Therapeutic methods for treating hepatitis b
A kind of technology of hepatitis B, use, applied in the therapeutic field for treating hepatitis B
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[1942] In one embodiment, the methods of the present invention exclude a method of treating hepatitis B in an animal comprising administering to the animal a synergistically effective amount of i) an inhibitor of formation of covalently closed circular DNA and ii) a nucleoside or nucleotide analogs.
[1943] In one embodiment, the pharmaceutical compositions of the present invention exclude compositions comprising as the only active hepatitis B therapeutics: i) inhibitors of covalently closed circular DNA formation and ii) nucleosides or nucleotides analog.
[1944] In one embodiment, the kits of the present invention exclude kits comprising as the sole hepatitis B agent: i) inhibitors of covalently closed circular DNA formation and ii) nucleoside or nucleotide analogs.
[1945] In one embodiment, the methods of the present invention exclude a method of treating hepatitis B in an animal comprising administering to the animal i) one or more siRNAs targeting hepatitis B virus a...
Embodiment 1-4
[2260] Materials and methods for combinatorial studies in primary human hepatocytes (PHH) are described in Examples 1-4 below.
[2261] PHH
[2262] Cryopreserved PHH (Lot IKB) was purchased from Bioreclamation IVT
[2263] test item
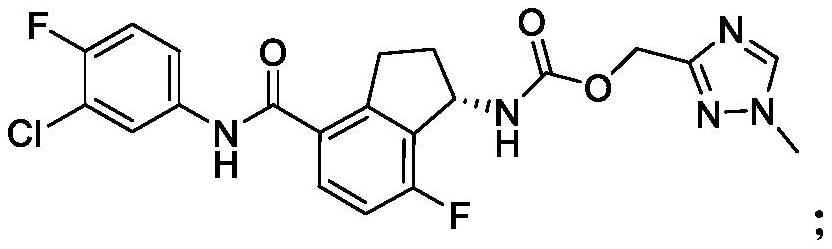
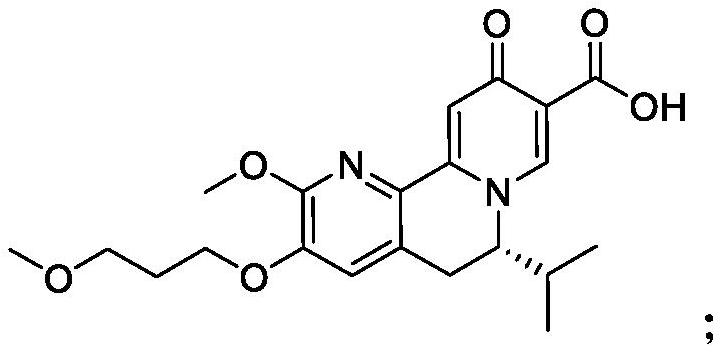
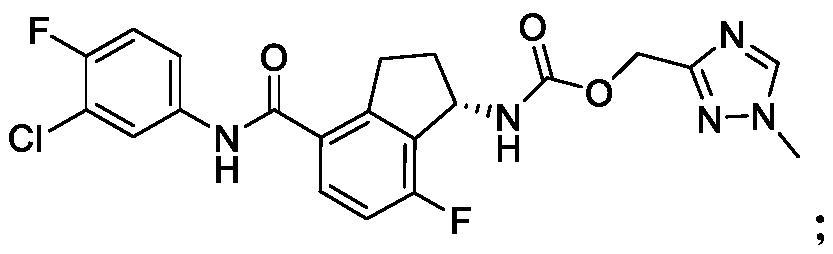
[2264] Compounds (V), (VI) and (VII) were produced by Arbutus Biopharma. Pegylated IFN-α2a and TAF are commercially available. Information on the compounds is shown in Table 1.
[2265] Table 1. Information on Test Items
[2266]
[2267] infectious virus stock
[2268] Type D HBV was concentrated from HepG2DE19 culture supernatants. Information about the virus is shown in Table 2.
[2269] Table 2. Information on HBV Virus Stock
[2270]
[2271]
[2272] *GE = HBV genome equivalent
[2273] reagent
[2274] The main reagents used in the study were QIAamp 96 DNA Blood Kit (QIAGEN #51162), FastStartUniversal Probe Master (Roche #04914058001), CellTiter-Glo (Promega #G7573) and HBsAg ELISA kit (Antu #CL 0310), and Lipofectamine 3...
Embodiment 1
[2324] Example 1. In vitro combination of compounds of formula (V) and (VI)
[2325] Research objectives
[2326] To determine the difference between compounds of formula (V) (GalNAc-conjugated siRNAs targeting the HBV genome and inhibiting production of HBV DNA, HBsAg and HBeAg and HBx) and compounds of formula (VI) (HBV RNA stabilization that inhibits HBV DNA, HBsAg and HBeAg) Whether dual-drug combinations of HBV-infected human primary hepatocytes in cell culture model systems are additive, synergistic, or antagonistic in vitro.
[2327] Results and Conclusions
[2328] Compounds of formula (VI) (concentration ranging from 4.00 μM to 0.05 μM in a 3-fold dilution series and 5-point titration) were combined with compounds of formula (V) (concentrations from 3.0 μg / mL to 0.04 μg / mL in a 3-fold dilution series) range and 5-point titration) combinations were tested on three replicate plates in each of two independent experimental trials. The mean % inhibition of HBV DNA and H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com