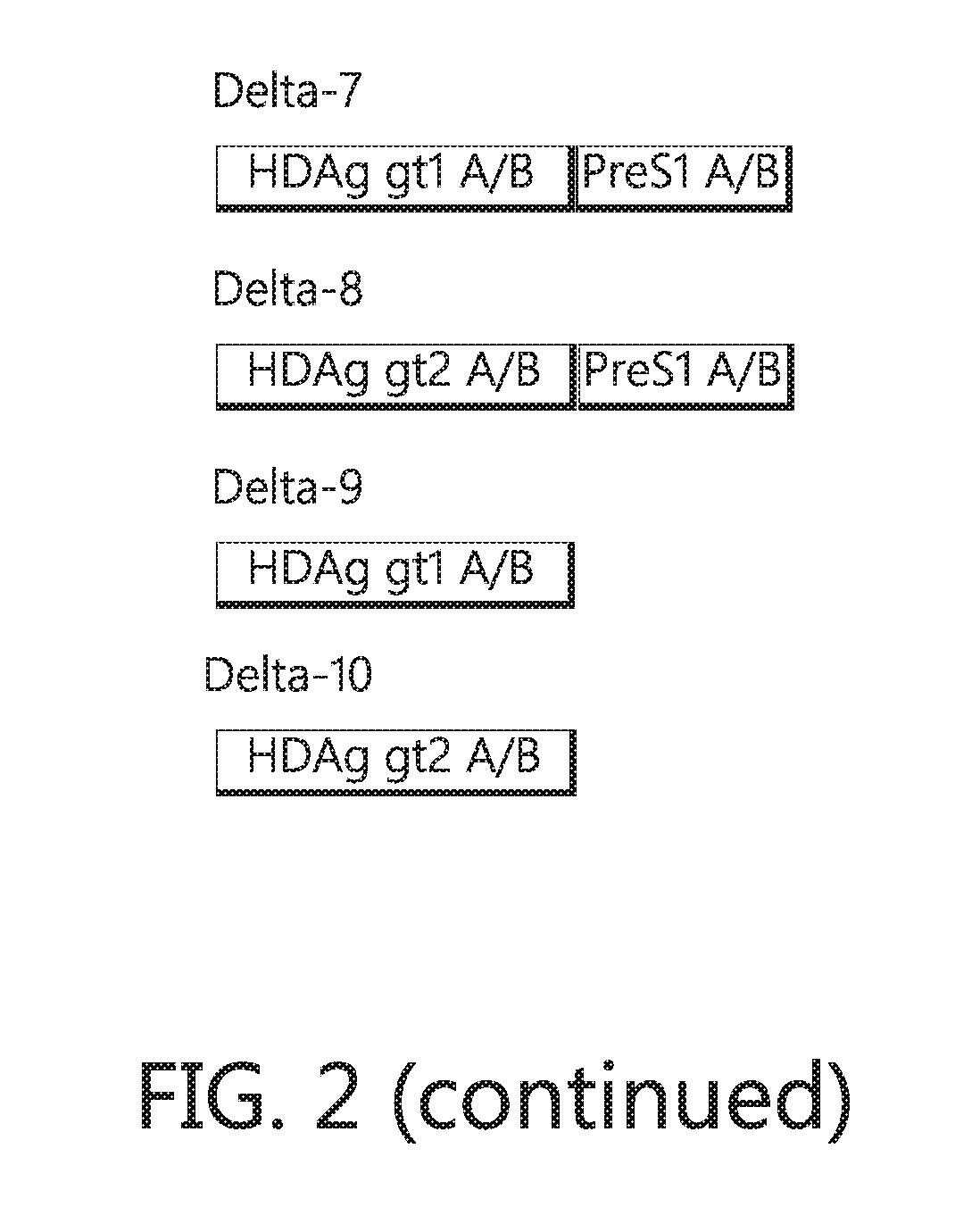
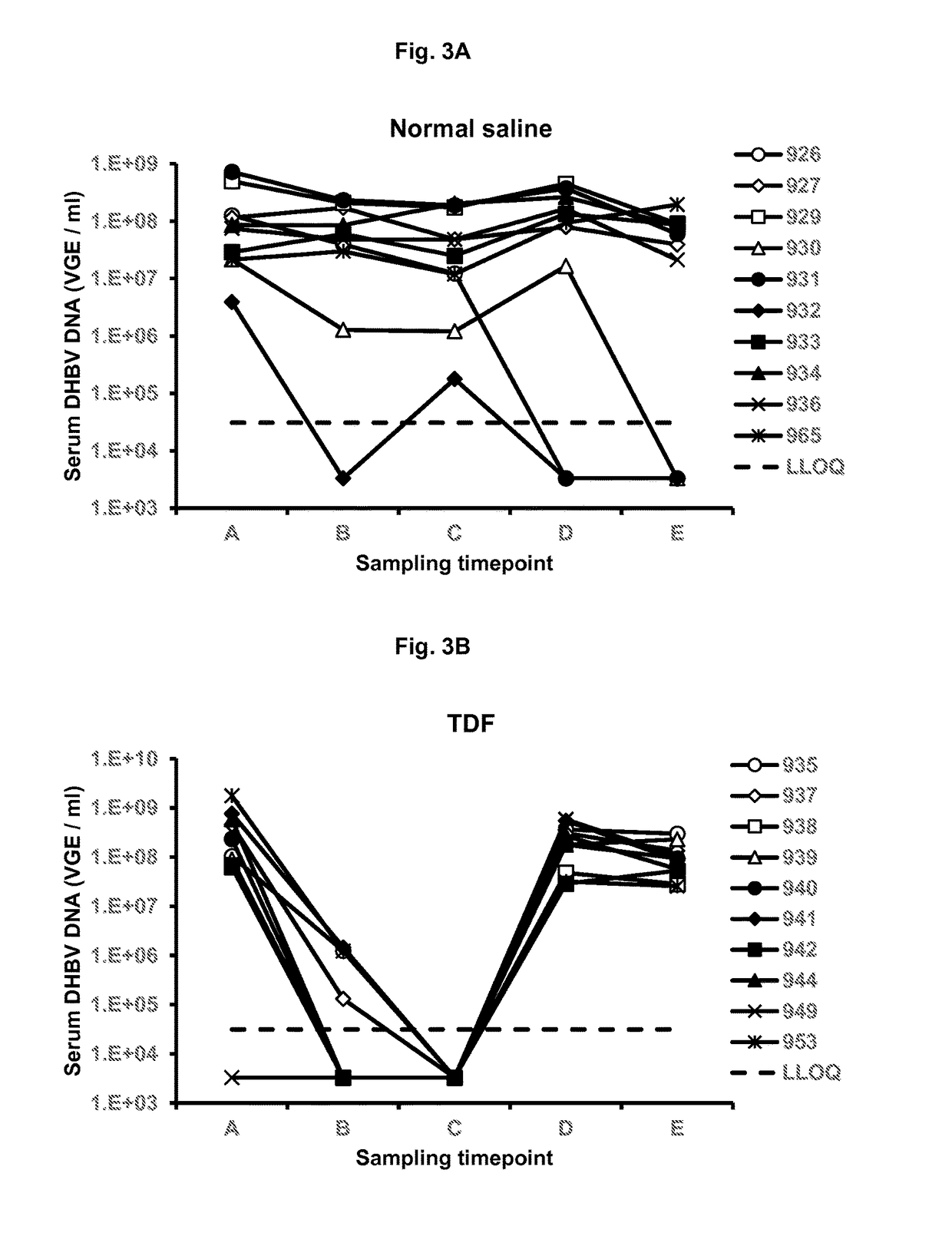
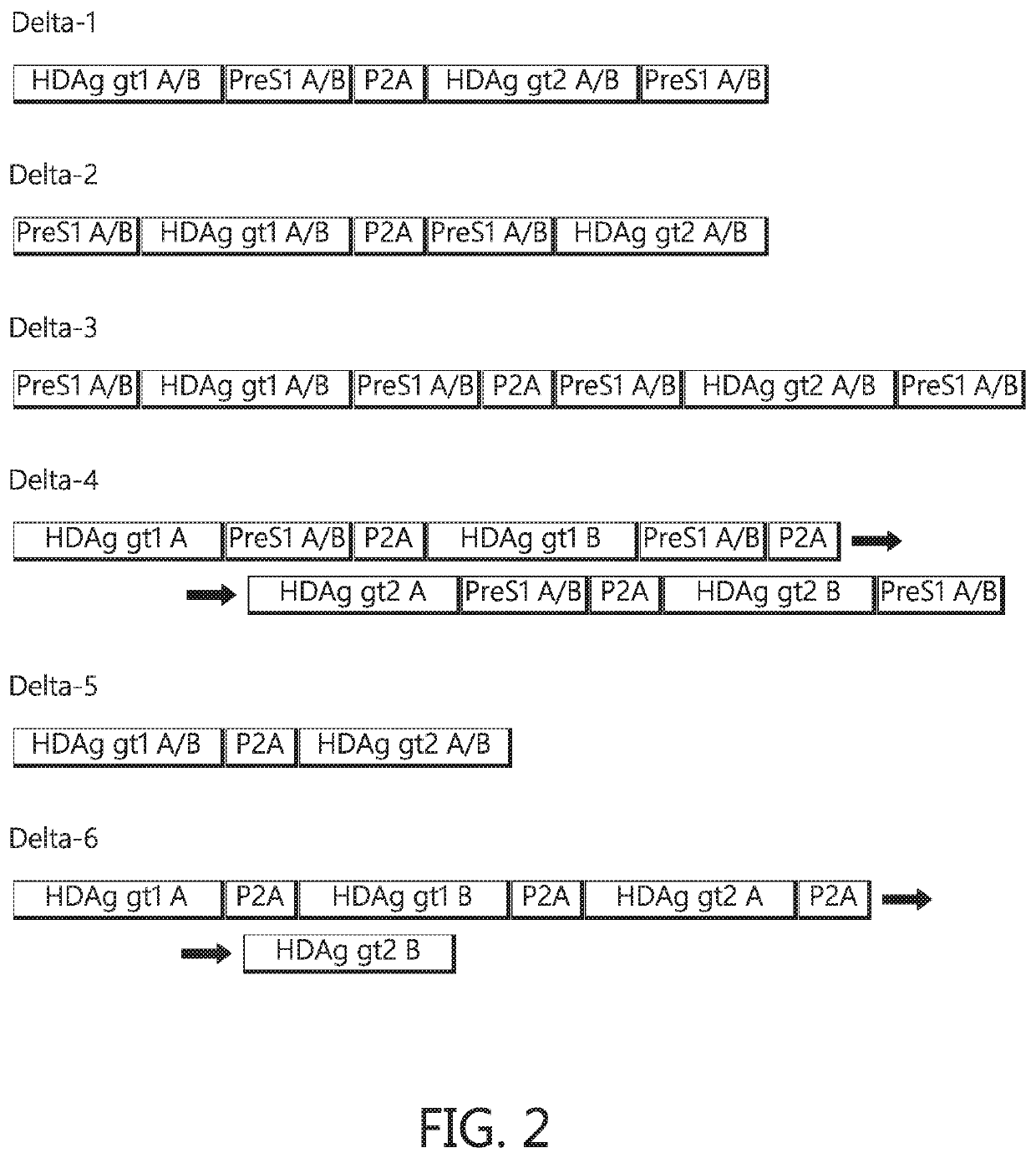
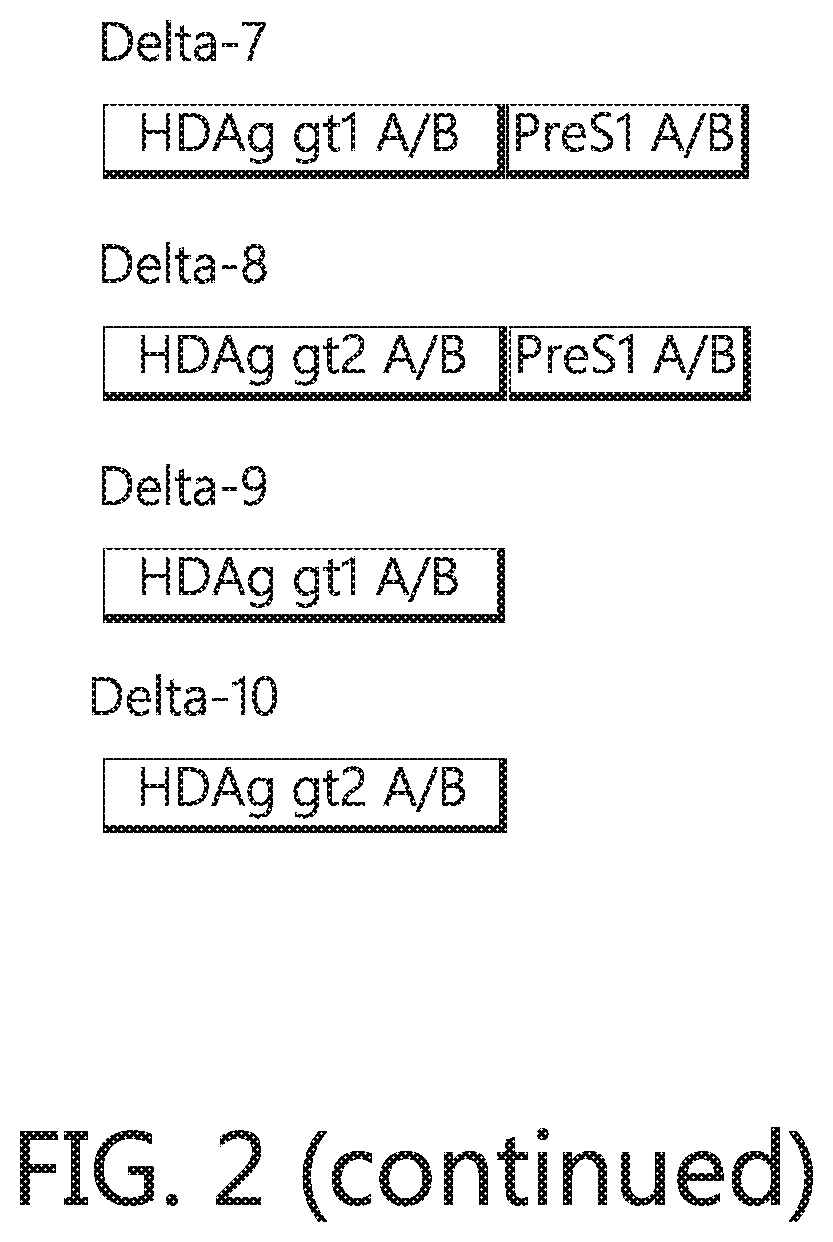
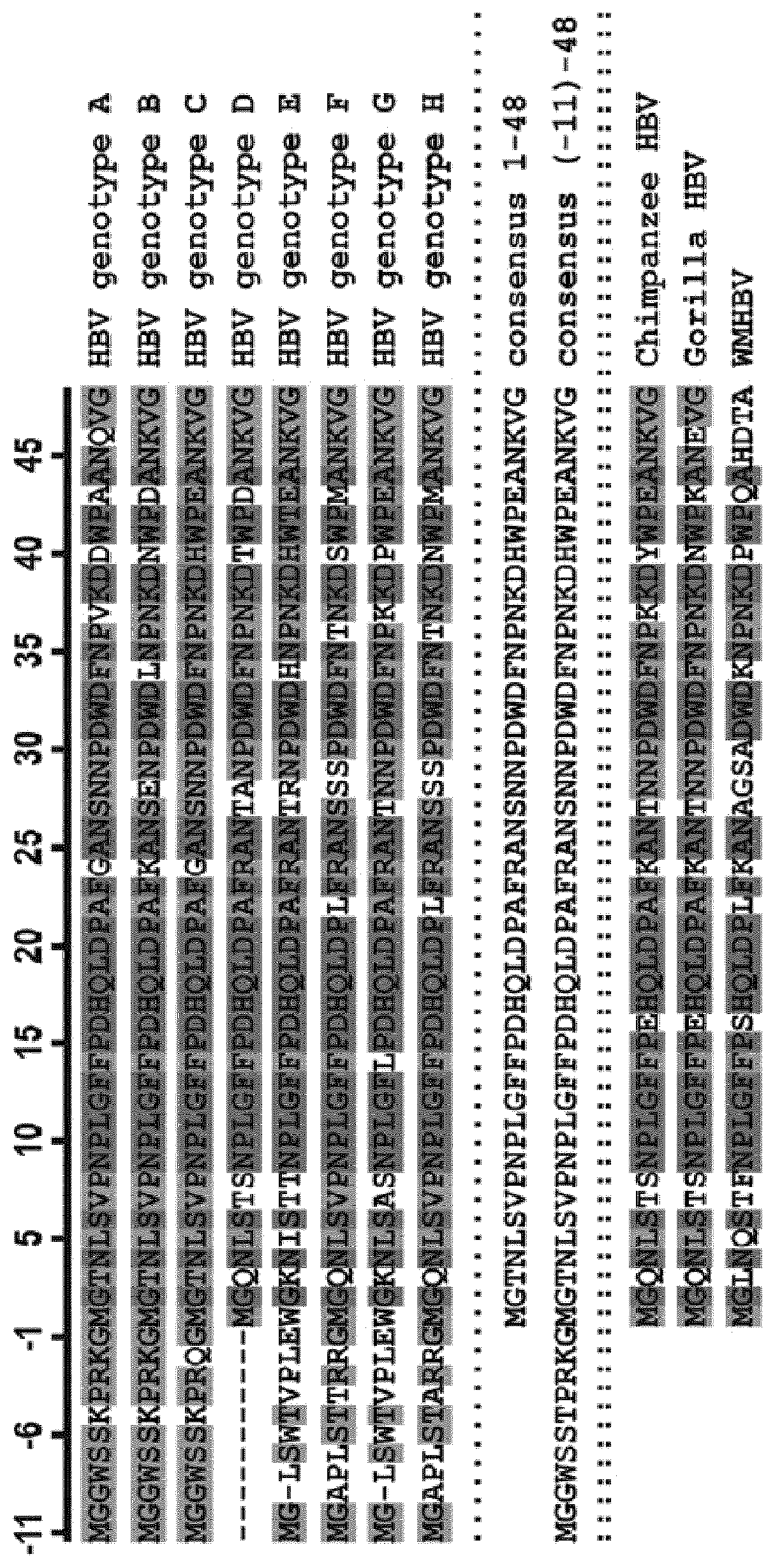
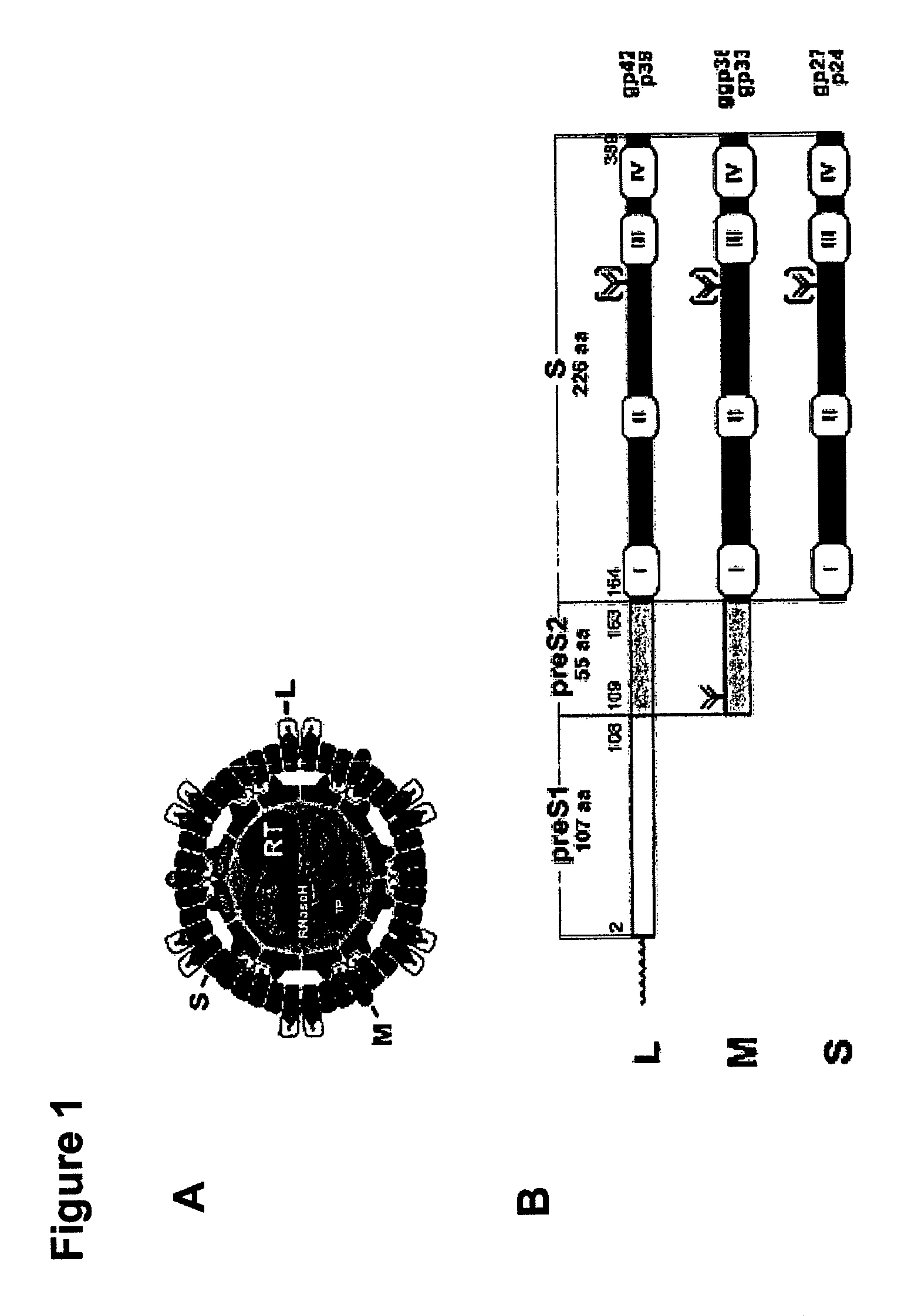
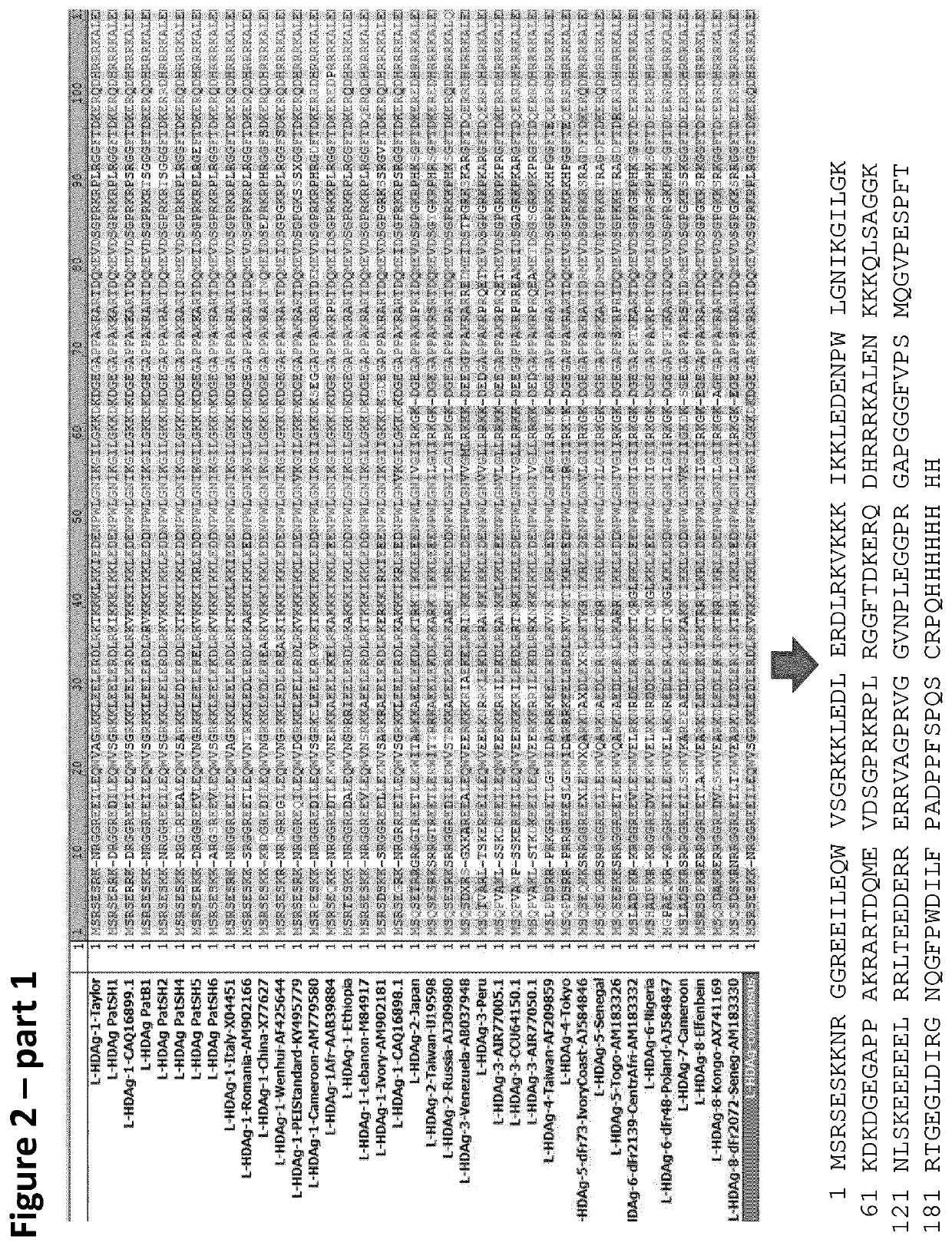
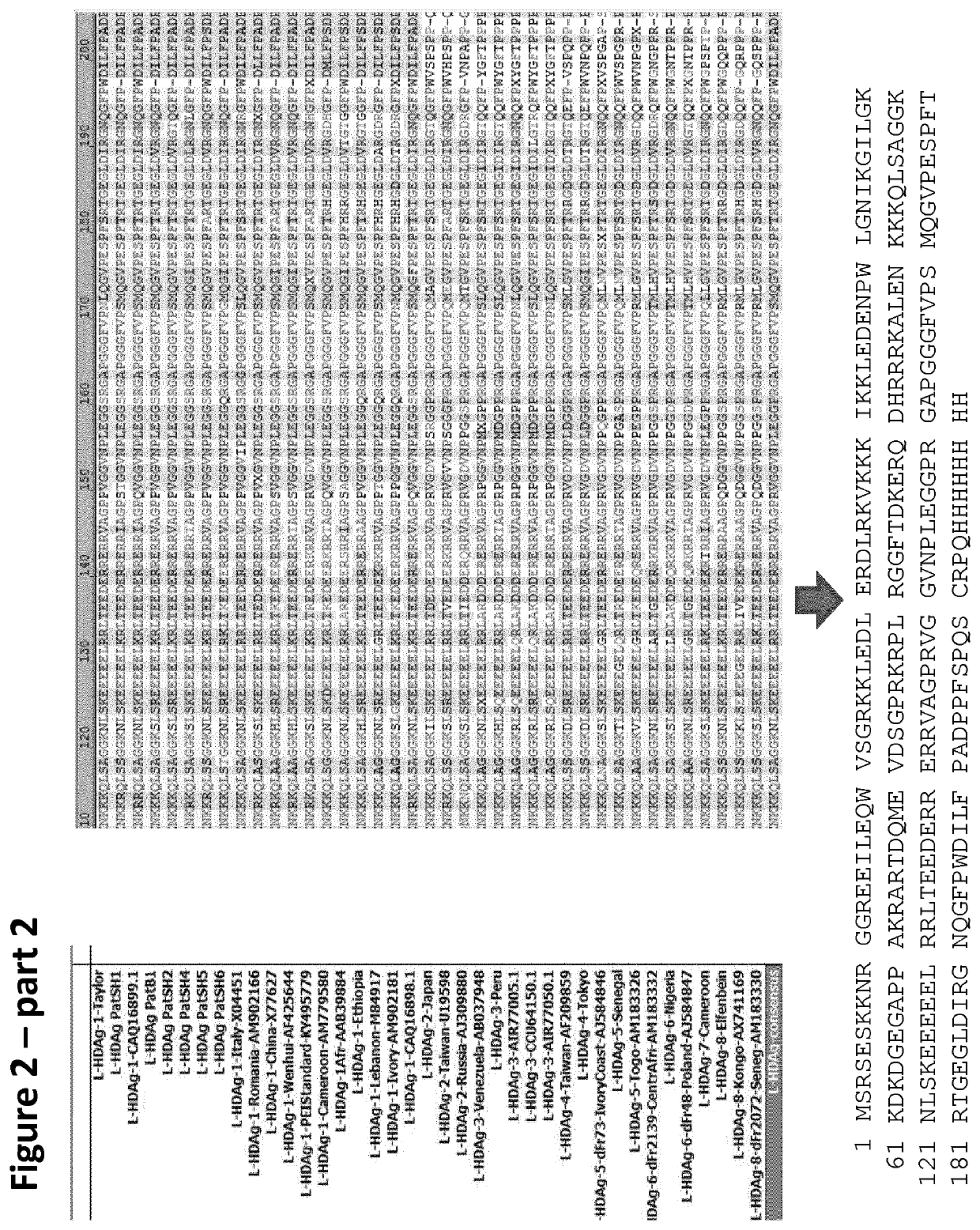
Chimeric hepatitis d virus antigen and hepatitis b virus pre s1 genes for use alone or in vaccines contaning hepatitis b virus genes
ActiveUS20190083607A1Stimulate immune responsePromotes adjuvant activitySsRNA viruses negative-senseAntibody mimetics/scaffoldsHepatitis DHepatitis B virus
Disclosed herein are chimeric genes, compositions of chimeric genes, and compositions of polypeptides that are useful for the generation, enhancement, or improvement of an immune response to a target antigen. Some embodiments of the compositions include chimeric genes encoding hepatitis D antigen (HDAg) protein in combination with one or more self-cleavage 2A polypeptides and a preS 1 polypeptide. In certain embodiments the self-cleavage polypeptide is P2A.
Owner:SVENSKA VACCINFABRIKEN PROD AB
Application of ozonized oil in liver disease prevention and treatment
InactiveCN106333960ANon-cytotoxicGood prevention effectInorganic active ingredientsDigestive systemFatty liverHepatitis D
The invention relates to application of ozonized oil in liver disease prevention and treatment and a drug combining with a liver disease treatment drug. Hepatitis B, hepatitis C, hepatitis D, hepatitis E, alcoholic hepatitis, drug-induced hepatitis, autoimmune hepatitis, liver fibrosis, liver cirrhosis and fatty liver are effectively prevented and treated.
Owner:侯建生
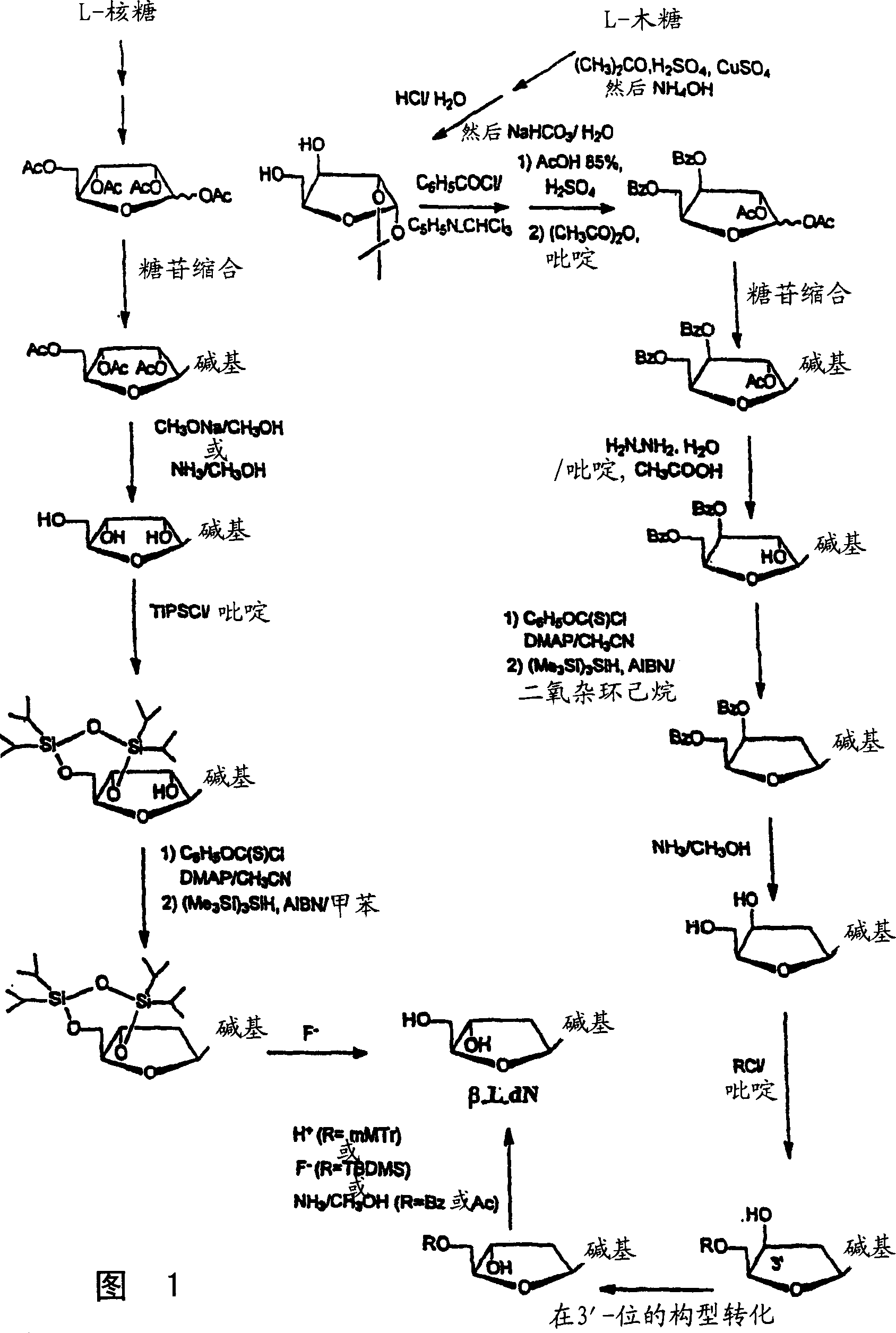
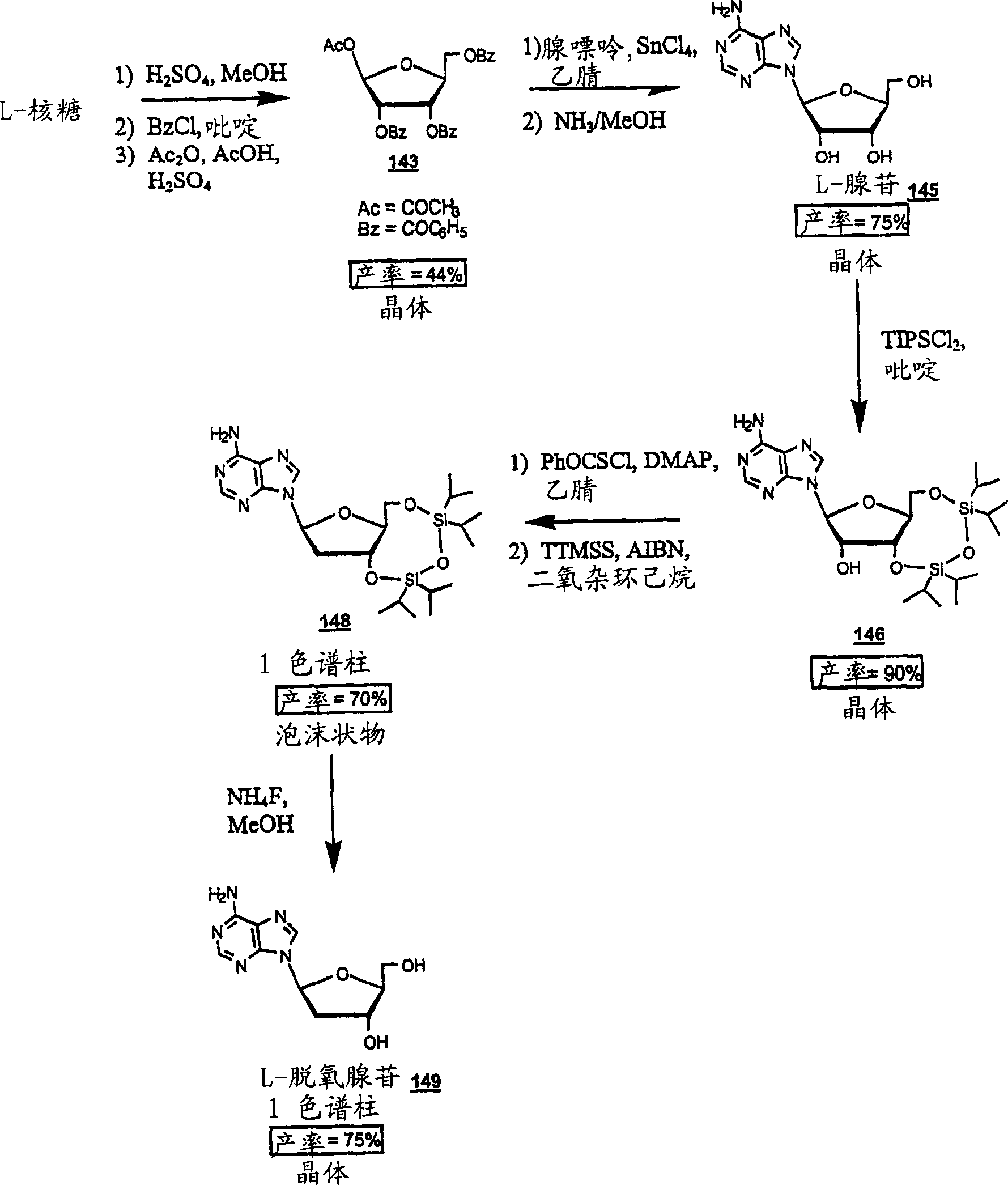
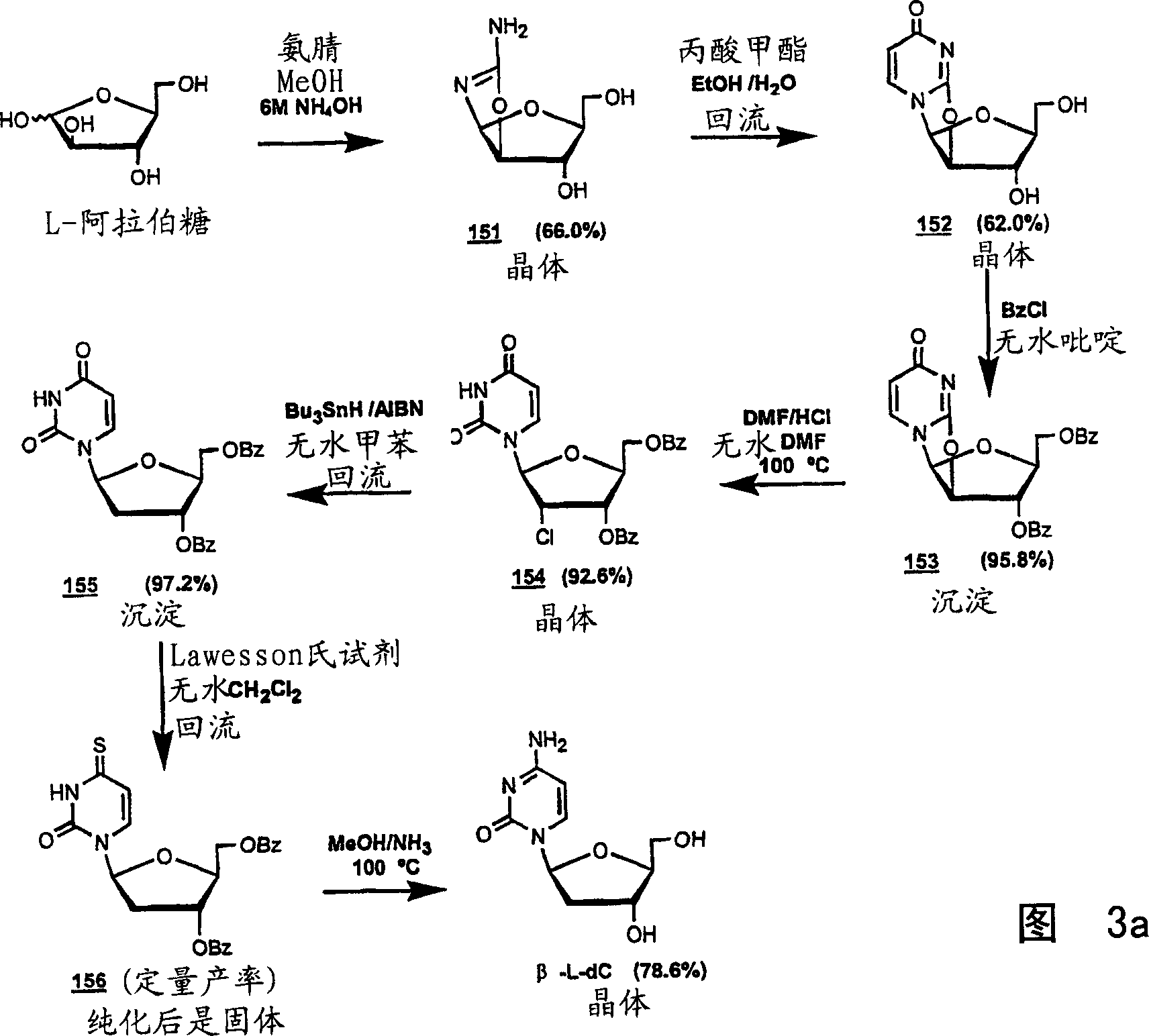
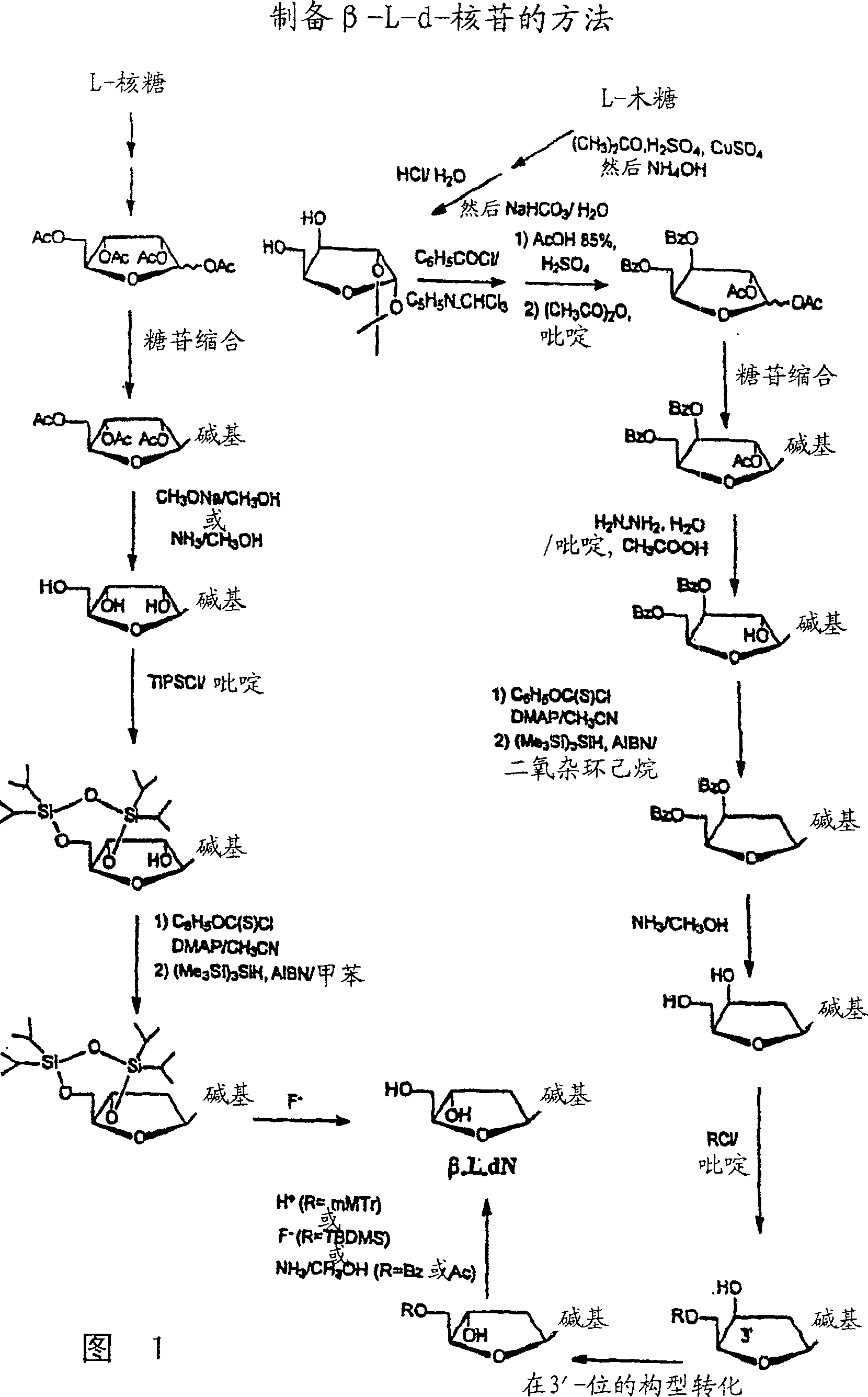
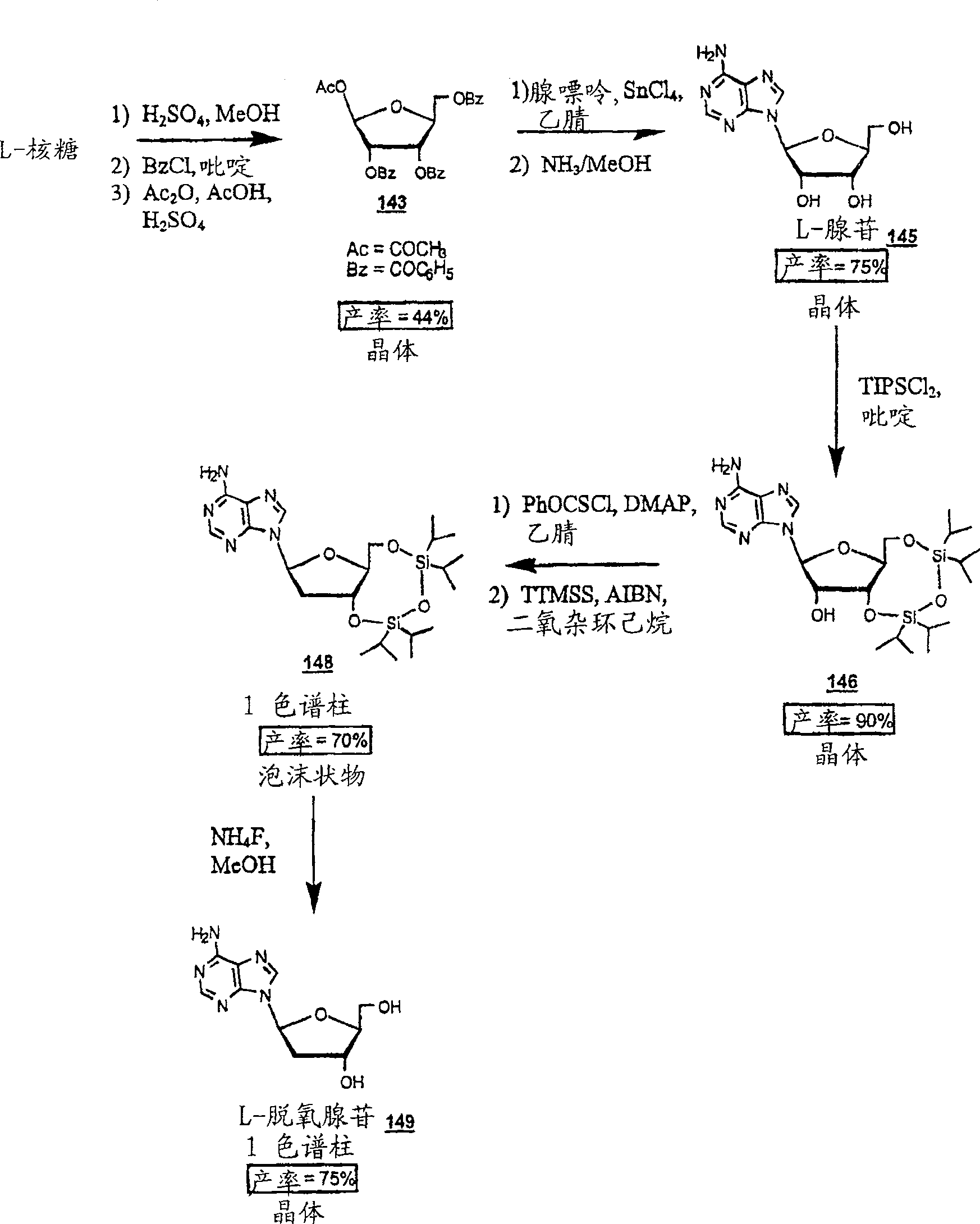
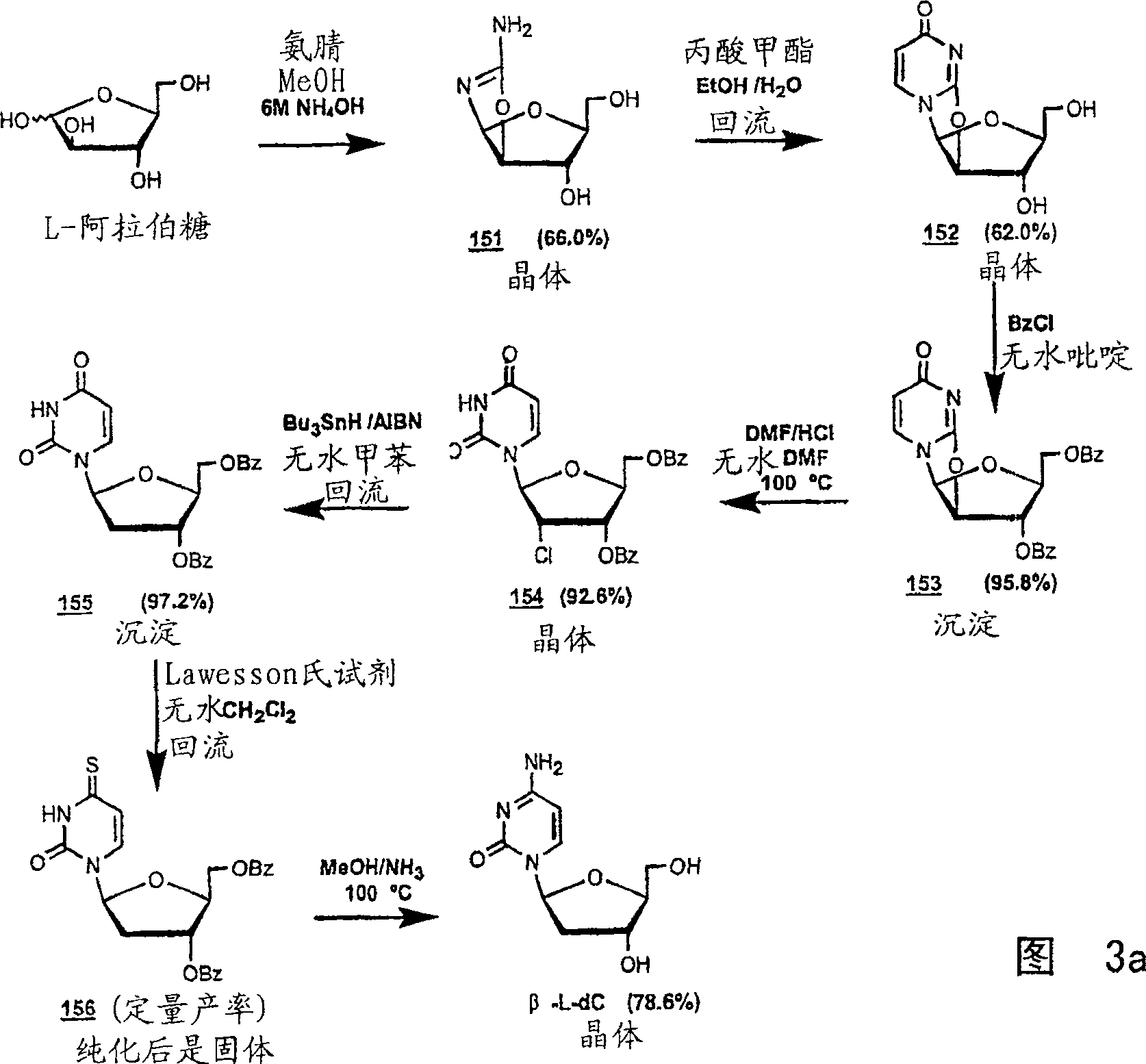
Methods for treating hepatitis delta virus infection with beta-L-2' deoxy-uncleosides
Owner:NOVARTIS AG
Methods for the treatment of hepatitis b and hepatitis d virus infections
It is disclosed a method for treating hepatitis B virus infection or hepatitis B virus / hepatits delta virus co-infection, the method comprising administering to a subject in need of such treatment a first pharmaceutically acceptable agent that comprises at least one phosphorothioated nucleic acid polymer and a second pharmaceutically acceptable agent that comprises at least one nucleoside / nucleotide analog HBV polymerase inhibitor.
Owner:REPLICOR INC
Medicine capable of treating hepatopathy
InactiveCN103948664AEffective treatmentImprove efficiencyDigestive systemUnknown materialsCalculus bovisToona sinensis
A disclosed medicine capable of treating hepatopathy comprises split-charged snorting composition, hot compress composition and oral composition; the snorting composition is melon pedicle powder; the hot compress composition is prepared by mixing and crushing 2-4 parts by weight of radix bupleuri, 2-4 parts by weight of catnip, 10-14 parts by weight of toona sinensis root bark, 0.02-0.1 part of calculus bovis and 0.5-2 parts of excrementum passeris; and the oral composition is a leaching liquid obtained by adding water into raw watermelon seeds and decocting. The provided medicine capable of treating hepatopathy is capable of effectively treating various hepatopathy, such as hepatitis A, hepatitis B, hepatitis C, hepatitis D, alcoholic fatty liver, liver cirrhosis early stage, icteric hepatitis and persistent hepatitis.
Owner:任德标
Methods for the treatment of hepatitis B and hepatitis D virus infections
It is disclosed a method for treating hepatitis B virus infection or hepatitis B virus / hepatitis delta virus co-infection, the method comprising administering to a subject in need of such treatment a first pharmaceutically acceptable agent that comprises at least one phosphorothioated nucleic acid polymer and a second pharmaceutically acceptable agent that comprises at least one nucleoside / nucleotide analog HBV polymerase inhibitor.
Owner:REPLICOR INC
Chimeric hepatitis D virus antigen and hepatitis B virus pre S1 genes for use alone or in vaccines containing hepatitis B virus genes
ActiveUS10905760B2SsRNA viruses negative-senseViral antigen ingredientsHepatitis B immunizationHepatitis D
Disclosed herein are chimeric genes, compositions of chimeric genes, and compositions of polypeptides that are useful for the generation, enhancement, or improvement of an immune response to a target antigen. Some embodiments of the compositions include chimeric genes encoding hepatitis D antigen (HDAg) protein in combination with one or more self-cleavage 2A polypeptides and a preS 1 polypeptide. In certain embodiments the self-cleavage polypeptide is P2A.
Owner:SVENSKA VACCINFABRIKEN PROD AB
Method for rapidly constructing reverse genetic strain of duck hepatitis A virus type 3
PendingCN110295148AGood genetic stabilityCapable of proliferatingSsRNA viruses positive-senseVirus peptidesDuck hepatitis A virusSubgenomic replicon
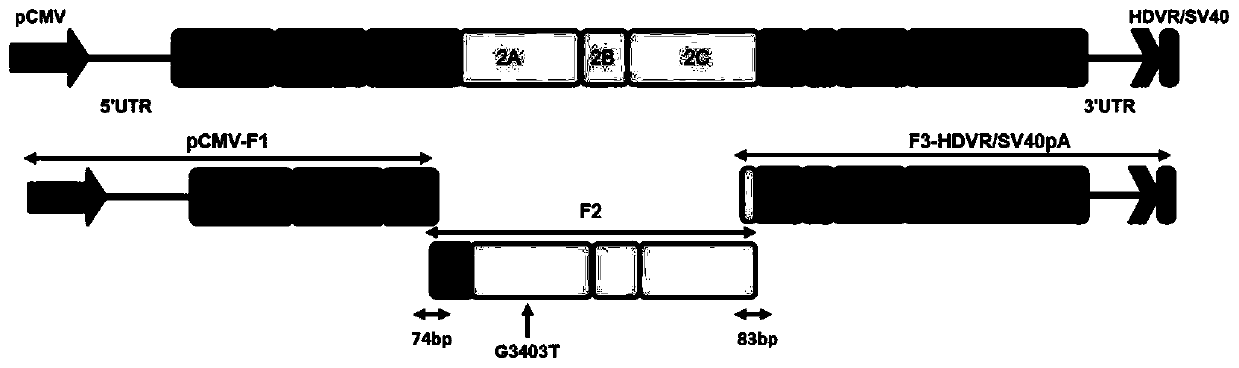
The invention relates to a method for rapidly constructing a reverse genetic strain of a duck hepatitis A virus type 3. A whole genome of the duck hepatitis A virus type 3 is divided into three segments with similar size for PCR amplification, a cytomegalovirus immediate early promoter is added at a 5' end of the first segment, synonymous mutation is introduced into a 2A gene of the second segmentto serve as a genetic marker locus, a hepatitis delta virus ribozyme sequence and an SV40 early mRNA polyadenylation signal sequence are added to a 3' end of the third segment of the genome of the virus, the construction of an infective subgenome replicon of the duck hepatitis A virus type 3 is completed, the infective subgenome replicon is mixed with a transfection reagent to transfect an original duck embryo fibroblast, the replicon is spontaneously recombined, and finally, a duck hepatitis A virus type 3 with a genetic marker is obtained. By means of the method, the mutant strain of the duck hepatitis A virus type 3 can be quickly obtained, and a favorable tool is provided for studying the pathogenic mechanism of the duck hepatitis A virus type 3 and developing a novel vaccine.
Owner:SICHUAN AGRI UNIV
Infectious cDNA clone of canine distemper virus cdv-3 strain and its construction method and application
ActiveCN110951778BFast growthStable and efficient rescueSsRNA viruses negative-senseVirus peptidesCanine distemper virus CDVTGE VACCINE
The invention discloses an infectious cDNA clone of canine distemper virus CDV‑3 strain, a construction method and application thereof. In the present invention, the canine distemper virus CDV-3 strain is used as a template, and the full length of CDV-3 is divided into five fragments for RT-PCR amplification. After enzyme digestion and splicing, the 5 fragments were sequentially inserted into the eukaryotic vector, and the sequences of hammerhead ribozyme and hepatitis D ribozyme were added to the head of F1 and the end of F5, respectively, to obtain the infection of canine distemper virus CDV‑3 strain sex cDNA clones. Then, construct three helper plasmids expressing CDV-3N, P, L protein. The infectious cDNA clone of canine distemper virus CDV‑3 strain and three helper plasmids were co-transfected into 293T cells to obtain rescued recombinant CDV‑3 virus (rCDV‑3). The study found that rCDV‑3 viral titers were obtained up to 10 7.667 TCID 50 / mL, 10 times higher than wtCDV‑3. After infection of Vero cells with rCDV‑3, the highest virus titer was rapidly reached at 36 hours after infection, while the virus content of wtCDV‑3 reached the highest at 72 hours after infection. The proposal of the invention lays a foundation for the development of a novel vaccine against canine distemper virus and the study of its pathogenic mechanism.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS
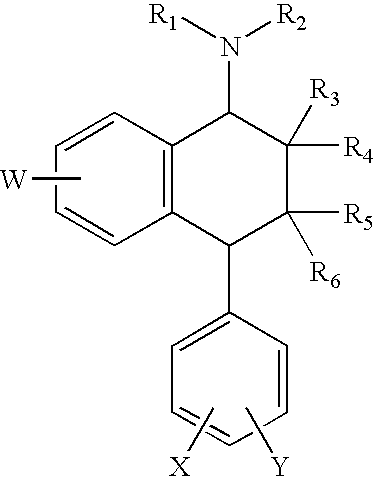
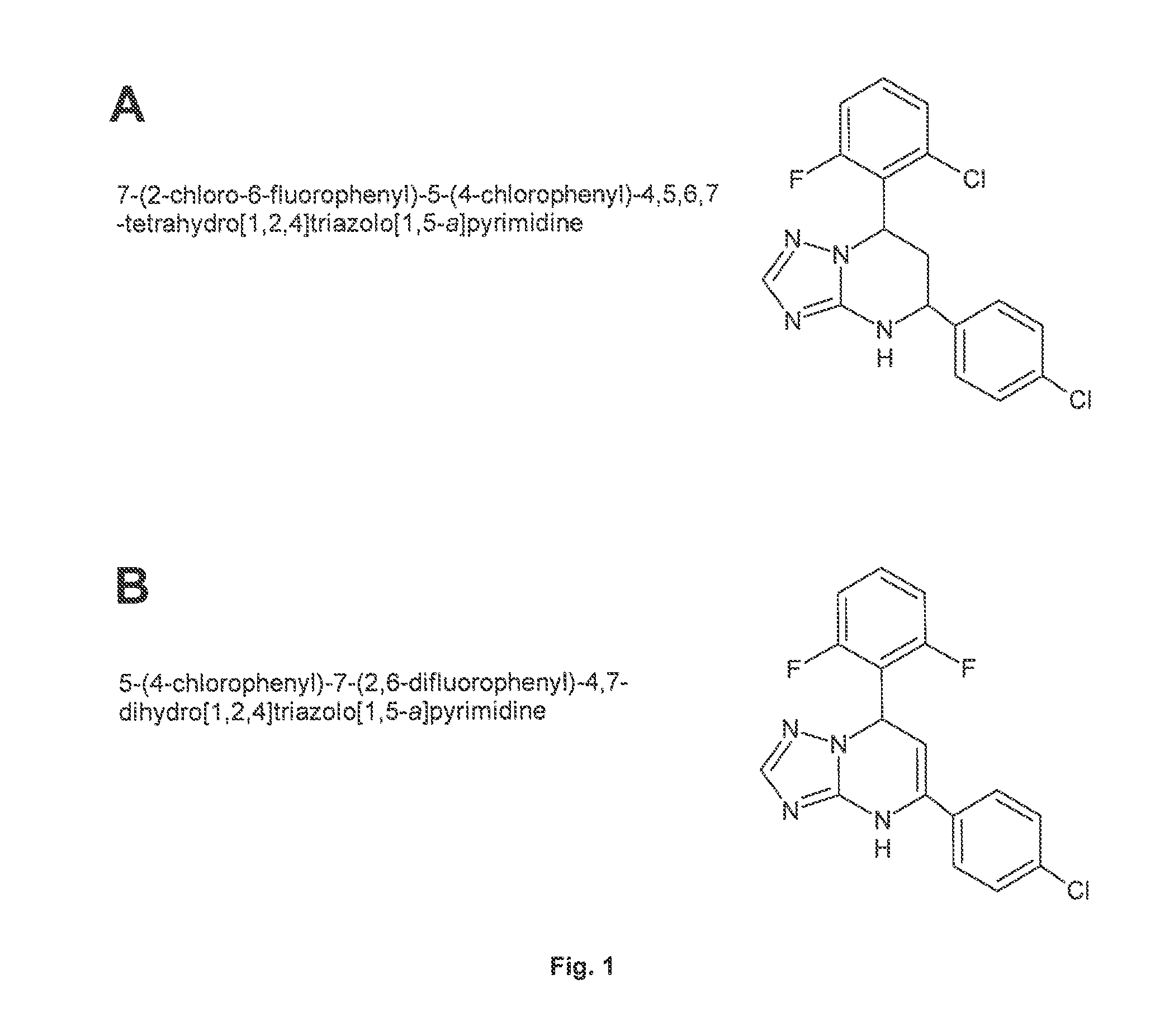
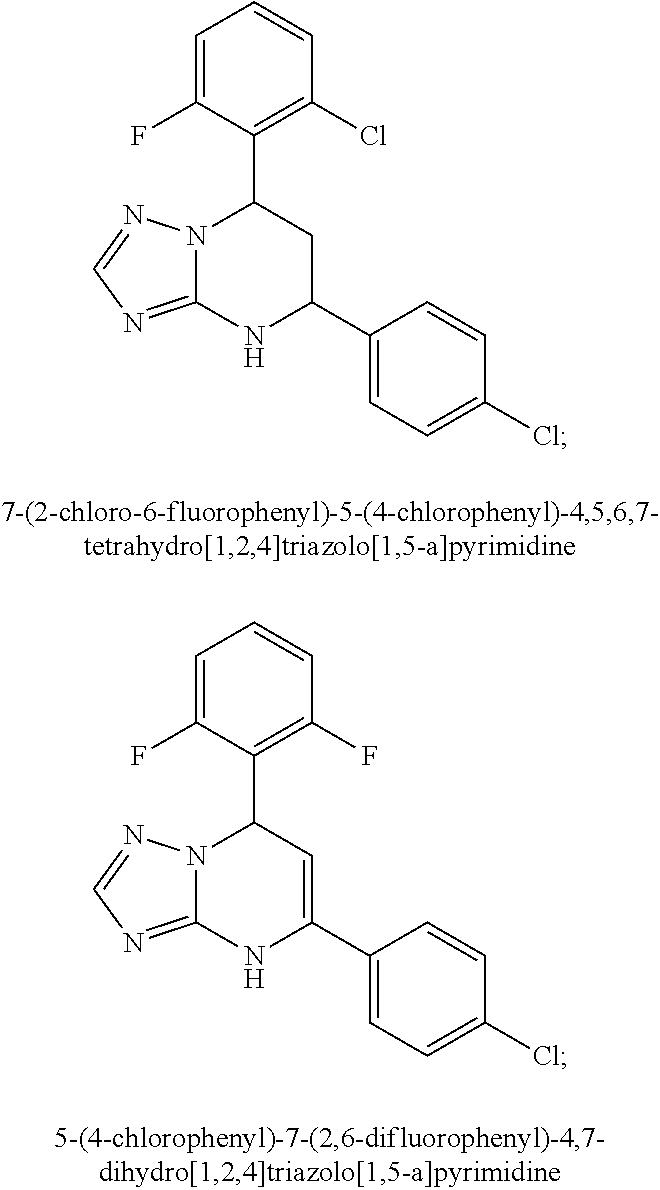
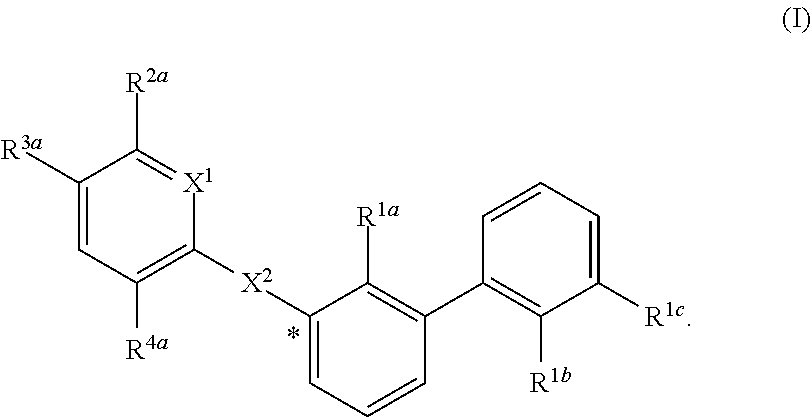
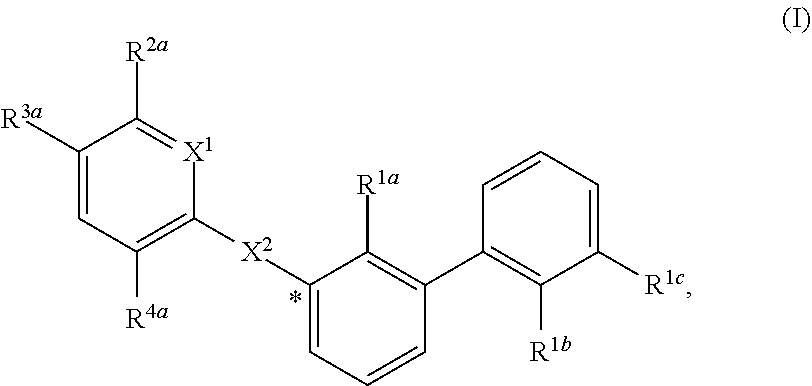
Substituted 1,1'-biphenyl compounds, analogues thereof, and methods using same
PendingUS20210052585A1Treating and preventing hepatitis virus infectionUseful in treatmentOrganic chemistryAntiviralsHepatitis B immunizationHepatitis D
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Compositions and methods for treating viral infections
PendingUS20210395751A1Reduce accumulationReduce concentrationSsRNA viruses negative-senseOrganic active ingredientsHepatitis DHepatitis
The present disclosure relates to compositions and methods for treating viral infections and in particular for treating hepatitis B and hepatitis D viral infections. A method of treating a hepatitis B virus infection in a subject the method comprising administering to the subject an inhibitor wherein the inhibitor inhibits or suppresses a regulator of liver lipid metabolism in the subject.
Owner:THE UNIV OF SYDNEY +2
Methods for treating hepatitis delta virus infection with beta-L-2' deoxy-uncleosides
Owner:NOVARTIS AG
Combination therapy of hbv and hdv infection
PendingUS20220040178A1SsRNA viruses negative-senseCell receptors/surface-antigens/surface-determinantsCholic acidNucleotide
Owner:MYR
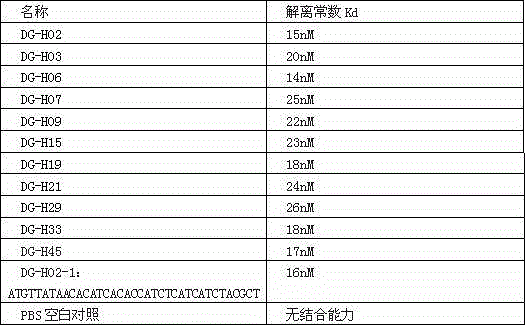
Nucleic acid aptamer combined with hepatitis delta virus and application thereof
InactiveCN104694544ALow costSimple methodPharmaceutical non-active ingredientsDNA/RNA fragmentationAntigenSingle-Stranded RNA
The invention discloses a nucleic acid aptamer targeted to the human-infected hepatitis delta virus and the sequence thereof and belongs to the field of genetic engineering and biological medicine. The new combinatorial chemistry technology SELEX is adopted, hepatitis delta virus protein serves as target protein, and the RNA aptamer capable of being combined with hepatitis delta virus surface antigen in a specific mode is screened out from a single-chain RNA random library. A special stem-loop structure can be formed by the aptamer in a random sequence area, and the aptamer can be combined with the hepatitis delta virus surface antigen in a specific and high-affinity mode or combined with a hepatic cell infected with the hepatitis delta virus in a targeted mode. The RNA aptamer provides a peculiarly efficient labelled molecule for the hepatitis delta diagnosis and treatment field and provides a new choice for developing a hepatitis delta diagnostic reagent and therapeutic drugs.
Owner:刘红卫
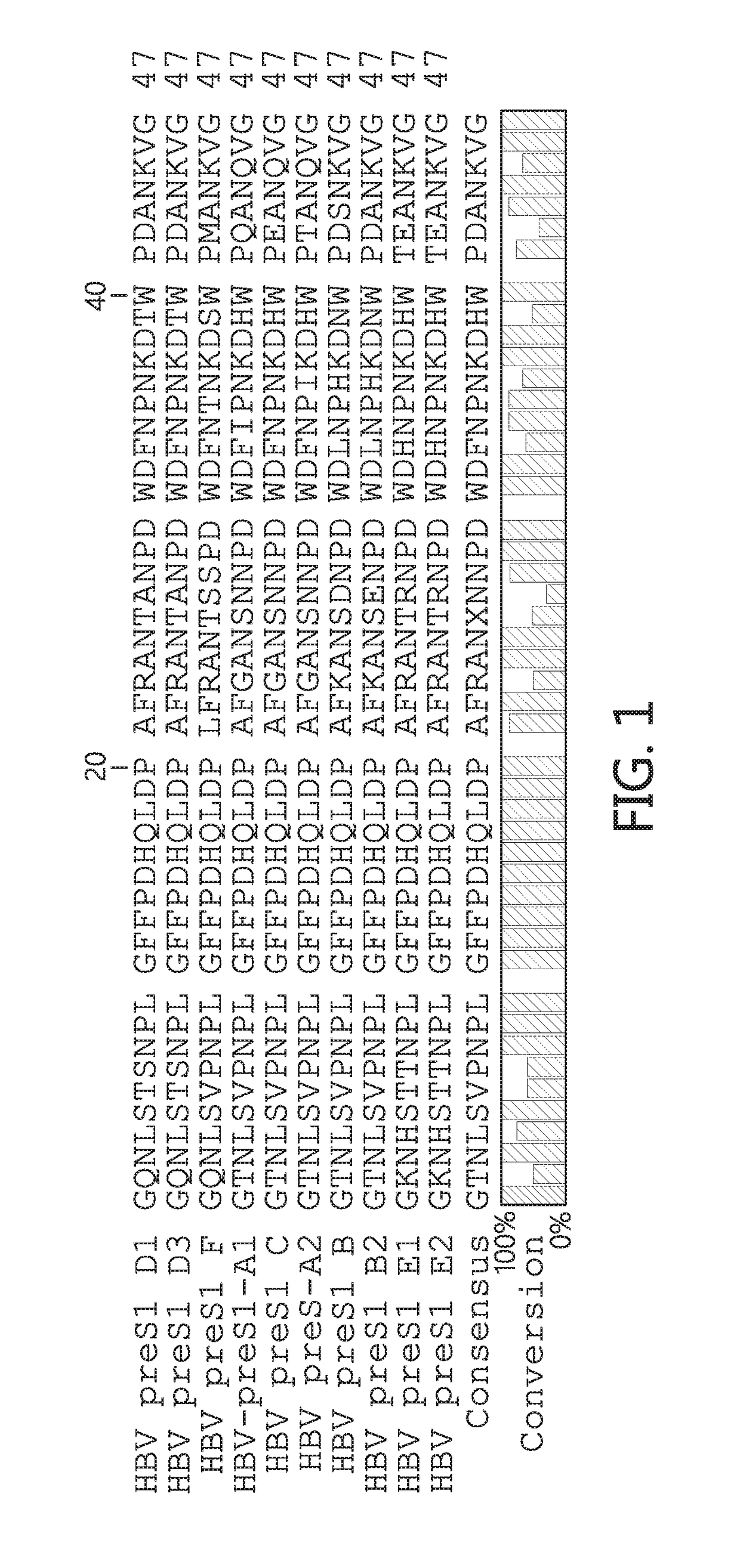
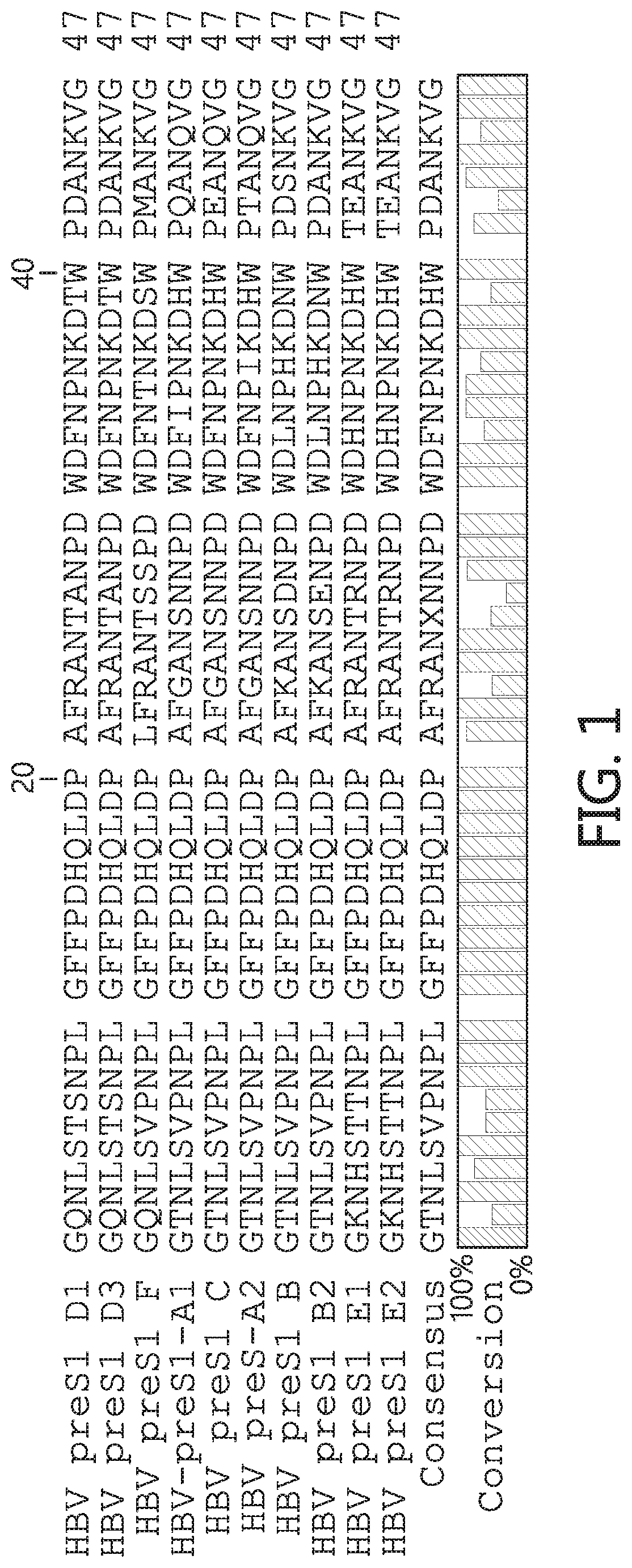
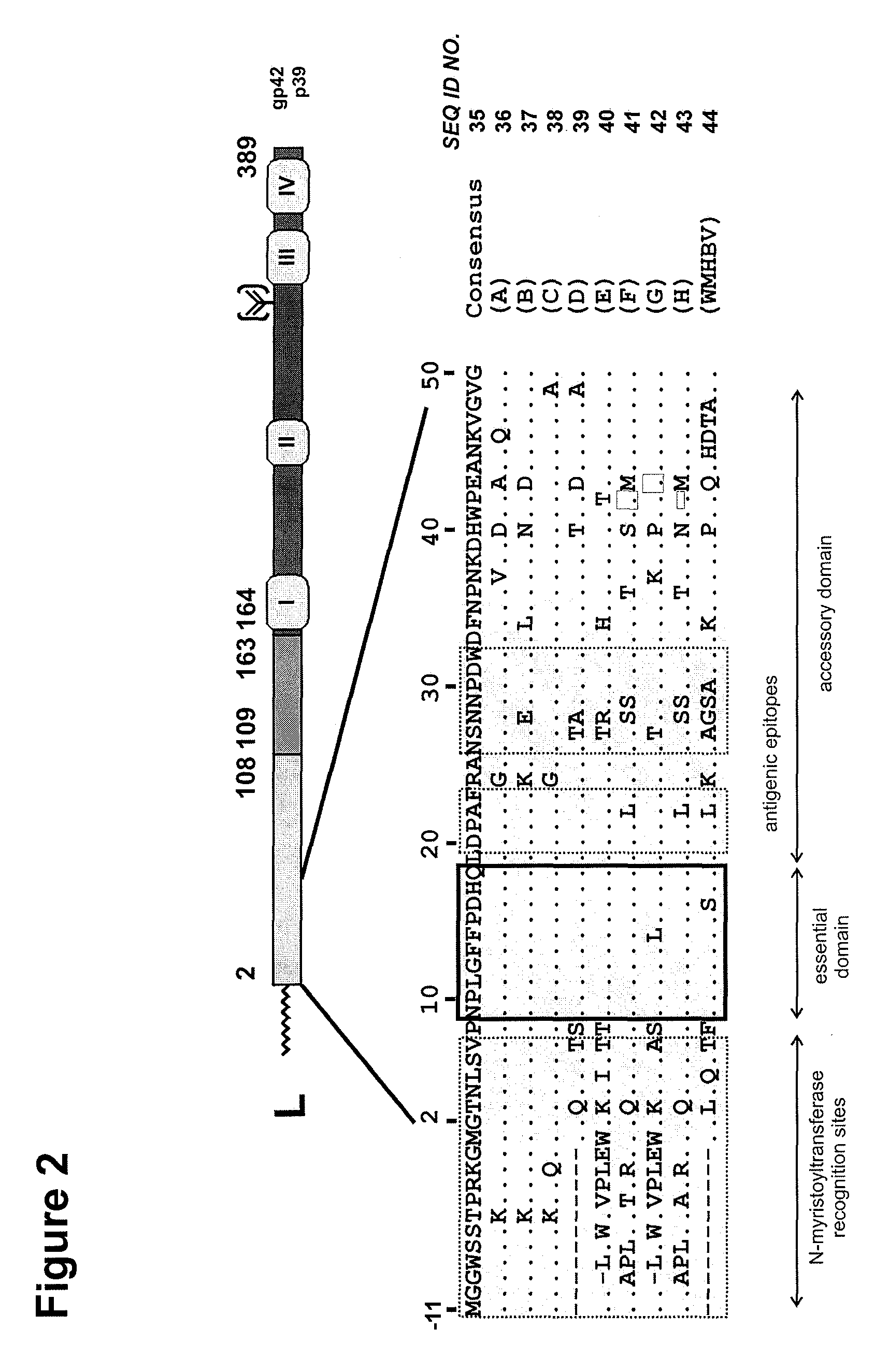
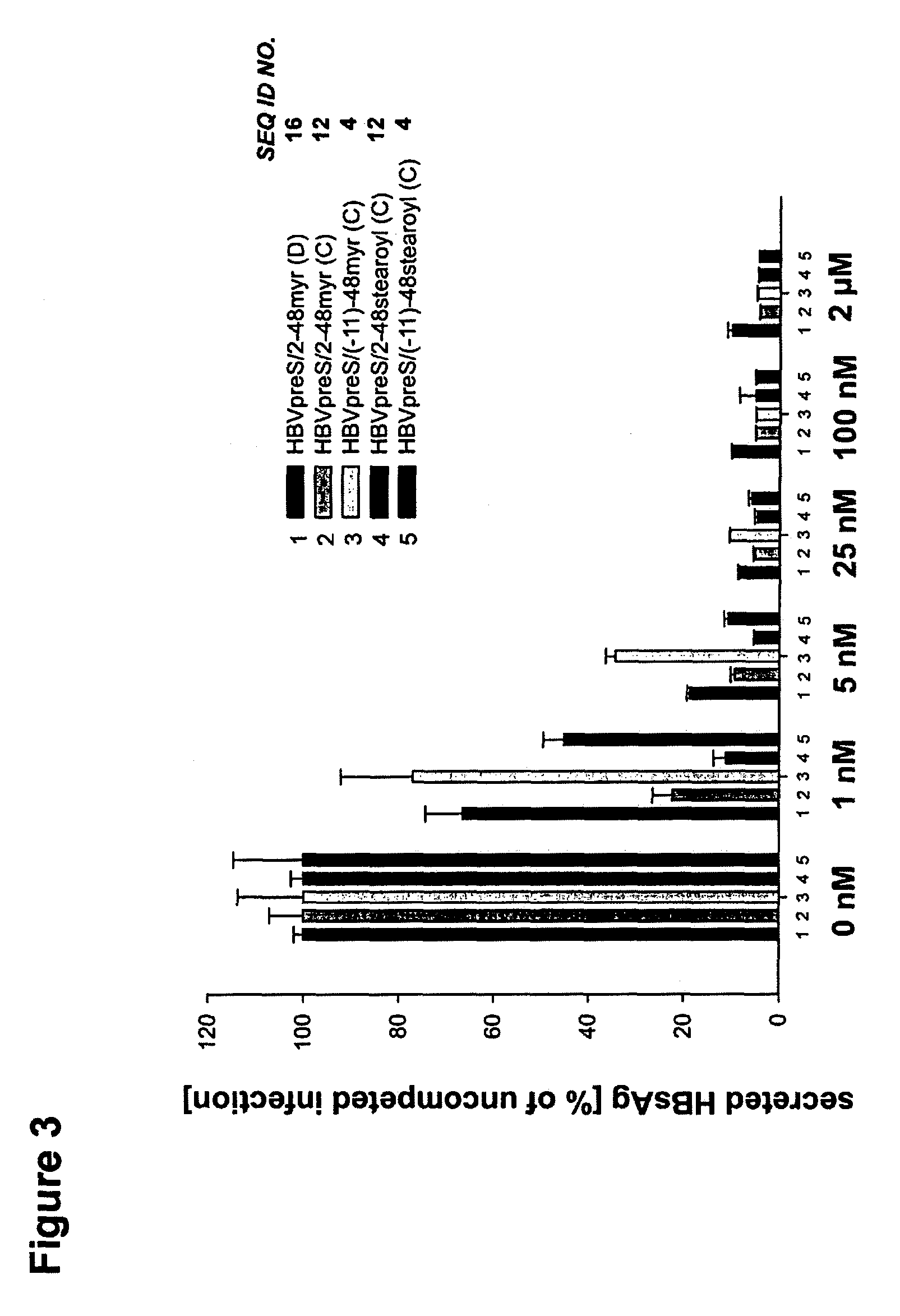
Hydrophobic modified pres-derived peptides of hepatitis B virus (HBV) and their use as HBV and HDV entry inhibitors
ActiveUS9562076B2Improving immunogenicityIncrease rangePeptide/protein ingredientsViral antigen ingredientsHepatitis B virusHepacivirus
The present invention relates to hydrophobic modified preS-derived peptides of hepatitis B virus (HBV) which are derived from a HBV preS consensus sequence and are N-terminal preferably acylated and optional C-terminal modified. These hydrophobic modified preS-derived peptides of HBV are very effective HBV entry inhibitors as well as HDV entry inhibitors and are, thus, suitable for the inhibition of HBV and / or HDV infection, prevention of primary HBV and / or HDV infection as well as treatment of (chronic) hepatitis B and / or D. The present invention further relates to pharmaceutical and vaccine compositions comprising these hydrophobic modified preS-derived peptides of HBV.
Owner:UNIVERSITY OF HEIDELBERG
Method and means for the rapid detection of hdv infections
The present invention relates to a polypeptide and a nucleic acid encoding the polypeptide for use in a method of detecting the presence of hepatitis D vims (HDV) and / or of diagnosing an HDV infection and / or of monitoring the treatment of an HDV infection. The present invention further relates to an in vitro method, an immunographic test device as well as a kit. In particular, the present invention relates to a point of care diagnostic for HDV infections.
Owner:UNIVERSITY OF HEIDELBERG
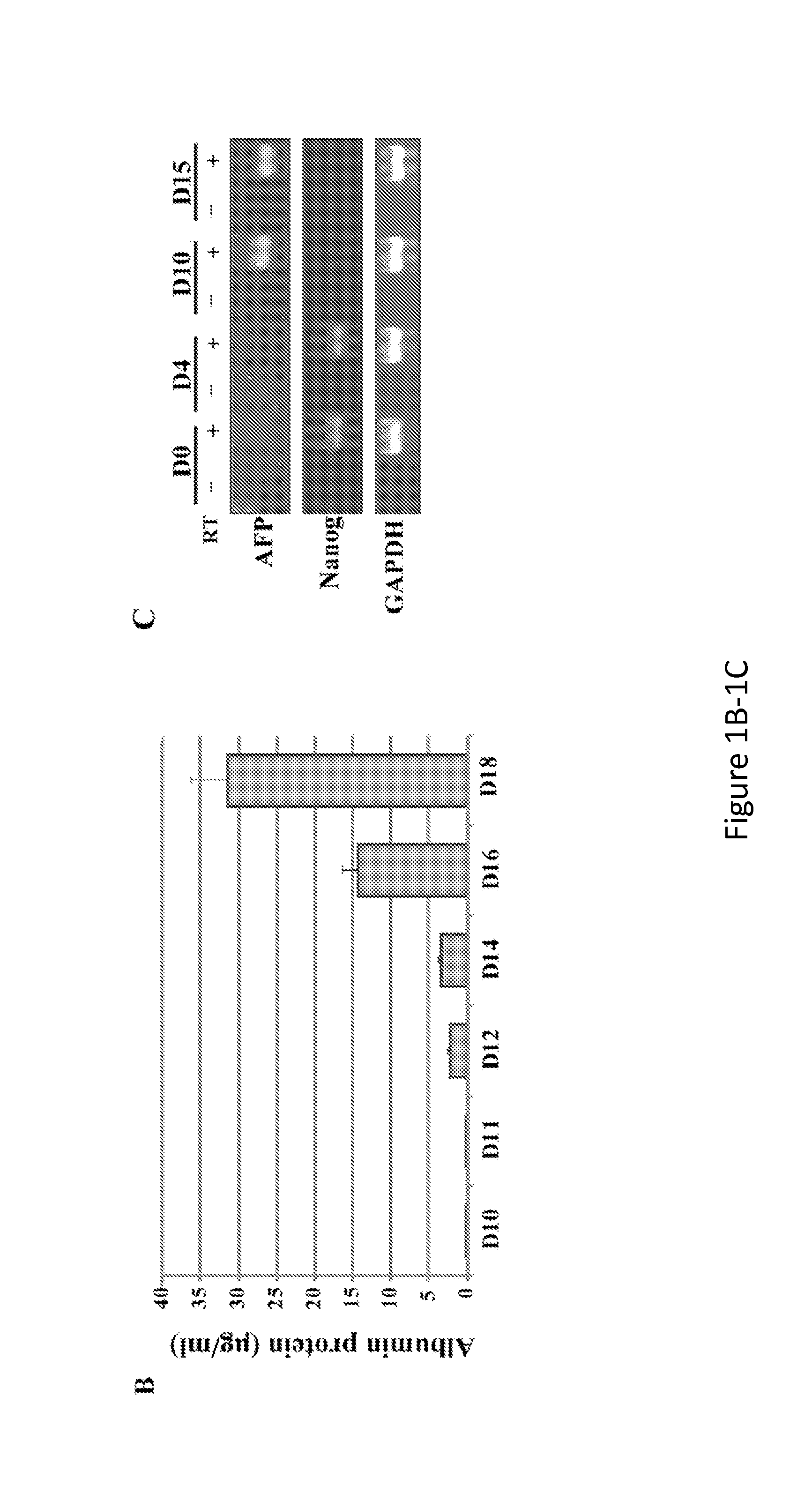
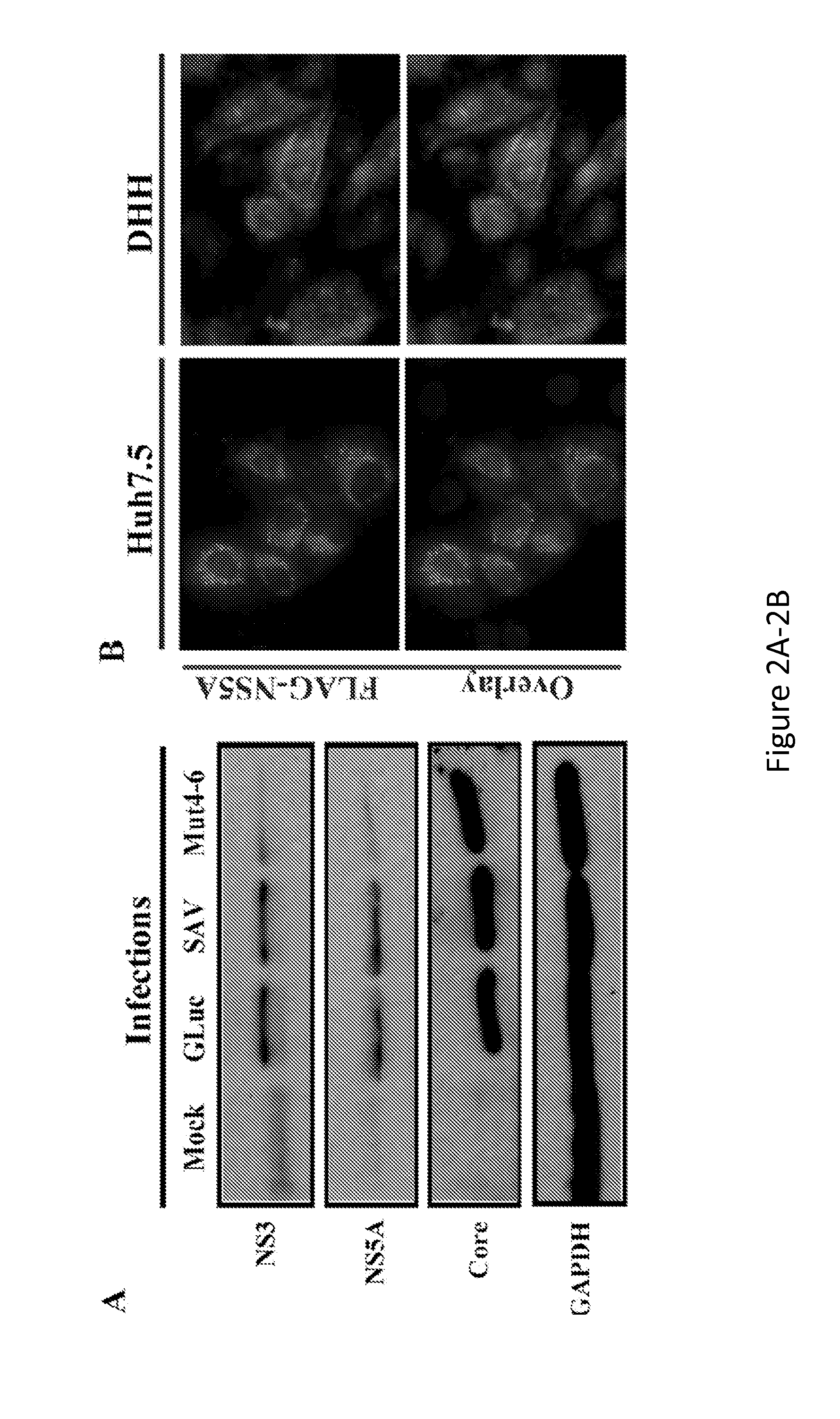
Hepatitis Virus Culture Systems Using Stem Cell-Derived Human Hepatocyte-Like Cells and Their Methods of Use
InactiveUS20140322704A1SsRNA viruses positive-senseMicrobiological testing/measurementHepatitis DHepatitis B virus
The invention relates to the discovery that DHH derived from stem cells are permissive for infection by hepatitis viruses (HV), such as hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis D virus (HDV) and hepatitis E virus (HEV). Included in the invention are HV-permissive DHHs and methods of making an HCV-permissive DHH derived from a stem cell. Also included is an HV culture system comprising at least one HV-permissive DHH. The HV-permissive DHH and HV culture system are useful for conducting HV life cycle analyses, diagnosing a subject as being infected with HV, genotyping and characterizing the HV of a subject infected with HV, detecting drug resistance of HV obtained from a subject infected with HV, screening for and identify modulators of HV infection, and monitoring the effect of a treatment of HV in a subject.
Owner:FLORIDA STATE UNIV RES FOUND INC
Antiviral compounds and method for treating hepatotropic viral infection, particularly hepatitis b and hepatitis d
PendingUS20210308137A1Avoid controlInhibits viral replicationOrganic active ingredientsAntiviralsHepatovirusHepatitis D
The present invention provides a compound or a method for treating a hepatotropic viral infection in a human, particularly hepatitis B and hepatitis D, wherein the compound is a certain tricyclic compound.
Owner:SENHWA BIOSCIENCES INC
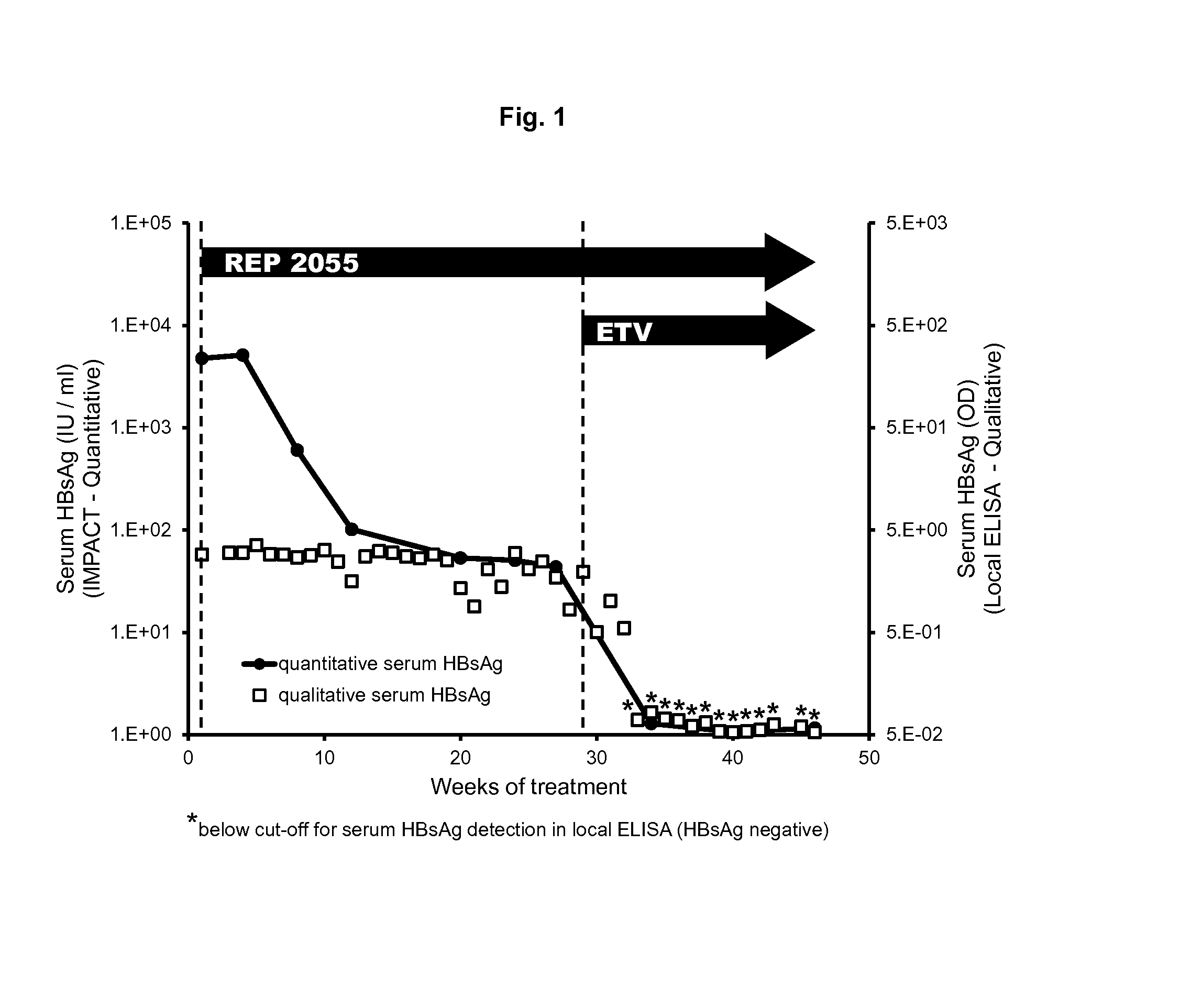
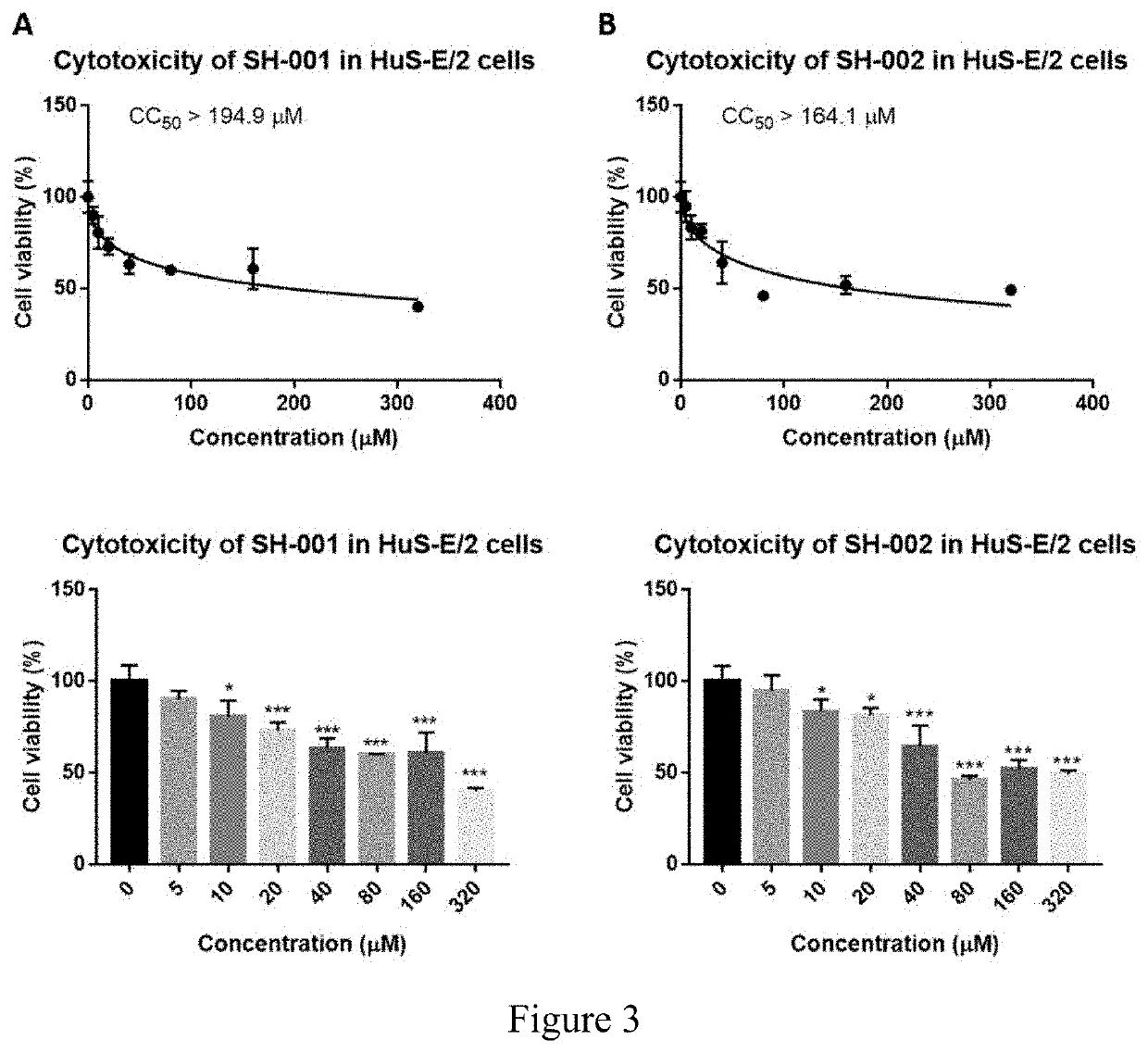
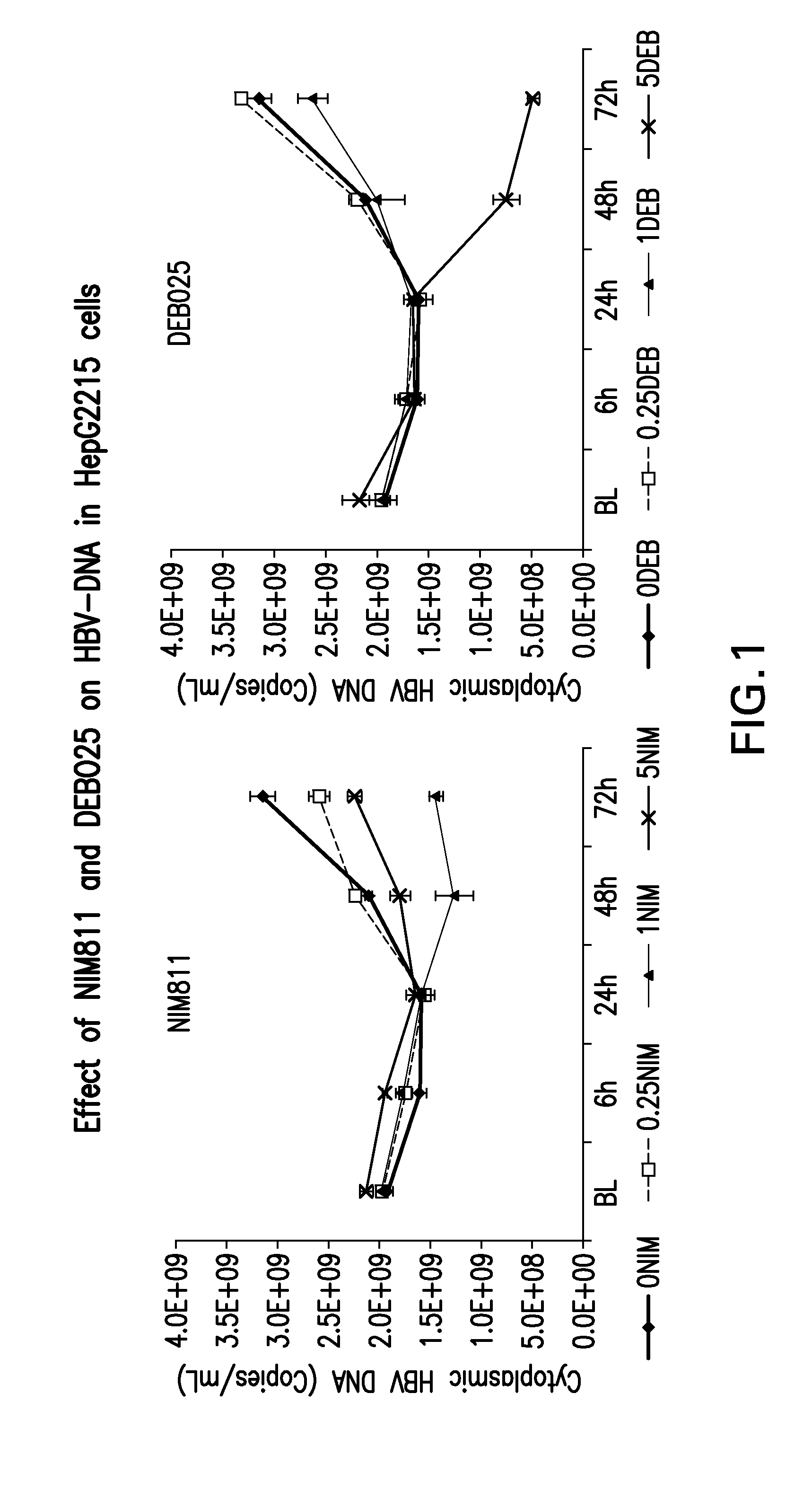
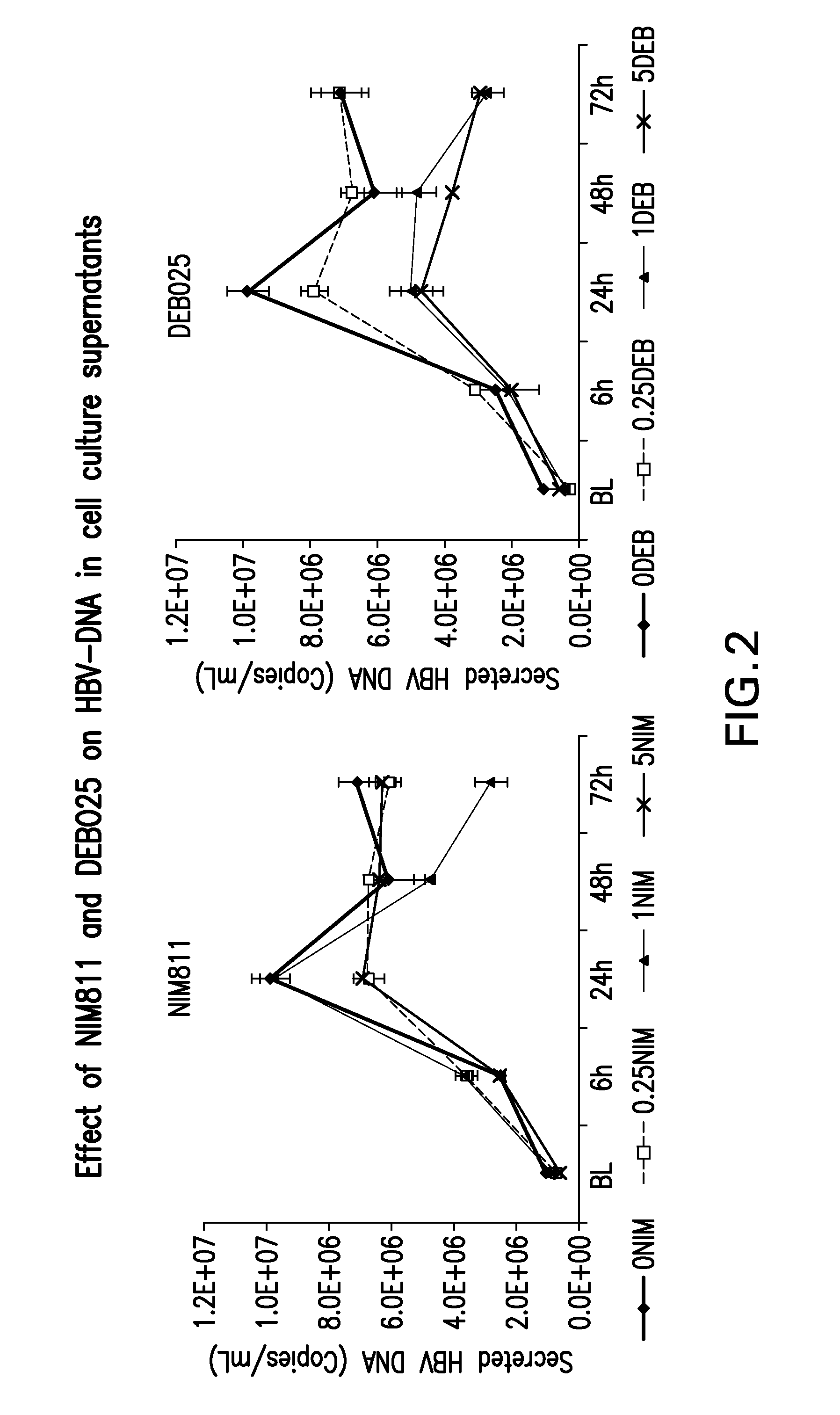
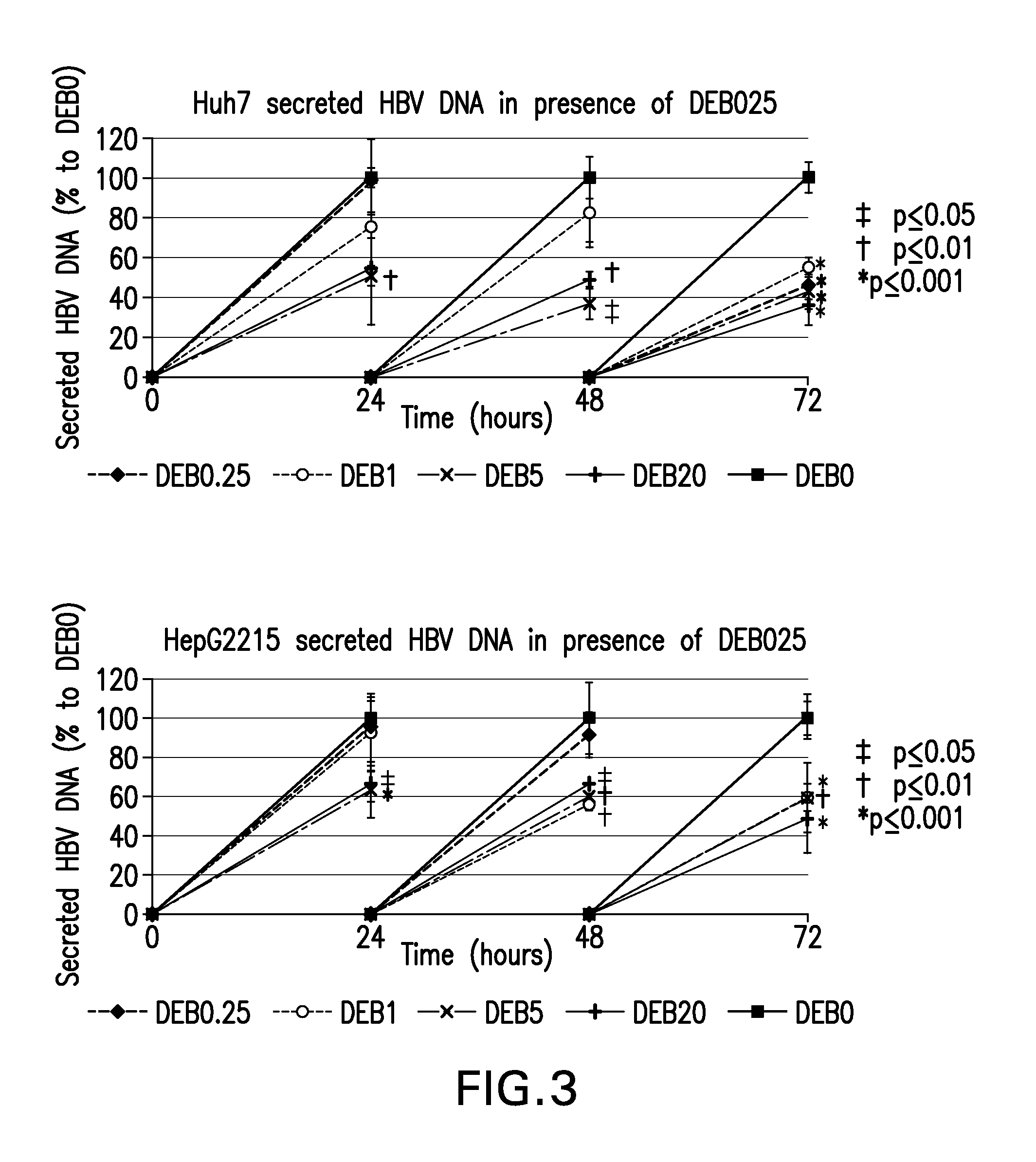
Treatment for infection with hepatitis b virus alone or in combination with hepatitis delta virus and associated liver diseases
The invention concerns the use of cyclophilin inhibitors in the treatment of Hepatitis B and Hepatitis D virus infections.
Owner:NOVARTIS AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com