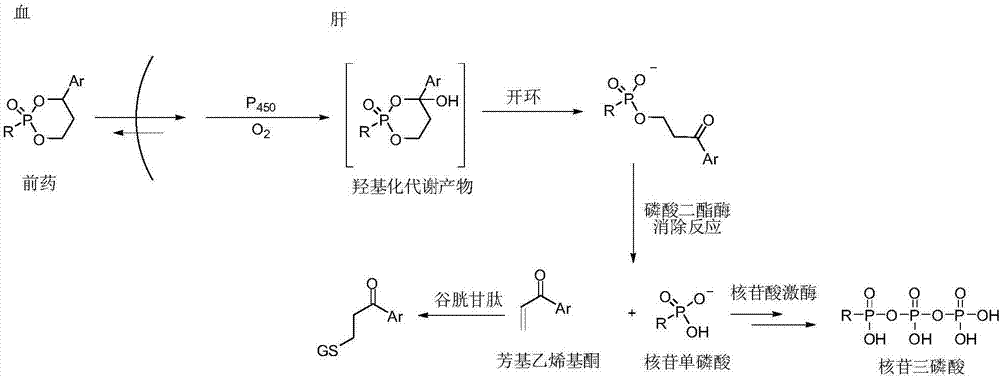
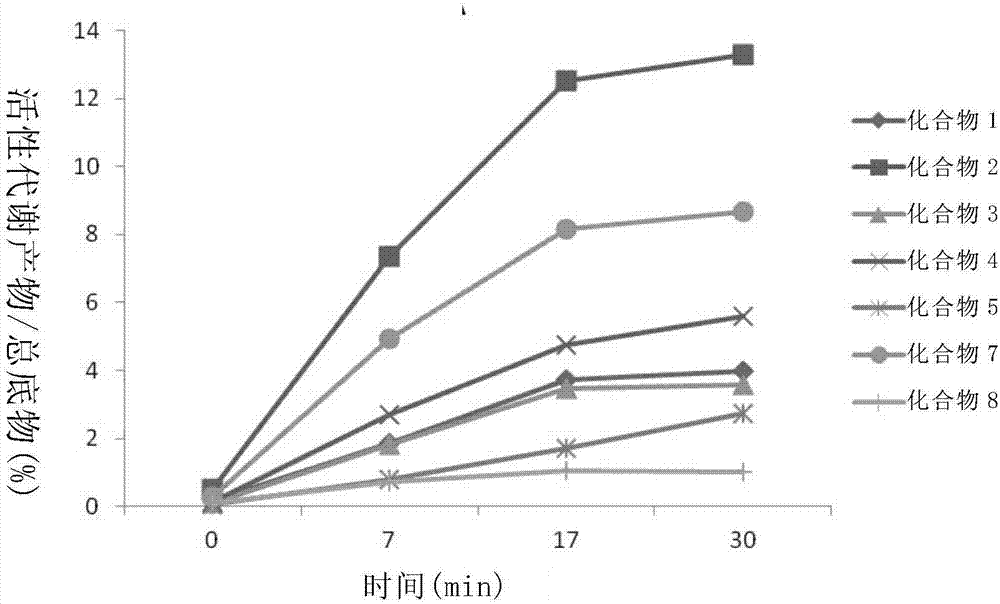
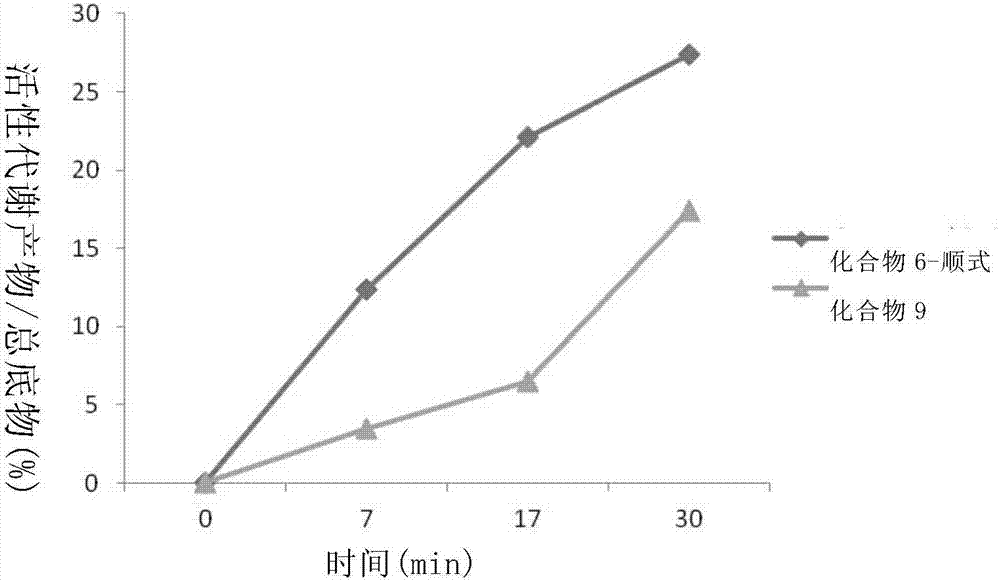
Liver specific delivery-based antiviral prodrug nucleoside cyclophosphate compound and use thereof
A compound and hydrate technology, applied in the field of pharmaceutically acceptable salts and pharmaceutical compositions, solvates, and optical isomers, can solve the problem of low toxicity and side effects, and achieve the effect of low toxicity and high curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0124] The preparation method of the compound (take the compound of formula III as an example):
[0125] In N,N-dimethylformamide and pyridine (5:1) solution, add monophosphoric acid derivative Va, 1,3-propanediol derivative Vd and dicyclohexylcarbodiimide, heat up to about 80 ℃, After the reaction was completed for 16 h, the reaction solution was rotary evaporated under reduced pressure to remove the organic solvent. The crude product was dissolved in ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, evaporated under reduced pressure to remove the solvent, and subjected to silica gel column chromatography to obtain Compounds of general formula II.
[0126]
[0127] Wherein, each reactant can be purchased through commercially available channels, and can also be prepared by conventional methods in the art using commercially available raw materials.
[0128] In a preferred embodiment of the present invention, the 1,3-propanediol derivative Vd (...
Embodiment 1
[0163] Example 1(2R)-9-{2-[(4S)-4-(3-chloro-2-fluorophenyl)-2-oxo-1,3,2-dioxaphosphacyclohexane Preparation of -2-yl]methoxypropyl}adenine
[0164] (R)-9-[2-(phosphonomethoxy)propyl]-adenine (84 mg, 0.294 mmol) was dissolved in N,N-dimethylformamide (15 mL) and pyridine (3 mL), respectively Dicyclohexylcarbodiimide (182 mg, 0.882 mmol) and (S)-3-(3-chloro-2-fluorophenyl)-1,3-propanediol (60 mg, 0.294 mmol) were added and the reaction mixture was heated to 80 After the reaction was completed, the reaction solution was rotary evaporated under reduced pressure to remove the organic solvent. The crude product was dissolved in ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, evaporated under reduced pressure to remove the solvent, and a silica gel column layer was formed. precipitation (dichloromethane:methanol=20:1 to 10:1) to obtain a white solid, yield: 41%, R f =0.4 (dichloromethane:methanol=10:1), 1 H NMR (400MHz, DMSO-d 6 )δ: 8.071-8.227(m,...
Embodiment 2
[0165] Example 2(2R)-9-{2-[(4S)-4-(3-chloro-5-fluorophenyl)-2-oxo-1,3,2-dioxaphosphacyclohexane Preparation of -2-yl]methoxypropyl}adenine
[0166] Prepared in a similar manner to Example 1, (R)-9-[2-(phosphonomethoxy)propyl]-adenine (84 mg, 0.294 mmol), dicyclohexylcarbodiimide (182 mg, 0.882mmol) and (S)-3-(3-chloro-5-fluorophenyl)-1,3-propanediol (60mg, 0.294mmol) were reacted to obtain 35mg white solid, yield: 26%, R f =0.4 (dichloromethane:methanol=10:1), 1 H NMR (400MHz, DMSO-d 6 ):δ:8.045-8.163(m,2H),7.173-7.455(m,5H),5.621-5.681(m,1H),4.228-4.271(m,1H),4.001-4.059(m,6H),1.952 -2.109(m,2H),1.097-1.196(m,3H)ppm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com