Methods for treating hepatitis delta virus infection with beta-L-2' deoxy-uncleosides
A technology for hepatitis D, virus, applied in the field of β-L-2'-deoxynucleoside or its pharmaceutically acceptable salt or prodrug, which can solve the problems of impact, lack of treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
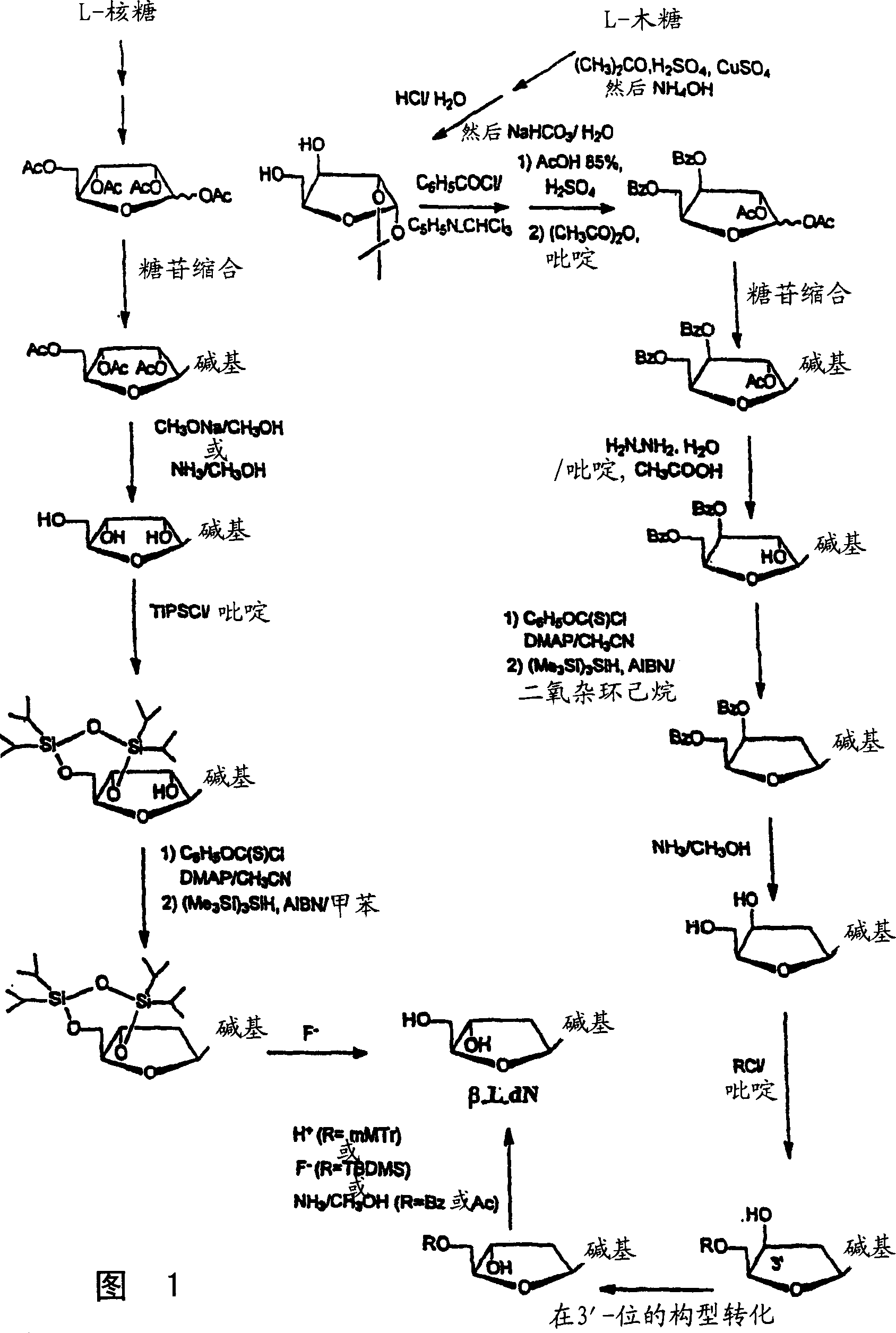
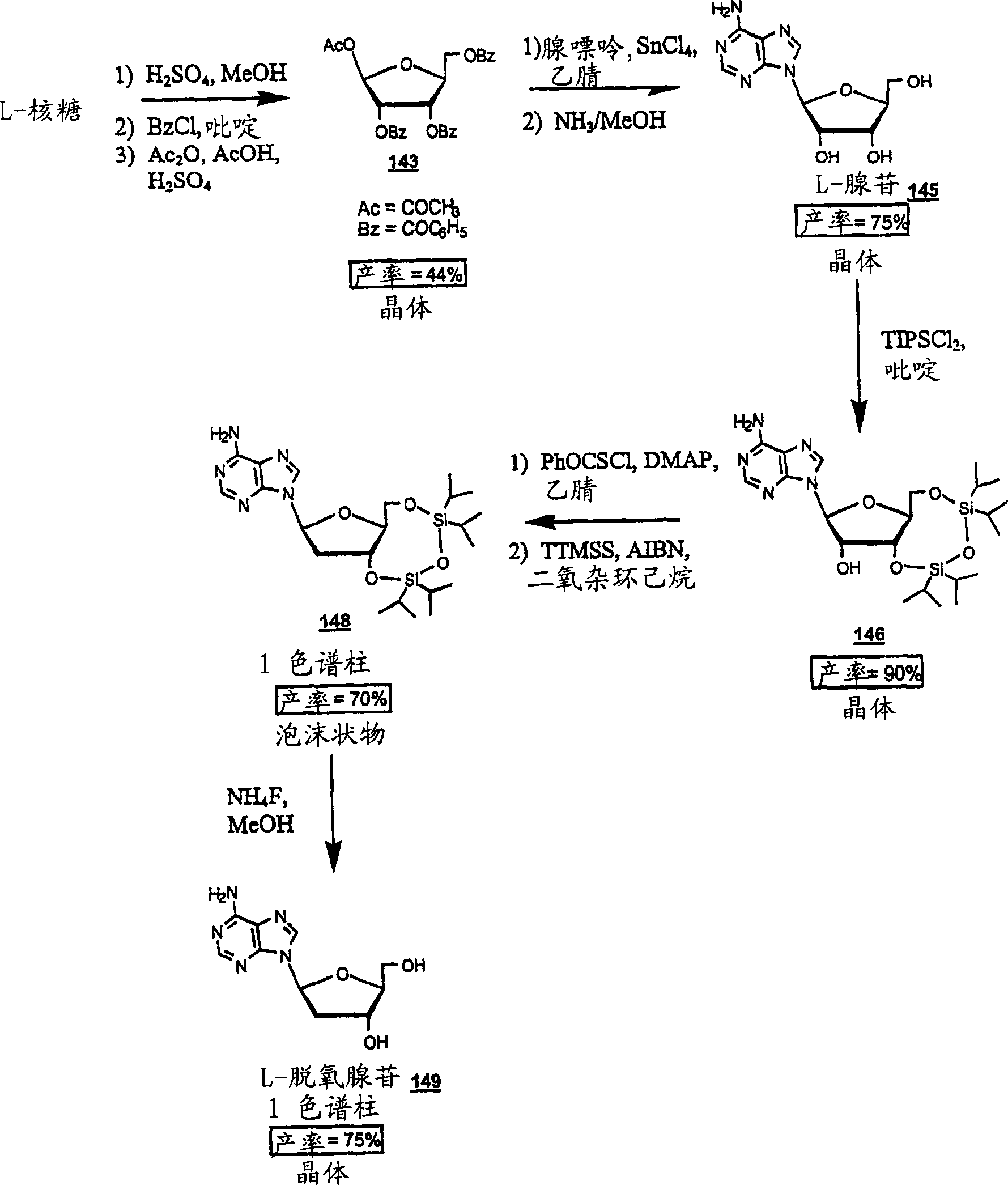
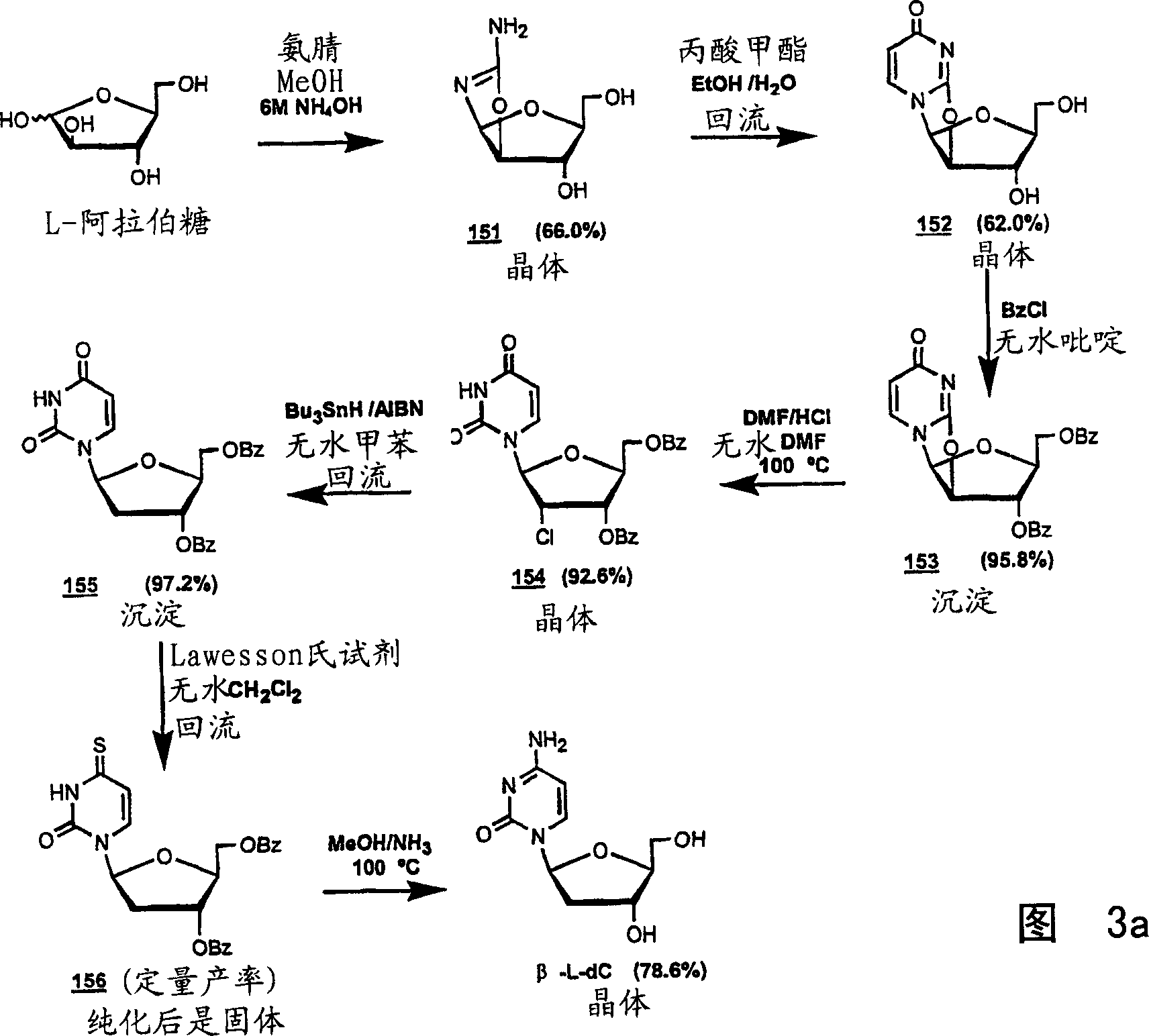
Image
Examples
Embodiment 1
[0106] 9-(2-O-acetyl-3,5-di-O-benzoyl-β-L-xylofuranosyl) adenine 2 [See: Gosselin, G.; Bergogne, M.-C.; Imbach, J.-L. "The synthesis and antiviral evaluation of β-L-xylofuranosyl nucleosides of five natural nucleic acid bases", Journal of Heterocyclic Chemistry .1993, 30, 1229-1233] (8.30g, 16.05mmol) and 98% hydrazine hydrate (234mL, 48.5mmol) in a pyridine / glacial acetic acid mixture (4 / 1, v / v, 170mL) at room temperature Stir for 22 hours. The reaction was stopped by adding acetone (40 mL) and continuing to stir for 1 hour. The volume of the reaction mixture was reduced to half, diluted with water (250 mL), and extracted with chloroform (2×150 mL). The organic layer was washed sequentially with saturated aqueous sodium bicarbonate (3×100 mL) and water (3×100 mL), dried, filtered, concentrated, and co-evaporated with toluene and methanol. The residue was purified by silica gel column chromatography (0-3% MeOH in dichloromethane mixture) to obtain 3 (5.2 g, 68%), which was precip...
Embodiment 2
[0107] 1 H NMR(DMSO-d 6 ): δ4.5-4.9 (m, 4H, H-2’, H-4’, H-5’ and H-5"), 5.64 (t, 1H, H-3’, J 2’3’ =J 3’,4’ =3.5Hz), 6.3(br s, 1H, OH-2’), 6.45(d, 1H, H-1’, J 1’,2’ =4.6Hz), 7.3(br s, 2H, NH 2 -6), 7.4-7.9 (m, 10H, 2 benzoyl), 8.07 and 8.34 (2s, 2H, H-2 and H-8); ms: matrix G / T, (FAB + )m / z 476[M+H] + , 136[BH 2 ] + , (FAB - )m / z 474[M-H] - , 134[B] - ; UV (95% ethanol): λ max 257nm(∈16400), 230nm(∈29300), λmin 246nm(∈14800); [α] D 20 =-64(c 1.07, CHCl 3 ). Elemental Analysis C 24 H 21 N 5 O 4 (M=475.45) Calculated value: C, 60.43; H, 4.45; N, 14.73. Measured value: C, 60.41; H, 4.68; N, 14.27. Example 2 9-(3,5-Di-O-benzene Formyl-2-deoxy-β-L-threo-pentofuranosyl) adenine (4)
[0108] To a solution of compound 3 (1.00g, 2.11mmol) in anhydrous acetonitrile (65mL) was added 4-(dimethylamino)pyridine (0.77g, 6.32mmol) and phenoxythioformyl chloride (0.44mL, 3.16mmol). The mixture was stirred at room temperature for 2 hours. After concentration, the residue was dissolved in dichlorome...
Embodiment 3
[0109] 1 H NMR(DMSO-d 6): δ2.9-3.1 (m, 2H, H-2' and H-2"), 4.6-4.7 (m, 3H, H-4', H-5' and H-5"), 5.8 (br s, 1H, H-3'), 6.43(dd, 1H, H-1', J 1’,2’ =3.1Hz, J 1’,2” =7.6Hz), 7.3(br s, 2H, NH 2 -6), 7.4-7.9 (m, 10H, 2 benzoyl), 8.05 and 8.33 (2s, 2H, H-2 and H-8); ms: matrix G / T, (FAB + )m / z 460[M+H] + , 325[S] + , 136[BH 2 ] + , (FAB - )m / z 458[M-H] - , 134[B] - ; UV (95% ethanol): λ max 261nm (∈14400), 231nm (∈26300), λ min 249nm(∈12000); [α] D 20 = -38 (c1.04, DMSO). Example 3 6-N-(4-methoxytrityl)-9-(3,5-di-O-benzoyl-2-deoxy- β-L-threo-pentofuranosyl) adenine (5)
[0110] To a solution of compound 4 (0.88 g, 1.92 mmol) in anhydrous pyridine (40 mL) was added 4-monomethoxytrityl chloride (1.18 g, 3.84 mmol). The mixture was stirred at 60°C for 24 hours. After adding methanol (5 mL), the solution was concentrated to dryness, the residue was dissolved in dichloromethane (50 mL), and washed with water (30 mL), saturated aqueous sodium bicarbonate solution (30 mL) and water (30 mL) su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com