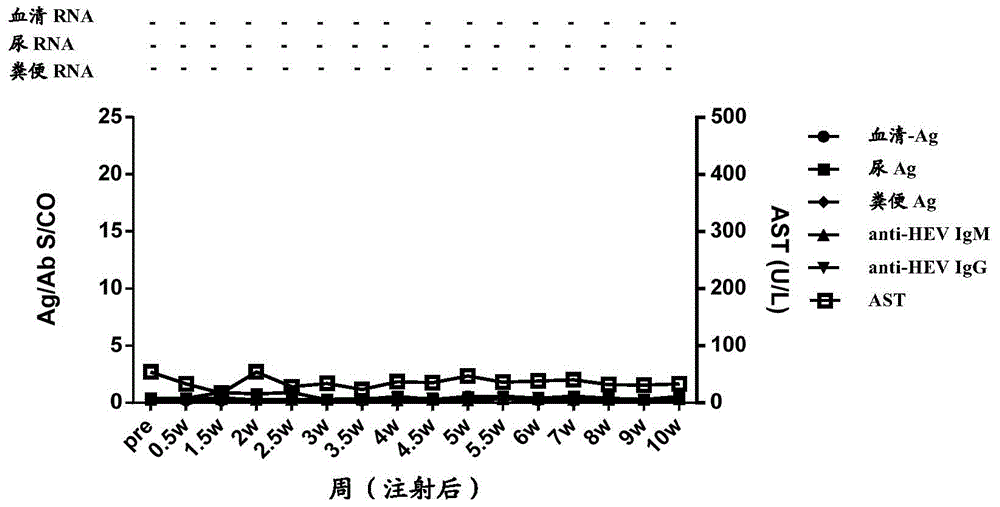
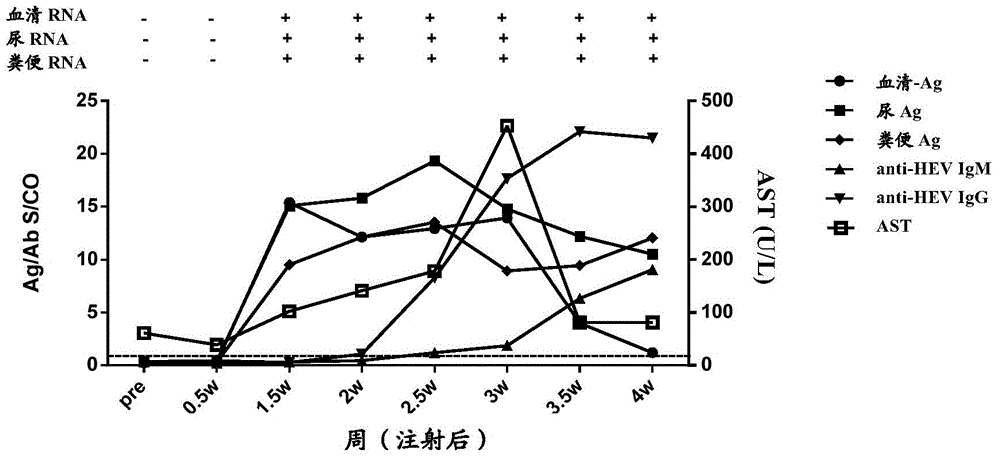
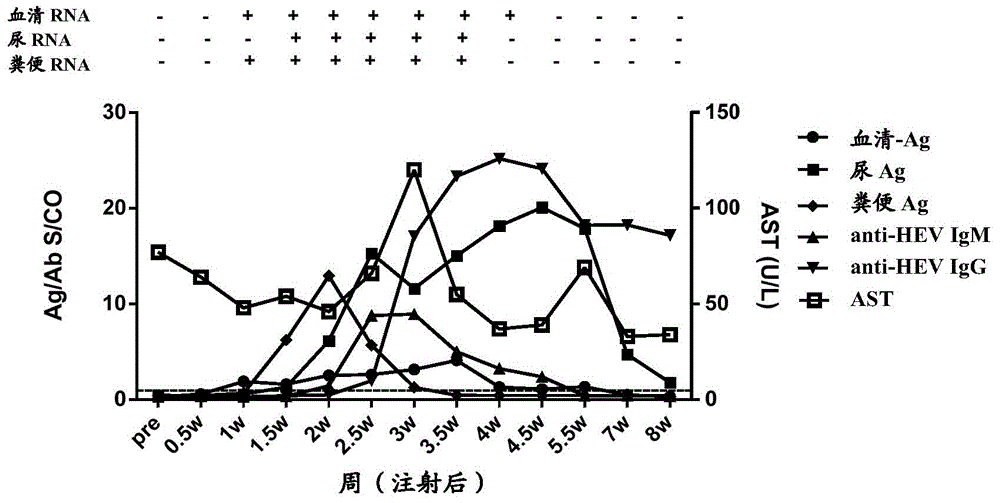
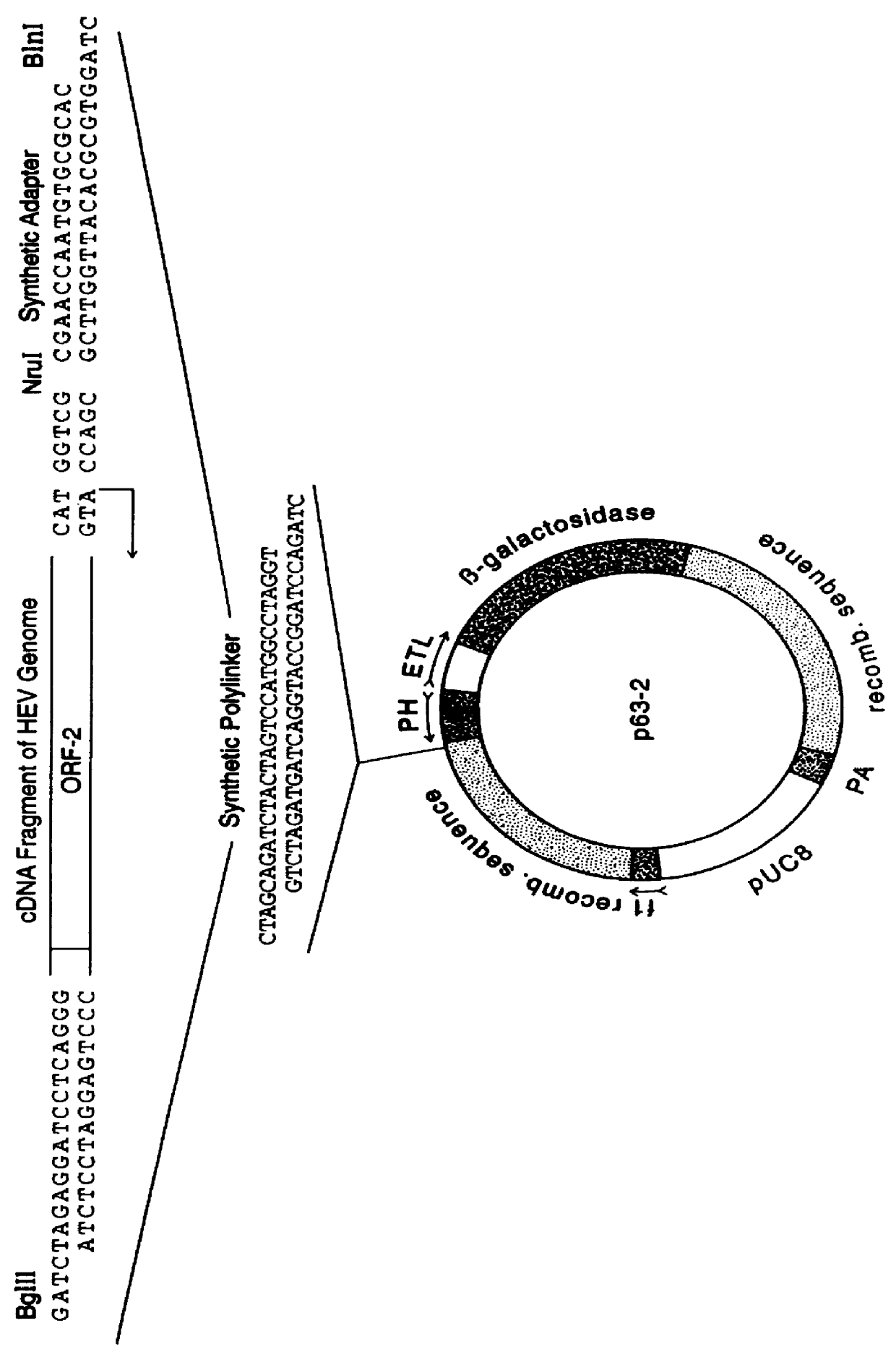
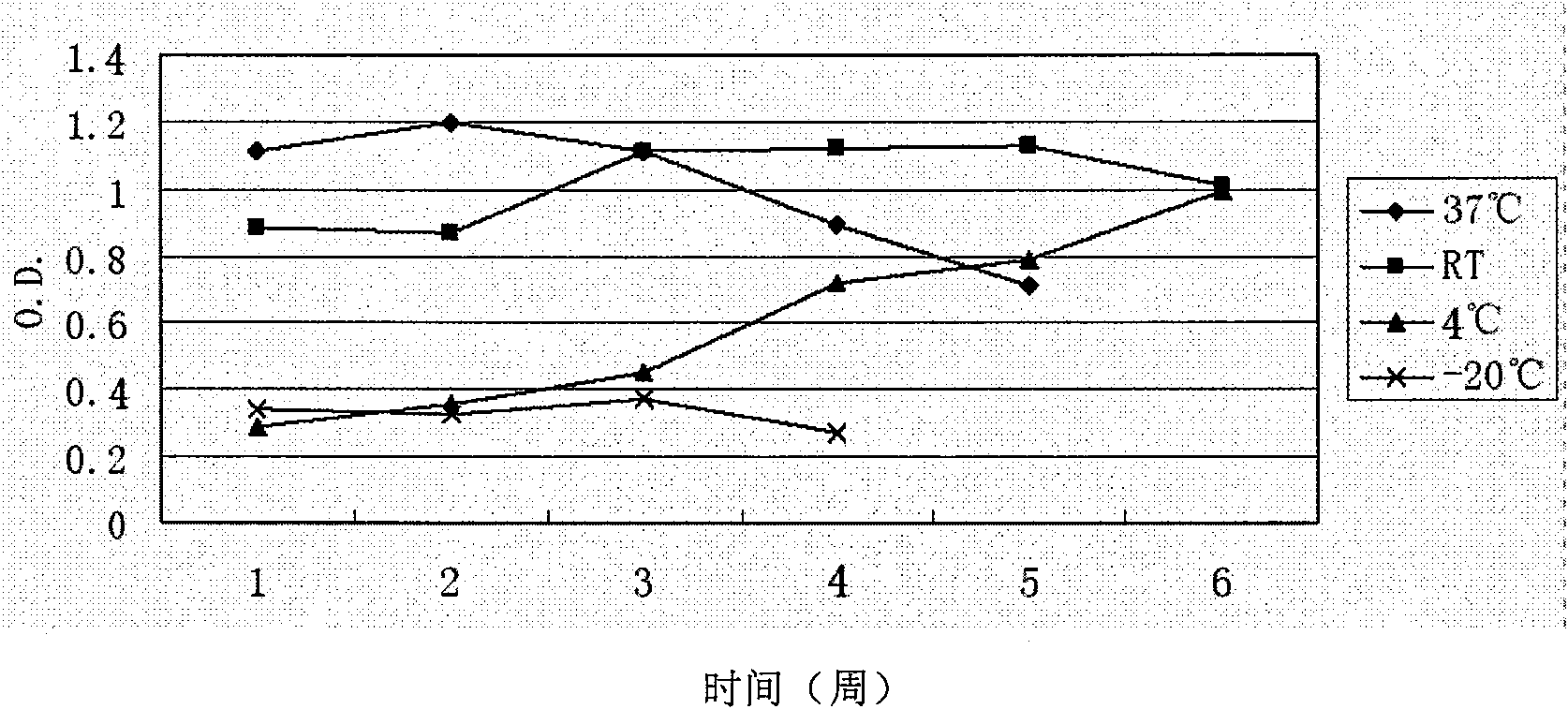
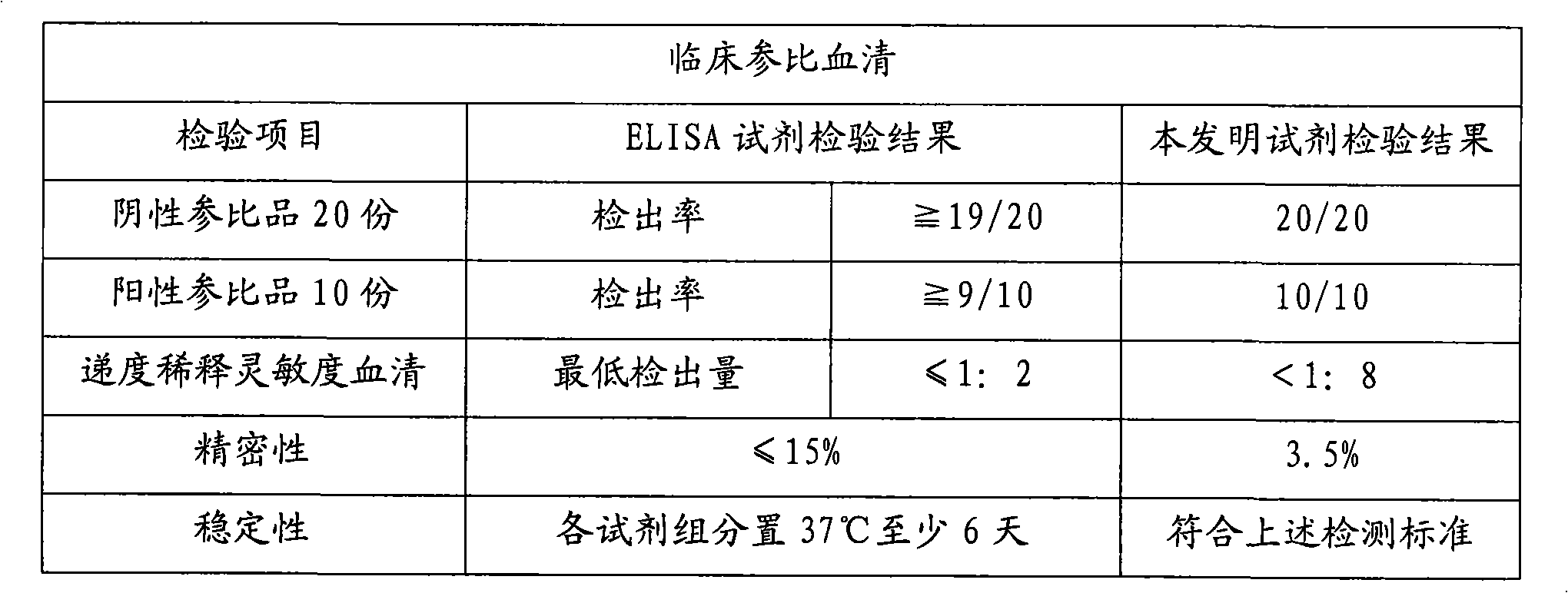
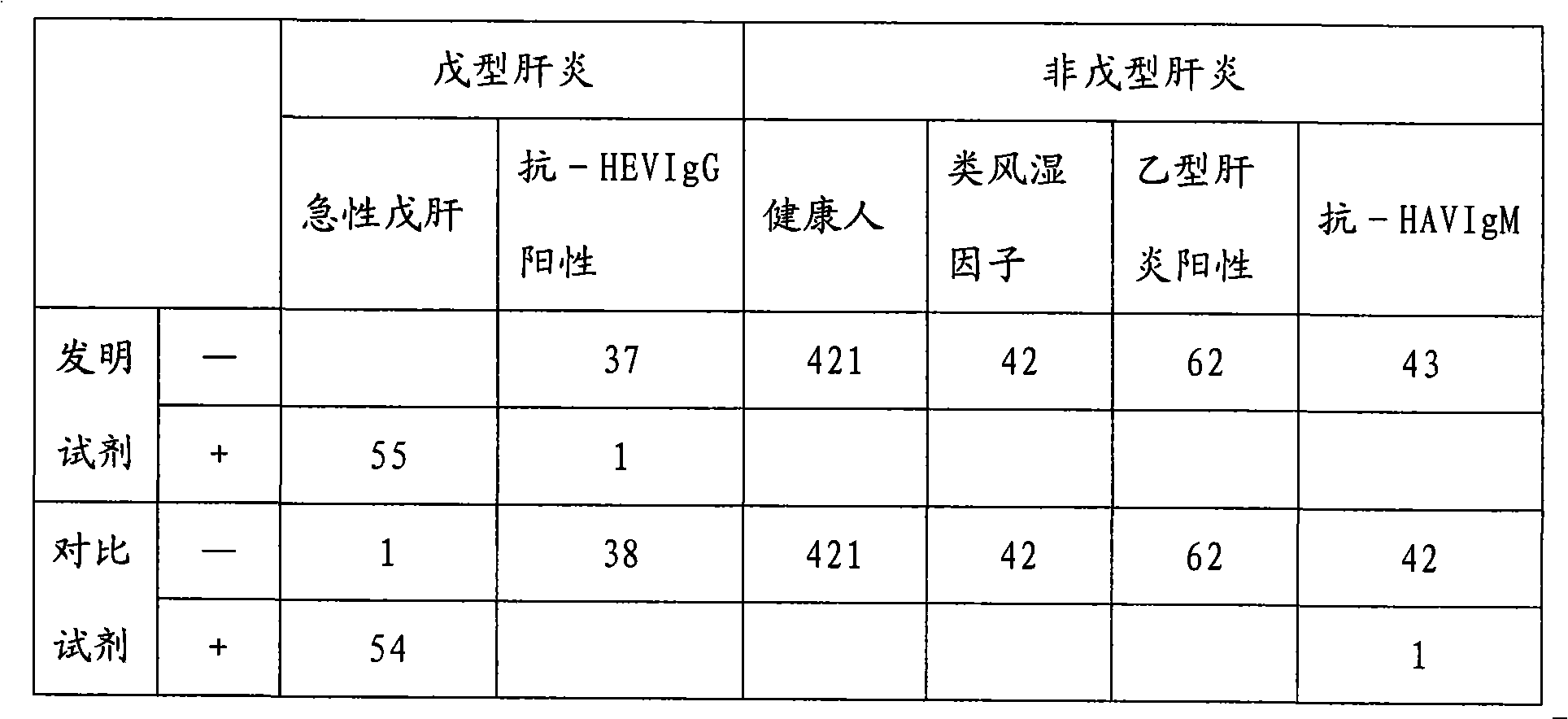
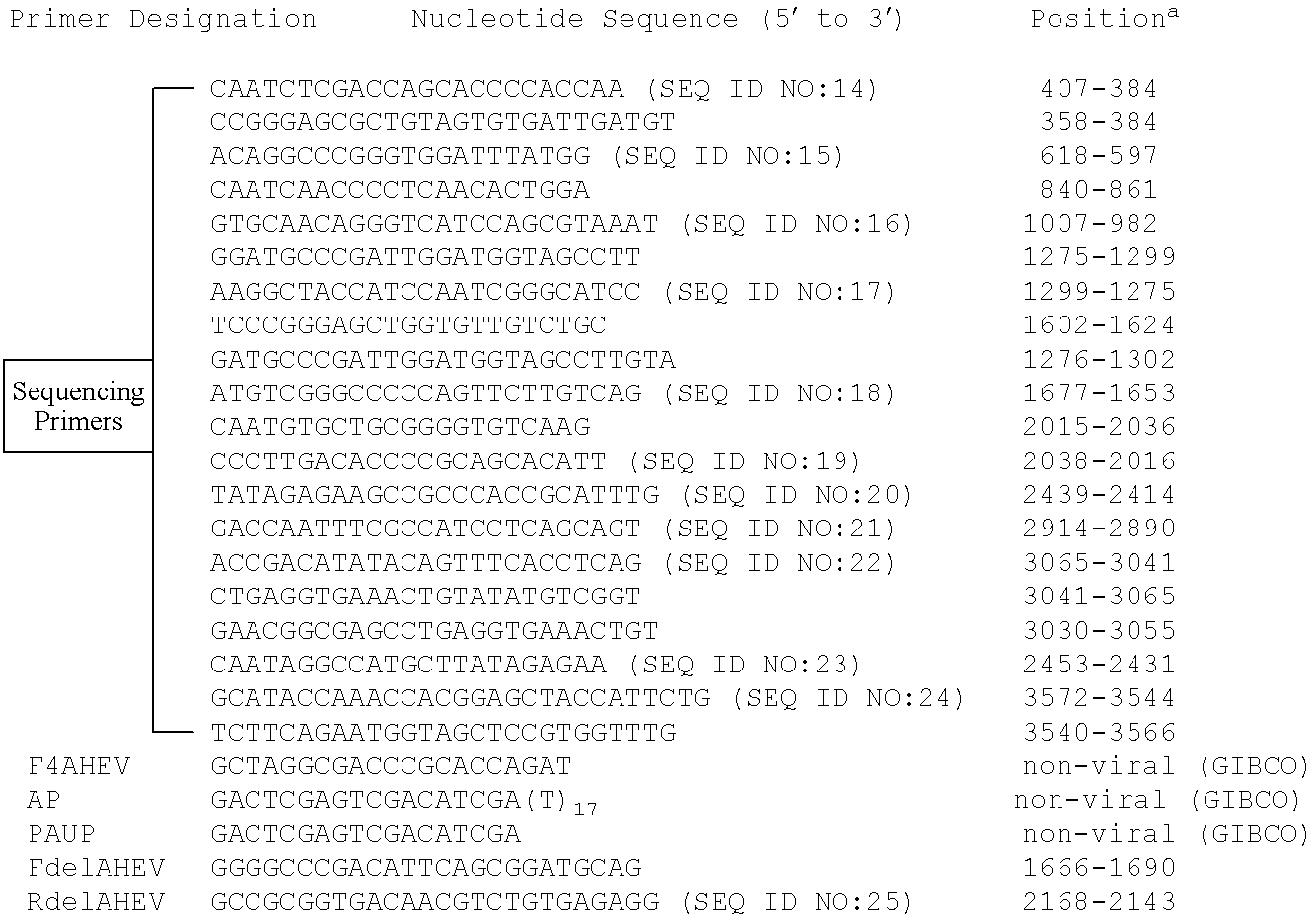
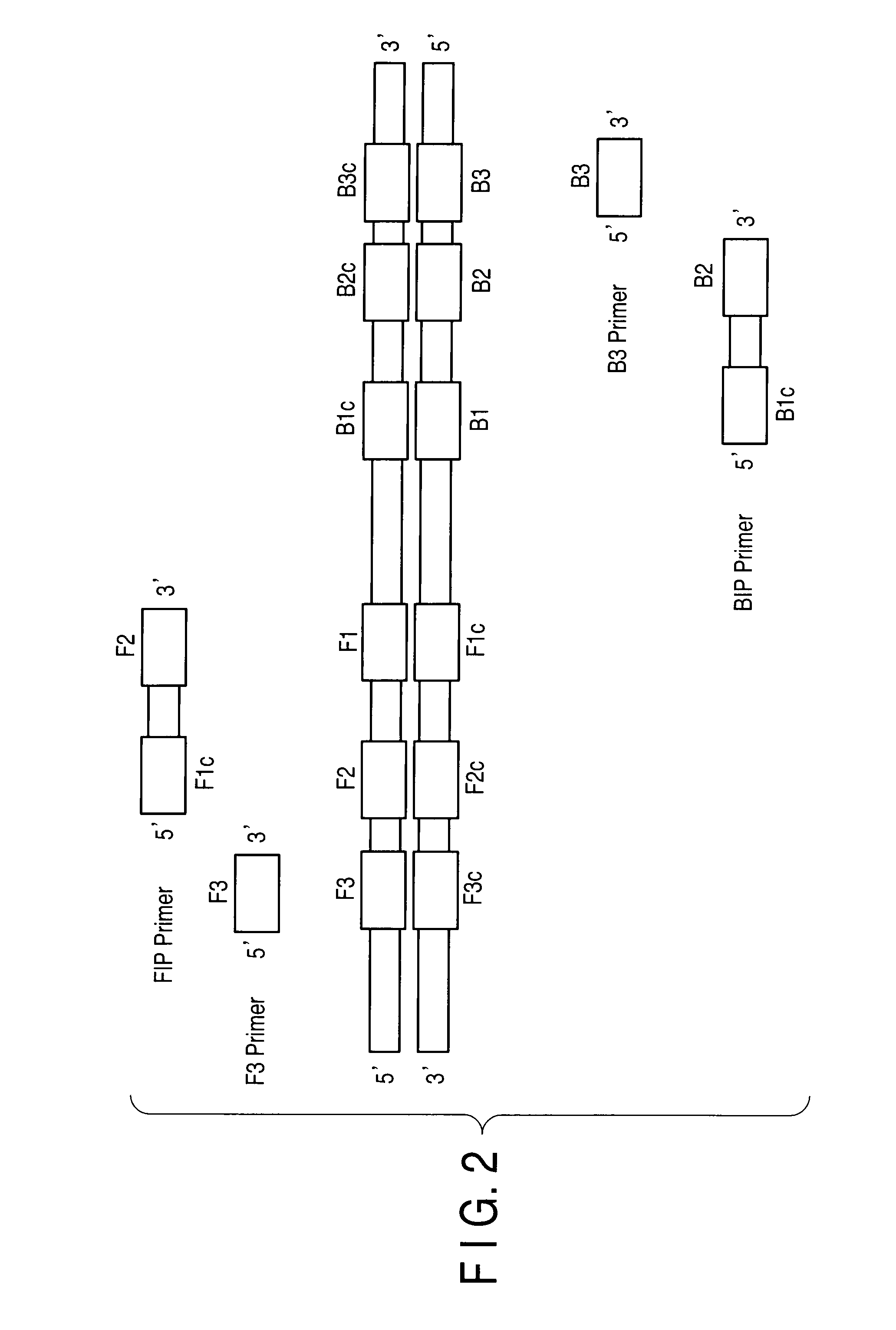
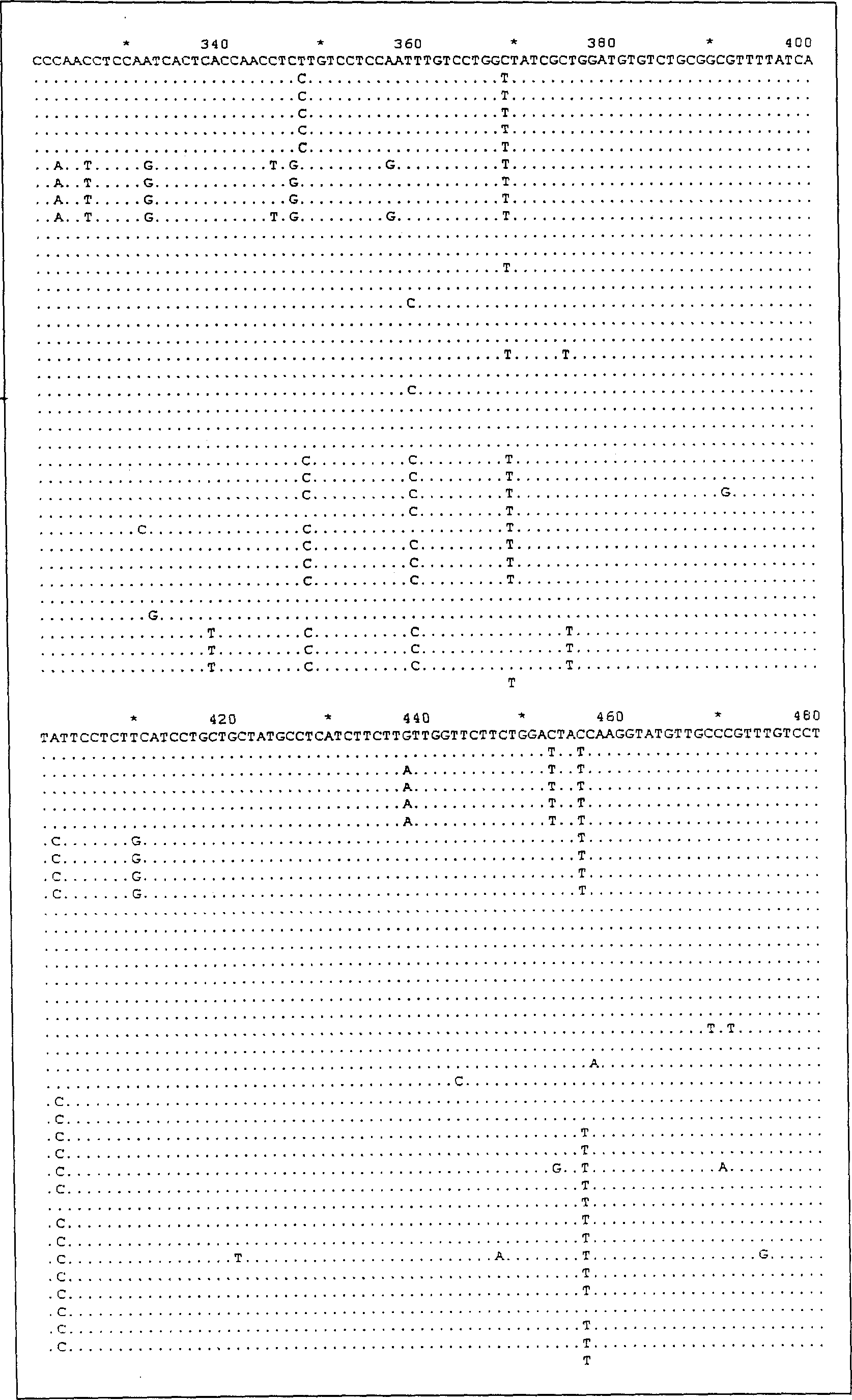
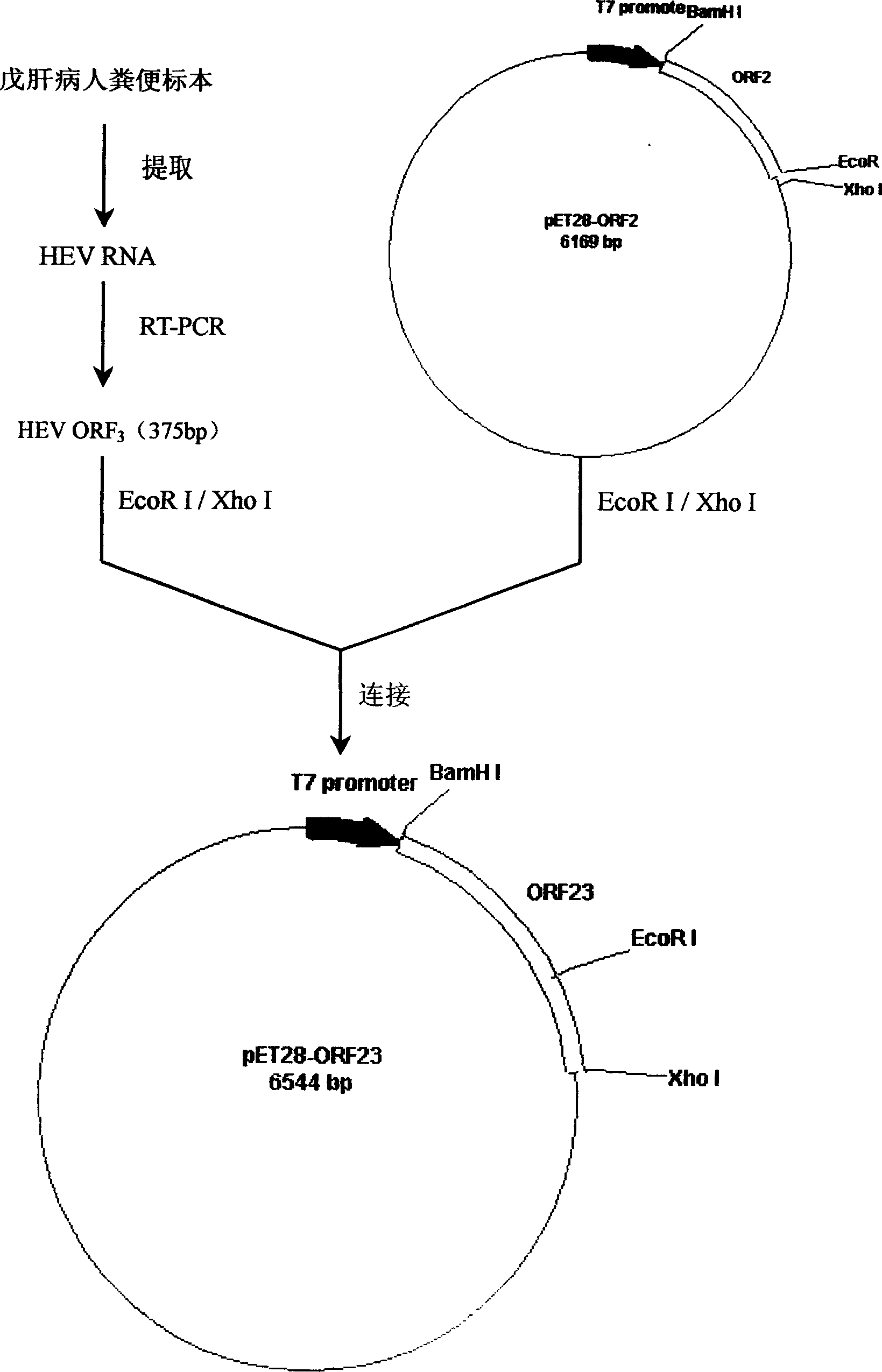
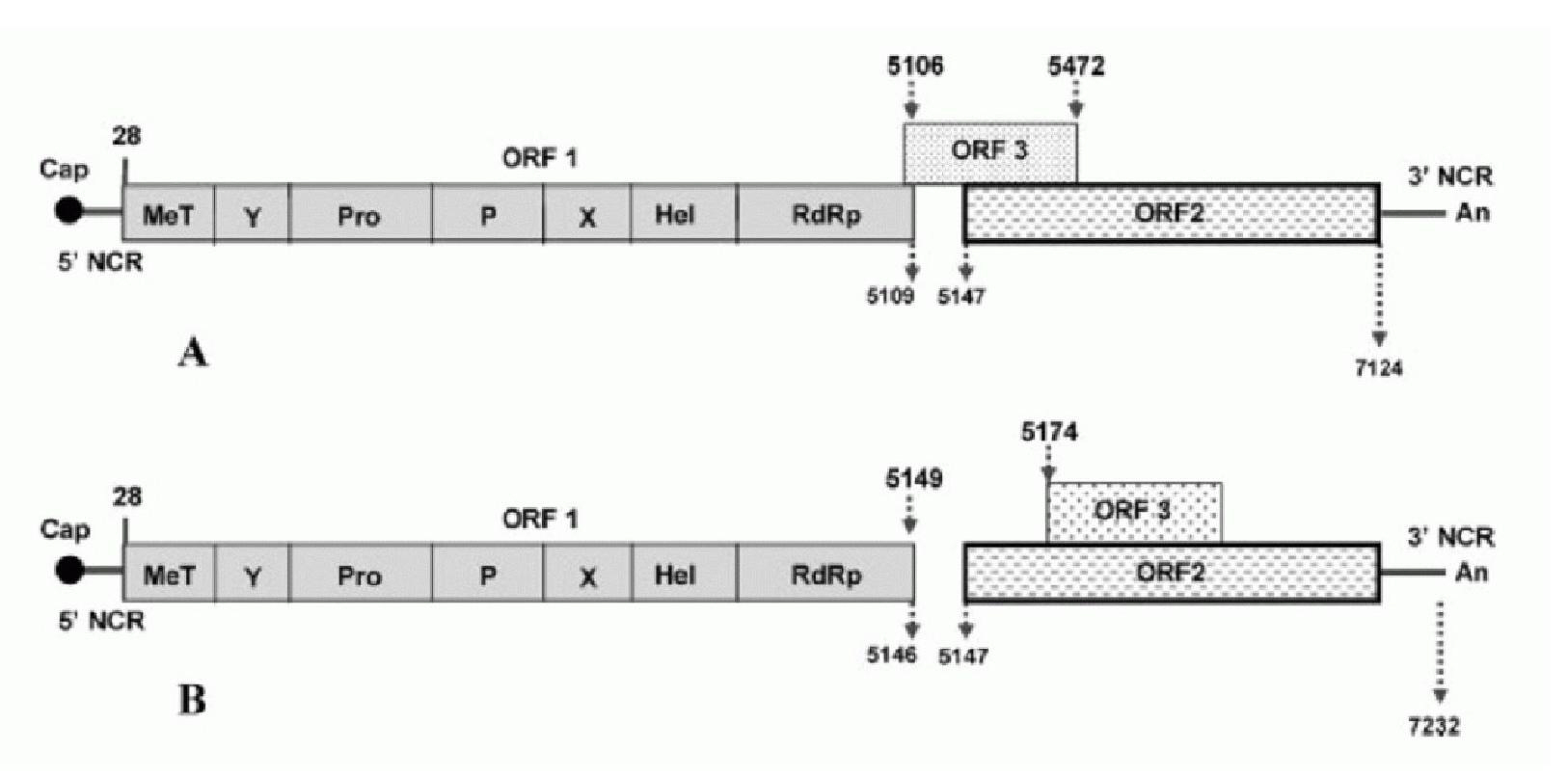
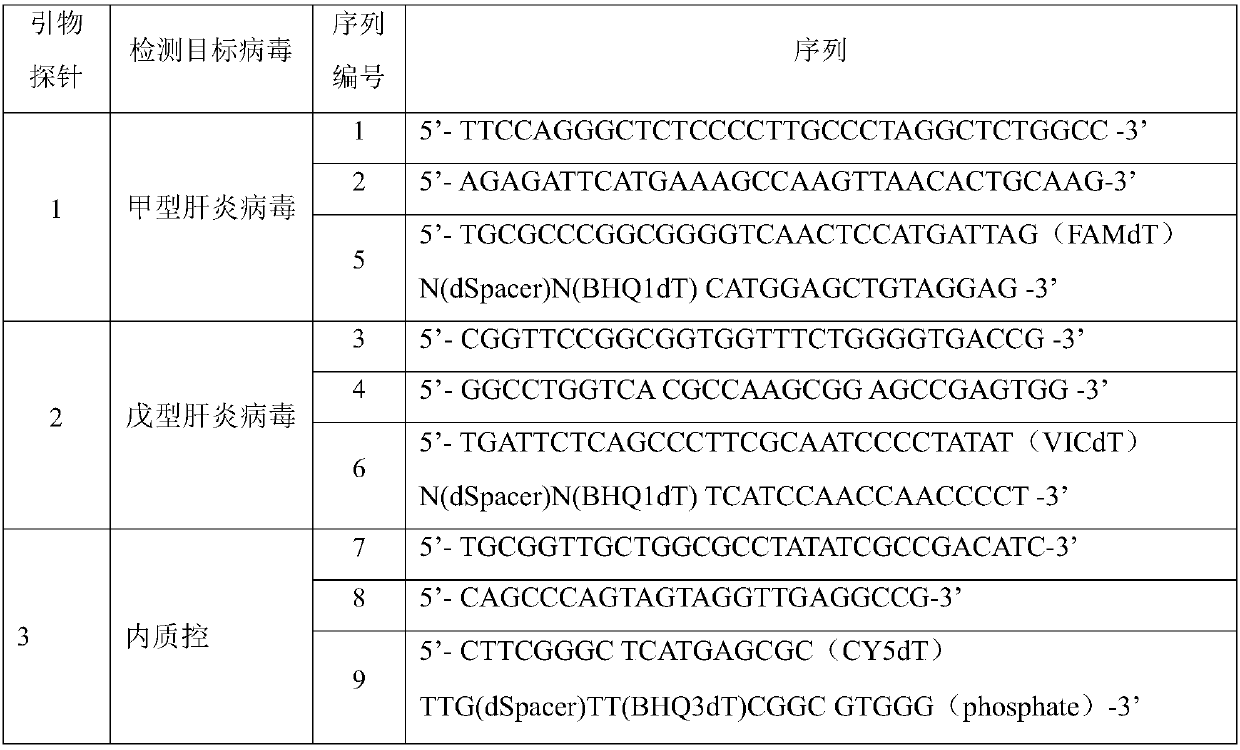
Patents
Literature
80 results about "Hepatitis E" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hepatitis E is inflammation of the liver caused by infection with the hepatitis E virus (HEV). Hepatitis E has mainly a fecal-oral transmission route that is similar to hepatitis A, but the viruses are unrelated. In retrospect, the earliest known epidemic of hepatitis E occurred in 1955 in New Delhi, but the virus was not isolated until 1983, by Russian scientists investigating an outbreak in Afghanistan. One of five known human hepatitis viruses: hepatitis A, B, C, D, and E, HEV is a positive-sense, single-stranded, nonenveloped, RNA icosahedral virus.
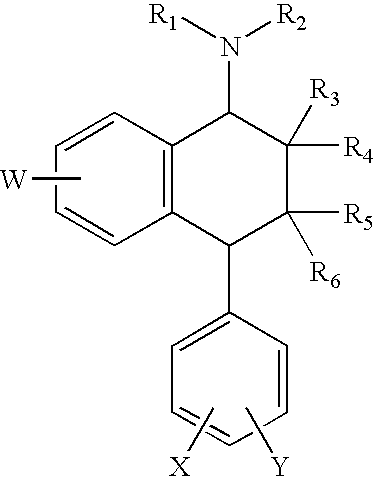
Compositions and methods for treatment of viral diseases
InactiveUS20080161324A1Slow and stop replicationReduce loadBiocideMicrobiological testing/measurementSingle-Stranded RNADisease
The present invention features compositions, methods, and kits useful in the treatment of viral diseases. In certain embodiments, the viral disease is caused by a single stranded RNA virus, a flaviviridae virus, or a hepatic virus. In particular embodiments, the viral disease is viral hepatitis (e.g., hepatitis A, hepatitis B, hepatitis C, hepatitis D, hepatitis E). Also featured are screening methods for identification of novel compounds that may be used to treat a viral disease.
Owner:EXCRX SINGAPORE PTE +1
Compositions and methods for treatment of viral diseases
InactiveUS20100009970A1Slow and stop replicationReduce loadBiocideNervous disorderSingle-Stranded RNADisease
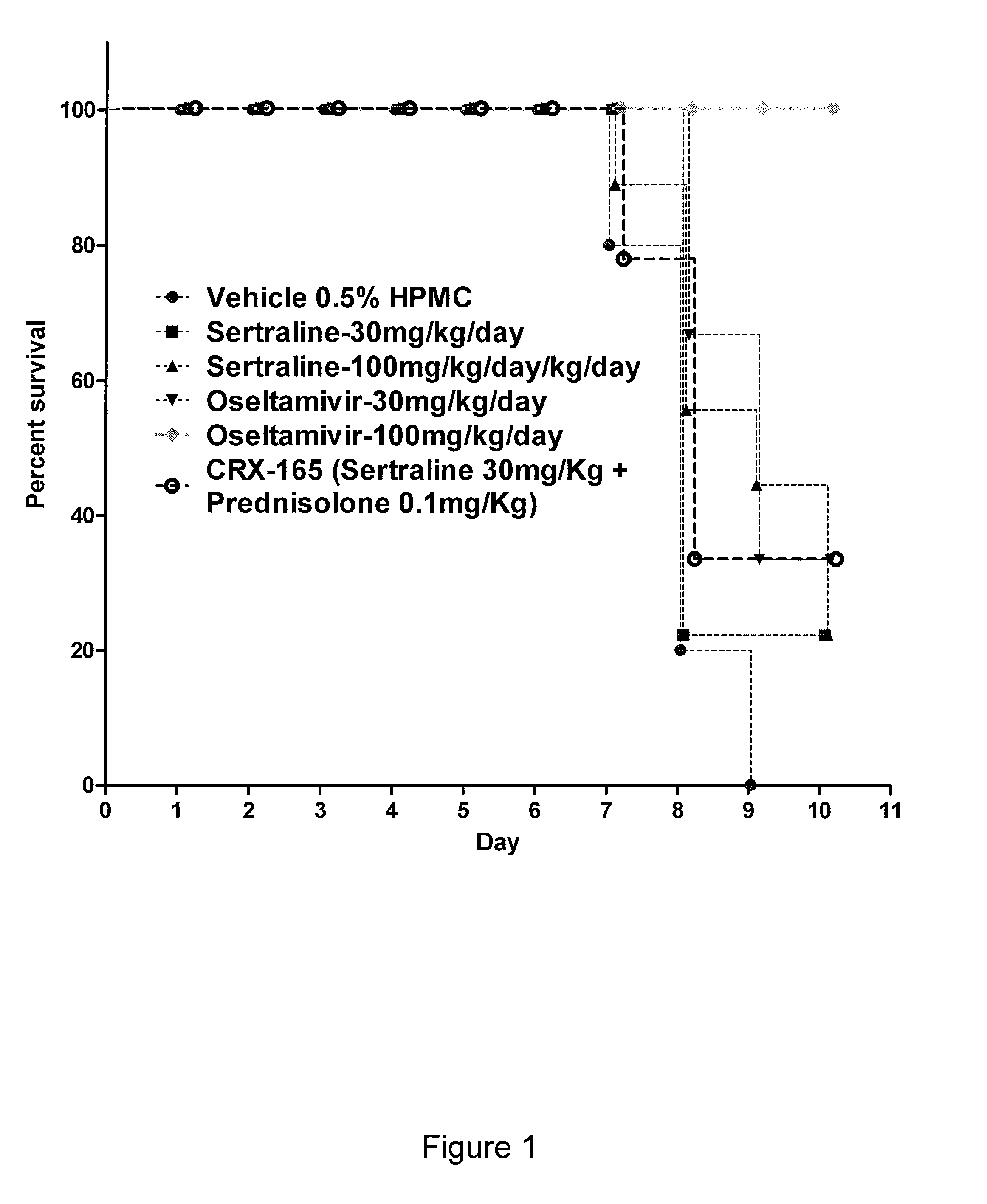
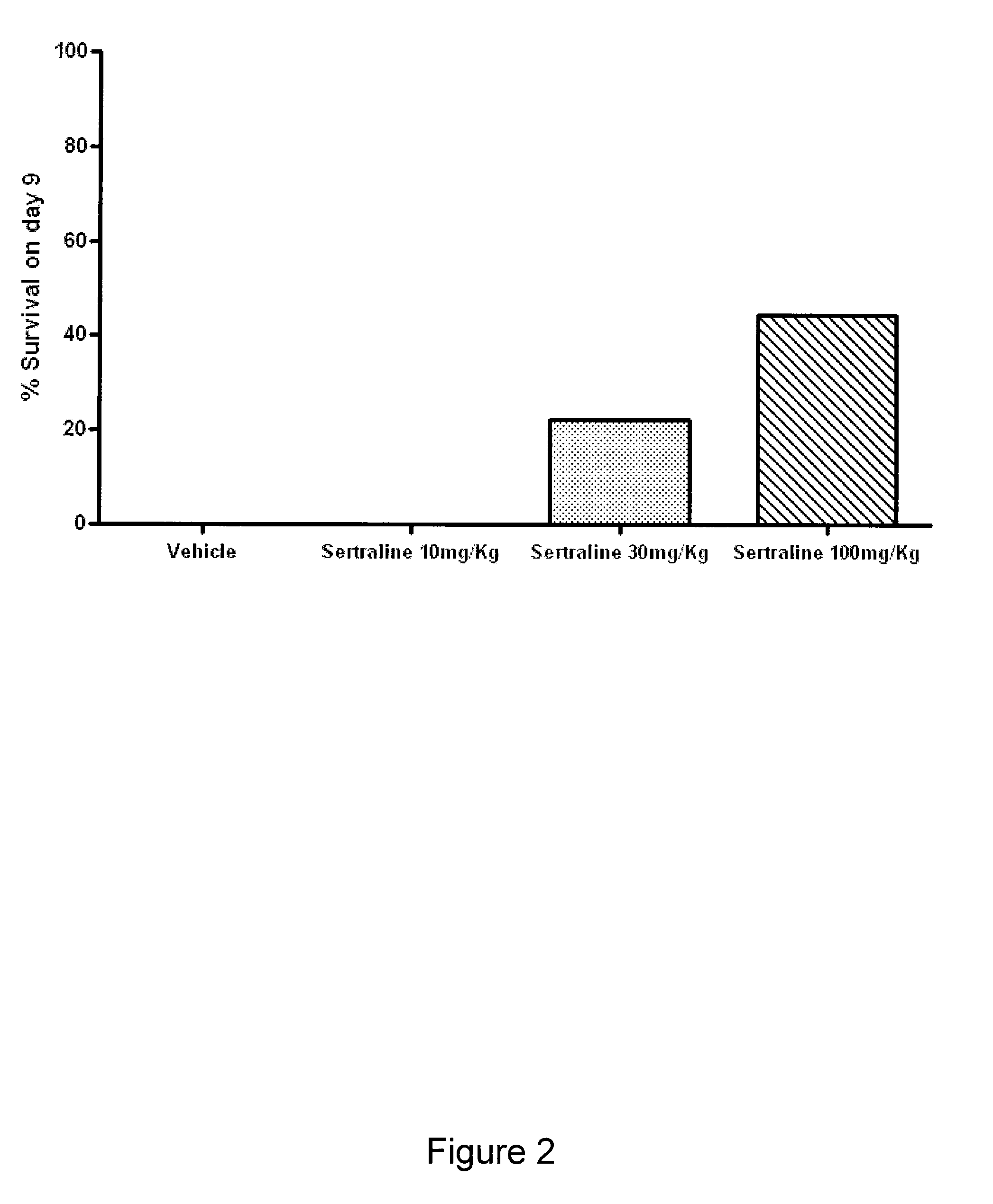
The present invention features compositions, methods, and kits useful in the treatment of viral diseases. In certain embodiments, the viral disease is caused by a single stranded RNA virus, a flaviviridae virus, or a hepatic virus. In particular embodiments, the viral disease is viral hepatitis (e.g., hepatitis A, hepatitis B, hepatitis C, hepatitis D, hepatitis E) and the agent or combination of agents includes sertraline, a sertraline analog, UK-416244, or a UK-416244 analog. Also featured are screening methods for identification of novel compounds that may be used to treat a viral disease.
Owner:EXCRX SINGAPORE PTE +1
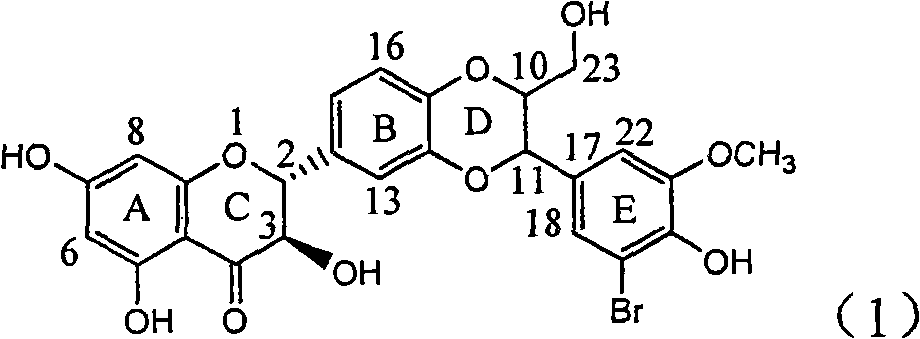
Application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829094AInhibitory activityInhibition of replicative activityOrganic active ingredientsAntiviralsDiseasePositive control
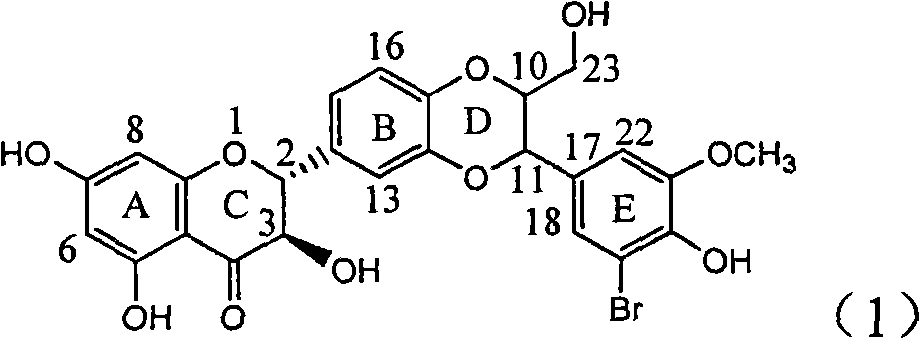
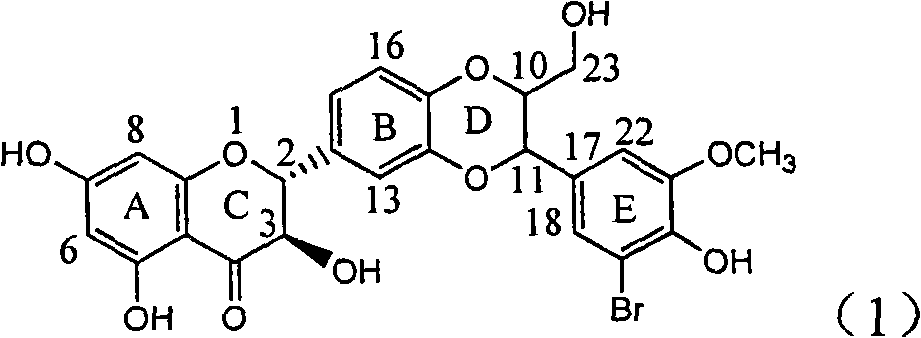
The invention relates to application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity on suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 38.2 percent and 39.1 percent which are respectively 2.4 times and 2.3 times of that of a positive control medicament (10,000 units / milliliter of alpha-interferon). Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 36 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
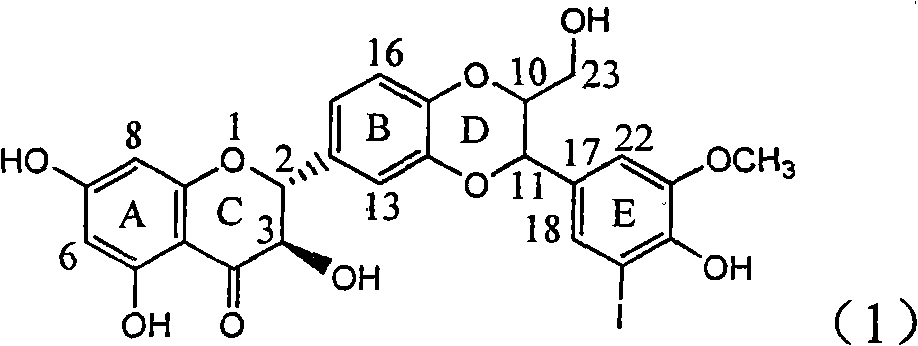
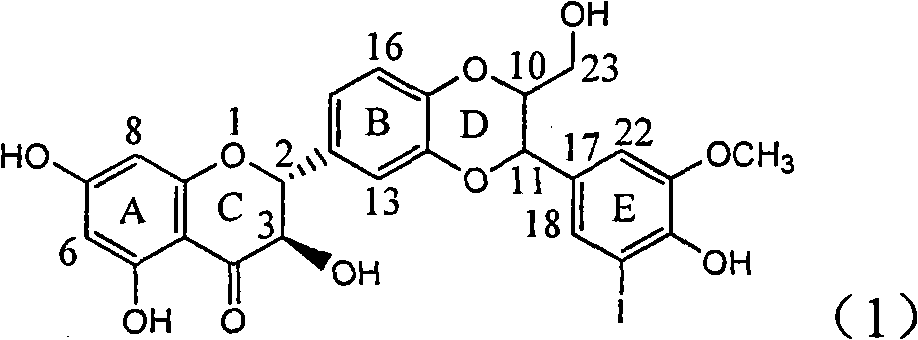
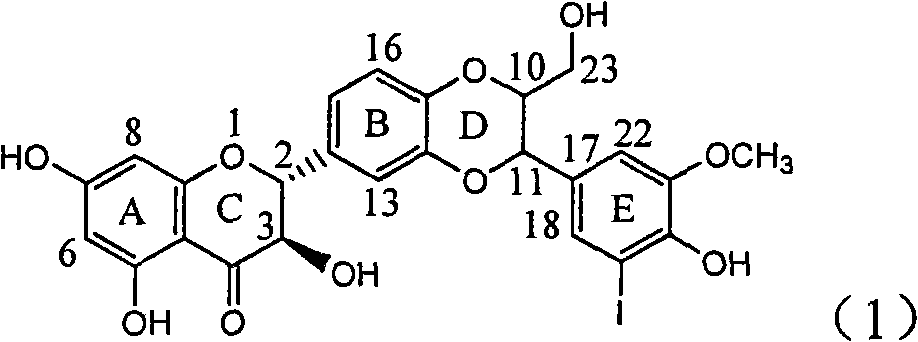
Application of ring E iodine substituted silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829096AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring E iodine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity of suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 20.0 percent and 29.0 percent which exceed that of a positive control medicament (10,000 units / milliliter of alpha-interferon) by 24 percent and 72 percent. Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 32.6 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
Method for diagnosing hepatitis e virus infection and kit
ActiveCN104792987ASignificant beneficial technical effectEasy to collectDisease diagnosisAntigenHepatitis E
The invention discloses a method for diagnosing whether a subject is infected with a hepatitis e virus (HEV) or suffered from hepatitis e or not. The method comprises the following steps: (1) determining existence of a hepatitis e virus antigen (HEV-Ag) in a urine sample from the subject; and (2) determining whether the subject is infected with the HEV or suffered from the hepatitis e or not, wherein the existence of the HEV-Ag in the urine sample of the subject shows that the subject is infected with the HEV or suffered from the hepatitis e. The invention further discloses a kit for the method. In addition, the invention further discloses a method for screening candidate medicines capable of resisting the hepatitis e virus infection or treating the hepatitis e, and a kit for the method.
Owner:NAT INST FOR FOOD & DRUG CONTROL +2
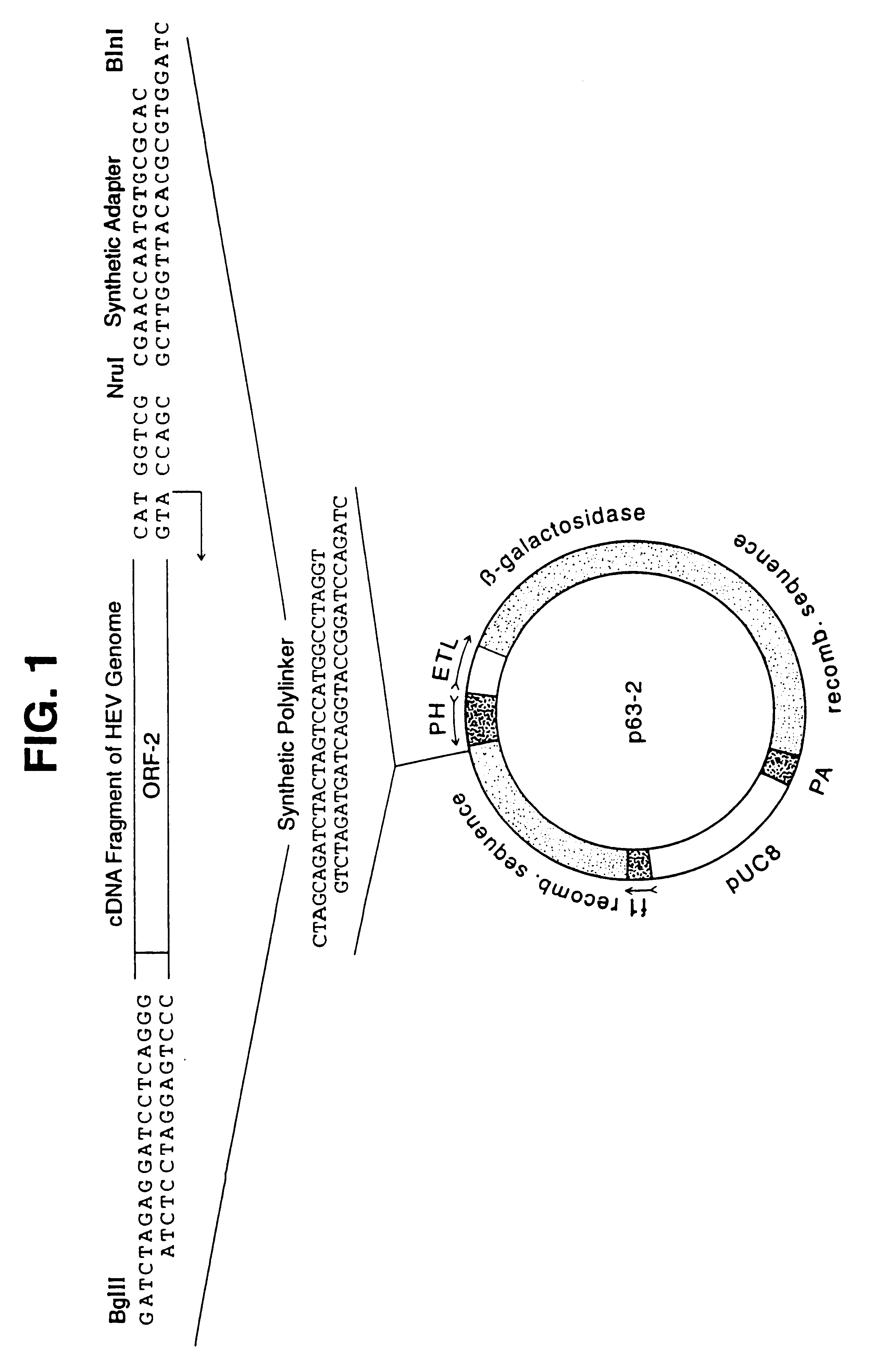
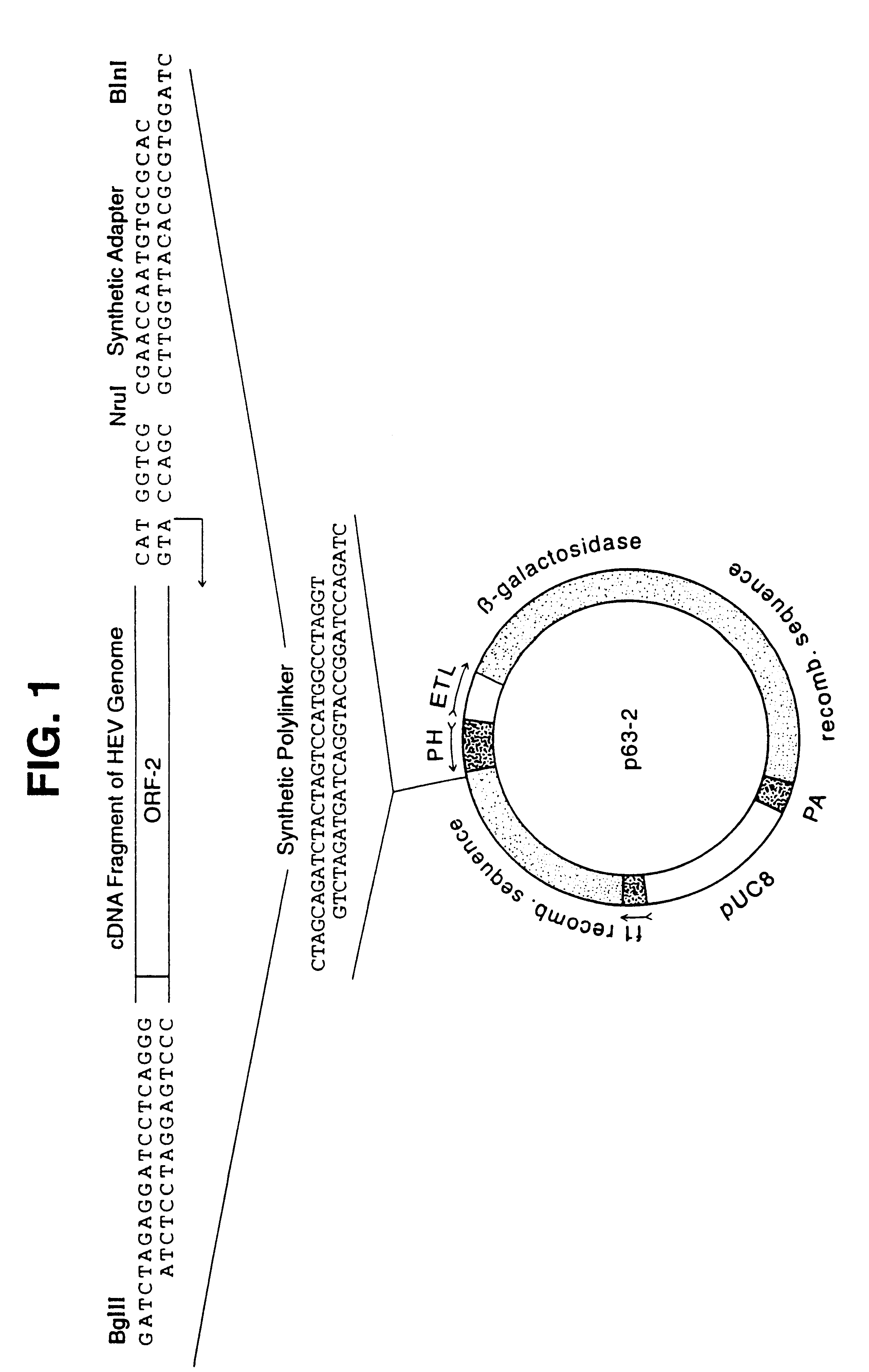
Recombinant proteins of a pakistani strain of hepatitis E and their use in diagnostic methods and vaccines
The invention relates to the expression of open reading frame 2 (ORF-2) proteins of a strain of hepatitis E virus from Pakistan (SAR-55) in a eukaryotic expression system. The expressed proteins can serve as an antigen in diagnostic immunoassays and / or as an immunogen or vaccine to protect against infection by hepatitis E.
Owner:NOVAVAX +1
Recombined hepatitis E hepatitis virus protein, preparation method and usage thereof
InactiveCN101062941AStrong targetingPracticalMicrobiological testing/measurementDigestive systemSolubilityInclusion bodies
The invention discloses a restructuring the fifth of the ten Heavenly Stems type hepatitis virus protein, coded sequence and usage, which is characterized by the following: the HEV virus strain to prepare restructuring the ten Heavenly Stems vaccine is the first gene type; the HEV p179 is based on popular production of the forth gene type HEV virus strain and possesses stronger placement and practical value; the HEV p179 can get highly effective expression in pronucleus system and 90% protein is nature solubility; the HEV p179-VLPs with diameter at about 20nm is constituted by 179aa; the HEV p179-VLPs is smallest restructuring HEV protein compared to now eucaryon system and HEV VLPs of pronucleus system expression.
Owner:SOUTHEAST UNIV
Recombinant proteins of a Pakistani strain of hepatitis E and their use in diagnostic methods and vaccines
A strain of hepatitis E virus from Pakistan (SAR-55) implicated in an epidemic of enterically transmitted non-A, non-B hepatitis, now called hepatitis E, is disclosed. The invention relates to the expression of the whole structural region of SAR-55, designated open reading frame 2 (ORF-2), in a eukaryotic expression system. The expressed protein is capable of forming HEV virus-like particles which can serve as an antigen in diagnostic immunoassays and as an immunogen or vaccine to protect against infection by hepatitis E.
Owner:UNITED STATES OF AMERICA
Application of stabilizer trehalose in pig hepatitis E diagnostic kit
The invention discloses an application of stabilizer trehalose in a pig hepatitis E diagnostic kit; 8-12wt percent of trehalose is added in enzyme combination diluent of the pig hepatitis E diagnostic kit, the function of the enzyme combination diluent is kept to be stable by utilizing the stabilizer trehalose, so as to keep the stabilization of the pig hepatitis E diagnostic kit.
Owner:SHANGHAI ACAD OF AGRI SCI
Protein for preparation of hepatitis E virus-like particles and method
ActiveCN104211784AAvoid damageReduce the cost of trainingSsRNA viruses positive-senseVirus peptidesDiseaseVirus-like particle
The present invention relates to the field of molecular biology and virology. In particular, the invention relates to a protein capable of being assembled in vitro into hepatitis E virus-like particles, a coding sequence and a preparation method thereof, and the virus-like particles comprising the protein; the protein and the virus like particles can be used for the prevention of or for the treatment of HEV (hepatitis E virus) infection and diseases caused by the HEV, such as hepatitis E and the like. The invention also relates to use of the protein and the virus like particles in the preparation of pharmaceutical compositions and vaccines, the pharmaceutical compositions or the vaccines are used for the prevention or treatment of the HEV infection and diseases caused by the HEV, such as hepatitis E and the like. In addition, the invention also provides a preparation method of the HEV virus like particles.
Owner:XIAMEN UNIV +1
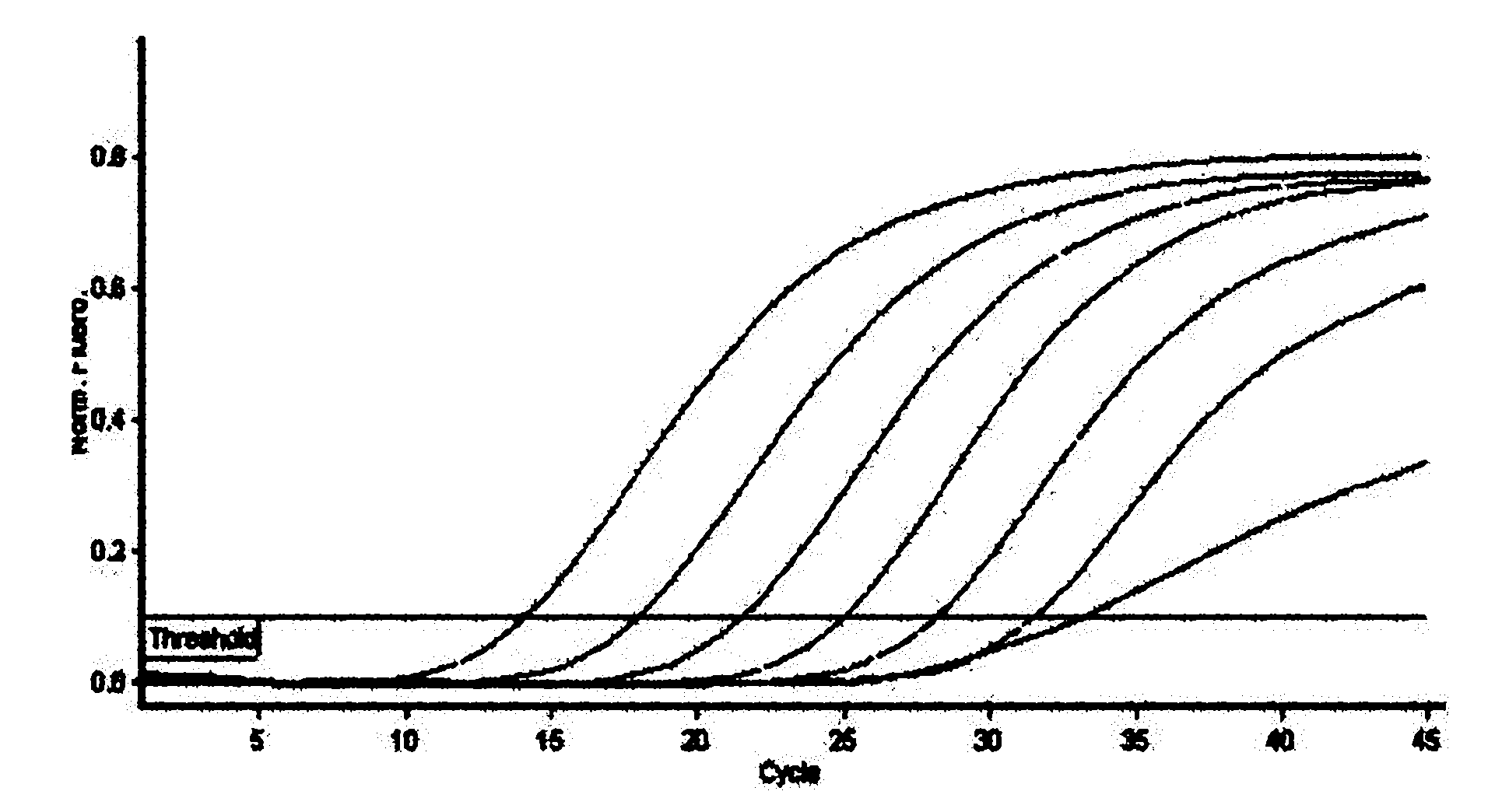
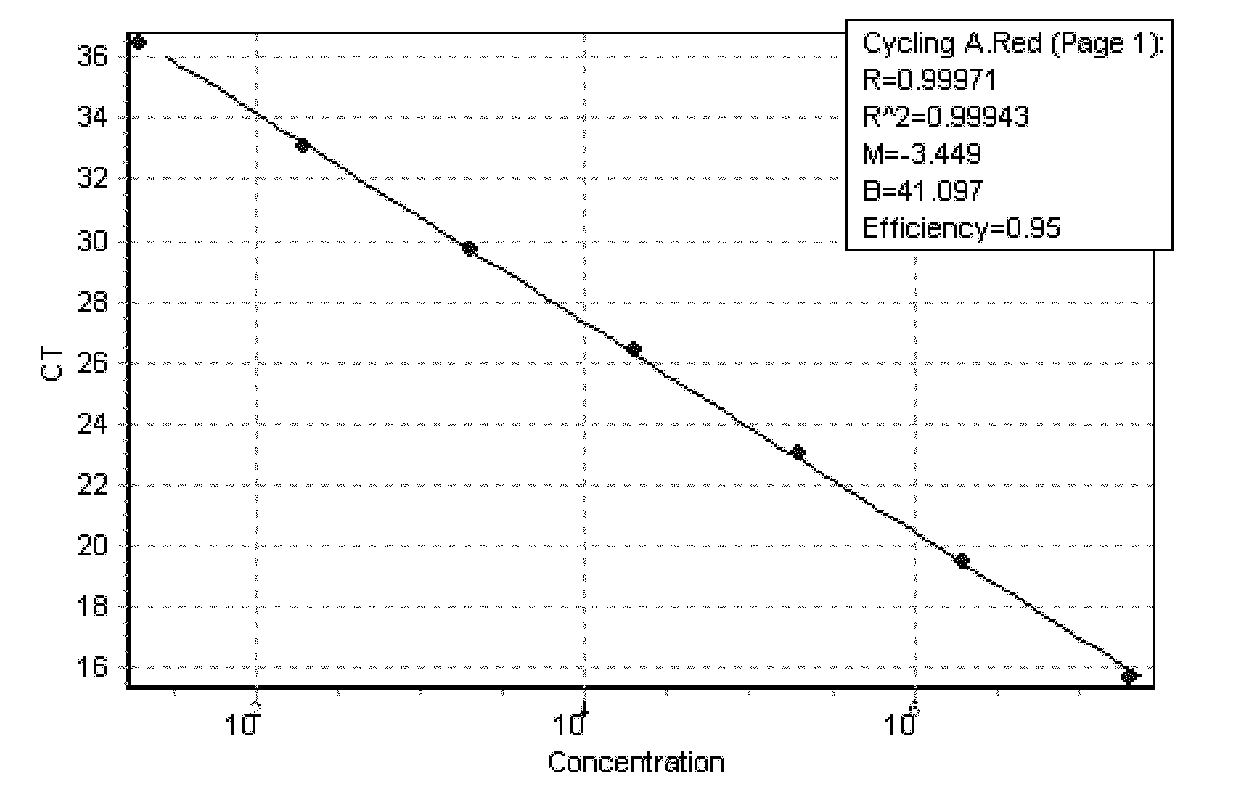
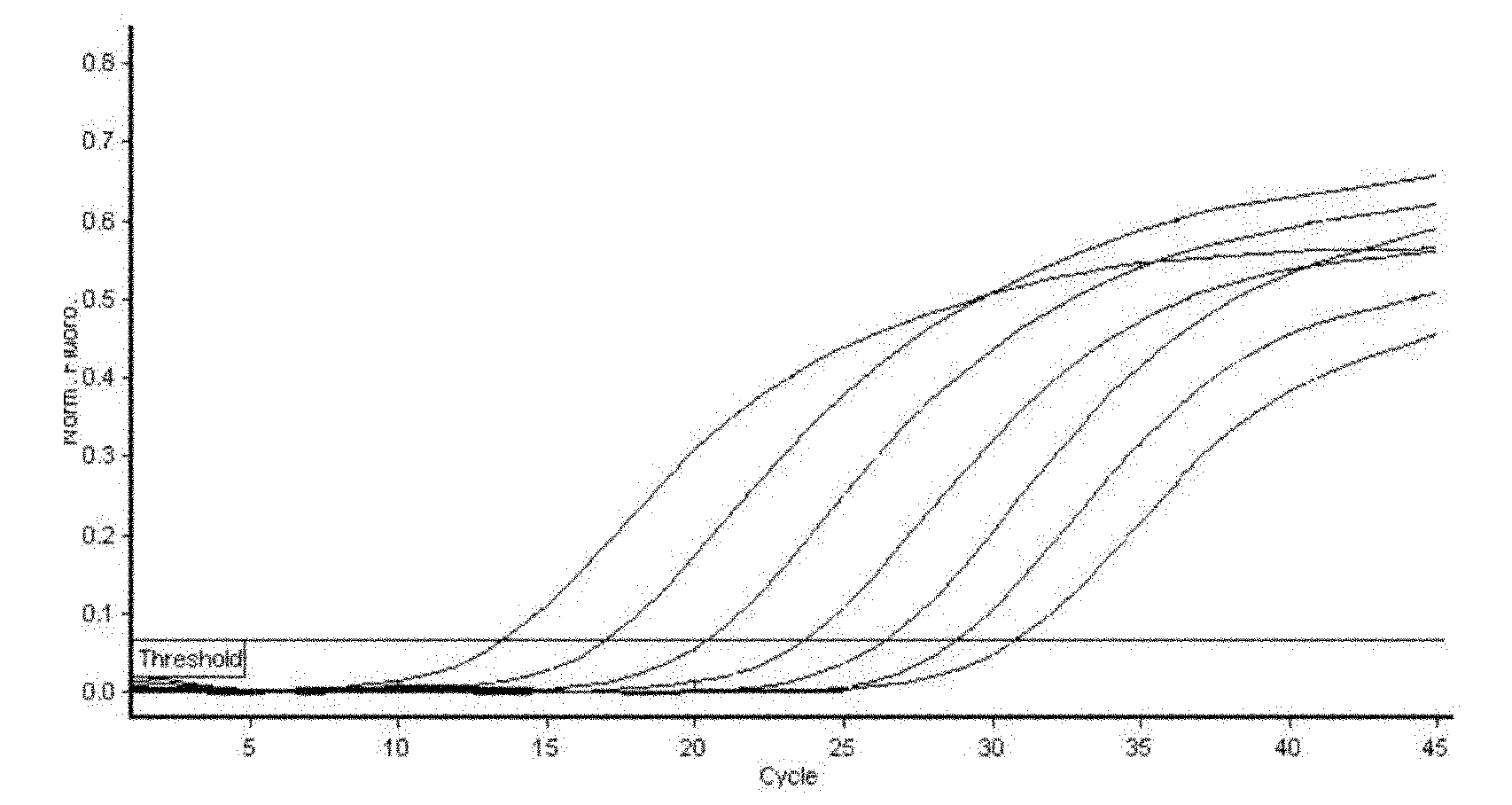
Identification and detection method for hepatitis E virus by utilizing quadruple fluorescence quantitative PCR (Polymerase Chain Reaction)
ActiveCN101962689AHigh sensitivityImprove accuracyMicrobiological testing/measurementFluorescence/phosphorescenceDiseaseFluorescence
The traditional pig source hepatitis E has no effective vaccine for prevention, which adopts a control measure that the finding is carried out as soon as possible and the epidemic condition in epidemic areas is monitored at any time to block the spread of the disease. The invention discloses a rapid detection method for identifying each gene type of hepatitis E virus by utilizing multiple fluorescence quantitative PCR, which is characterized in that the a pair of conservative amplification primers and 4 strip-shaped specificity TaqMan probes are designed aiming at an HEVORF 3 sequence; the four probes are respectively designed aiming at the respective ORF 3 metamorphosis region sequence in a type specificity mode; and the identification and the detection of different gene types can be realized. The invention maintains the characteristics of high sensitivity and high accuracy of a PCR method and has the advantages that the quadruple fluorescence quantitative PCR enhances the detection efficiency and reduces the detection cost; the purposes of identification and diagnosis can be simultaneously realized; and the invention lays a foundation for work of infection source survey, spread environment, virus source tracing, etc.
Owner:THE INSPECTION & QUARANTINE TECH CENT ZHEJIANG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Preparation method of diagnostic kit for pig hepatitis E virus ELISA
The invention discloses a preparation method of a diagnostic kit for pig hepatitis E virus ELISA, which comprises the following steps: (1) selecting antigen epitopes by a computer; (2) preparing antigens; (3) collecting serum; (4) establishing an indirect ELISA method; (5) verifying the sensibility and the specificity of the indirect ELISA method; (6) verifying the stability of the kit; and (7) carrying out clinical detection on a slaughterhouse. The method has the advantages of simplicity, high speed, high sensitivity, strong specificity, qualified stability and good repeatability. The kit prepared by the invention can effectively detect the hepatitis E virus of infected pigs, is suitable for detection of a large amount of pathogenetic samples, and can be used for quickly diagnosing the pig hepatitis E, preventing and controlling the spread of the pig hepatitis E virus and preventing the hepatitis E virus from spreading to people from pigs.
Owner:SHANGHAI ACAD OF AGRI SCI
Method for cultivating hepatitis E viruses
InactiveCN105671004AImprove replication efficiencyEasy to copySsRNA viruses positive-senseMicroorganism based processesBiological propertyWestern blot
The invention discloses a method for cultivating hepatitis E viruses in an in-vitro manner. The method has the advantages that miRNA-A6 [micro-RNA (ribonucleic acid)-A6] is transfected by cells for 24 h, then viruses are inoculated and can be massively replicated, and mass replication of offspring viruses of the HEV (hepatitis E viruses) can be promoted by the transfected miRNA-A6 as revealed by means of Real-Time qPCR (quantitative polymerase chain reaction) and Western Blot analysis; the method is major breakthrough in the aspect of HEV in-vitro cultivation, and excellent foundation is laid for further researching biological characteristics and immunological characteristics of the HEV, researching and developing HEV vaccine and screening anti-HEV medicines.
Owner:KUNMING UNIV OF SCI & TECH
Chemiluminscence immunoassay kit of hepatitis E virus IgG antibody and preparation method thereof
InactiveCN101551396AHigh sensitivityEfficient use ofChemiluminescene/bioluminescenceAntigenAntiendomysial antibodies
The invention discloses a chemiluminscence immunoassay kit of a hepatitis E virus IgG antibody and a preparation method thereof. The kit comprises negative and positive reference substances of the hepatitis E virus IgG antibody, a coated solid phase carrier, an enzyme labeling combination, sample diluent, chemiluminescent substrate solution and concentrated cleaning solution. The preparation method of the kit comprises the following steps: the reference substances are prepared according to the negative and the positive serums of the hepatitis E virus IgG antibody; the solid phase carrier is coated by recombining the antigen of the hepatitis E virus; the special anti-human IgG antibody is labeled by enzyme; the sample diluent is prepared; the chemiluminescent substrate solution is prepared; the concentrated cleaning solution is prepared; all the components are subpackaged; and the components are assembled into finished products. The kit can provide the most direct clinical basis for the prevention, the diagnosis and the treatment of the hepatitis E.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Preparation method of E hepatitis rabbit-human chimeric antibody quality control substance
The invention discloses an E hepatitis rabbit-human chimeric antibody quality control substance and preparation method thereof, belonging to the clinical examination and biotechnology field. The E hepatitis rabbit-human chimeric antibody quality control substance is characterized in that:the E hepatitis rabbit-human chimeric antibody quality control substance is chimeric antibody formed by cross-linking rabbit-anti-human HEV-IgG and human IgM using 1-ethyl-3[3-dimethyl amino propyl] carbodiimide. The innovation point of the invention is that: the chimeric antibody quality control substance for iImmunological analysis of HEV is suitable for EQA and IQC. The anti HEV IgM quality control substance is constructed using a chemical crosslink method for the first time at home and abroad. The quality control substance is repetitively produced on the premise of relative uniform and relatively stable and economic and easy to obtain a large amount at high titer without latent biology infection danger and reference to medical ethics problem and with higher affinity with the antigen. The E hepatitis rabbit-human chimeric antibody quality control substance is a good positive quality control substance.
Owner:BEIJING HOSPITAL
Inactivated Poliomyelitis Vaccine Derived From Sabin Strain Of Polio Virus
InactiveUS20080193478A1Effective immunizationEffective and stableSsRNA viruses positive-senseViral antigen ingredientsAntigenHuman immunodeficiency
An inactivated Polio Vaccine derived from Sabin strain for safe and effective immunization against Poliomyelitis is provided. A process of preparation for such vaccine and formulations thereof are also provided. Administration of the vaccine of the present invention along with other antigens provides immunization not only against polio infection but also against other pathogens causing Hepatitis C. Hepatitis D. Hepatitis E. Meningitis A. Meningitis B. Meningitis C. Meningitis W. Meningitis Y. Pnemococcal (23 valent or more). Smallpox, Typhoid, Bacille Calmette Guerin, Tuberculosis. Human Immunodeficiency Virus. Anthrax or the like, to which children or adults not immunized earlier are susceptible, particularly to which children are susceptible.
Owner:PANACEA BIOTEC
Chemiluminescence immunoassay kit of hepatitis E virus IgM antibody and preparation method thereof
InactiveCN101551395AEfficient use ofGuaranteed SensitivityChemiluminescene/bioluminescenceIgm antibodyPerformance index
The invention relates to a chemiluminescence immunoassay kit of a hepatitis E virus IgM antibody and a preparation method thereof, which belongs to the technical field of clinical in-vitro diagnosis immunoassay. The preparation method of the kit comprises the following steps: the reference substance is prepared according to the negative and the positive serums of the hepatitis E virus IgM antibody; a solid phase carrier is coated by an anti-human-mu chain anti-body (monoclonal antibody or polyclonal antibody); the recombinant antigen of the hepatitis E virus is labeled by enzyme; a chemiluminescent substrate solution acted by the enzyme is prepared; a concentrated cleaning solution is prepared; the negative reference substance, the positive reference substance, the labeled combination, the chemiluminescent substrate and the concentrated cleaning solution of the hepatitis E virus IgM antibody are subpackaged; and all the components are assembled into finished products. The prepared kit has stable performance indexes (specificity, sensitivity and stability) and can be applied to the early diagnosis of the hepatitis E.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Avian hepatitis E virus, vaccines and methods of protecting against avian hepatitis-splenomegaly syndrome and mammalian hepatitis E
InactiveUS7005130B2SsRNA viruses positive-senseSugar derivativesNucleic acid hybridisationAvian hepatitis E virus
The present invention relates to a novel isolated avian hepatitis E virus having a nucleotide sequence set forth in SEQ ID NO:1 or its complementary strand. The invention further concerns immunogenic compositions comprising this new virus or recombinant products such as the nucleic acid and vaccines that protect an avian or mammalian species from viral infection or hepatitis-splenomegaly syndrome caused by the hepatitis E virus. Also included in the scope of the invention is a method for propagating, inactivating or attenuating a hepatitis E virus comprising inoculating an embryonated chicken egg with a live, pathogenic hepatitis E virus and recovering the virus or serially passing the pathogenic virus through additional embryonated chicken eggs until the virus is rendered inactivated or attenuated. Further, this invention concerns diagnostic reagents for detecting an avian hepatitis E viral infection or diagnosing hepatitis-splenomegaly syndrome in an avian or mammalian species comprising an antibody raised or produced against the immunogenic compositions and antigens such as ORF2 proteins expressed in a baculovirus vector, E. coli, etc. The invention additionally encompasses methods for detecting avian HEV nucleic acid sequences using nucleic acid hybridization probes or oligonucleotide primers for polymerase chain reaction (PCR).
Owner:VIRGINIA TECH INTPROP INC
Method of predicting potential severity of hepatitis e, probe sets, and primer sets
InactiveUS20100173283A1SsRNA viruses positive-senseMicrobiological testing/measurementGenotypeAmino acid mutation
The present invention provides a method of predicting potential severity of hepatitis, includes determining that hepatitis in a subject is potentially severe when it is detected that any amino acid is mutated to an amino acid of genotype-4, the any amino acid being amino acid of an amino acid sequence in a region encoded by ORF1 of an HEV genome RNA of genotype 3, and the HEV genome RNA being contained in a specimen nucleic acid taken from the subject infected with genotype-3 HEV.
Owner:TAKAHASHI KAZUAKI +3
Detection type gene chip for detecting various peptitis
InactiveCN1392268AImprove detection efficiencyReduce dosageMicrobiological testing/measurementGenotypeInfective disorder
The present invention provides a detection gene chip for diagnosing serveral kinds of hepatitis and new primer with high detection rate for using the gene chip in various subtype amplification. The gene chip includes detection quality controlling system and disease diagnosing system. The chip can be used to detect the virus ites of hepatitis B and hepatitis C and detect hepatitis D, hepatitis E, hepatitis G and TTV simultaneously. It has short detection period, high diagnosis accuracy and low diagnosis cost and may be used in epidemiological investigation and blood detection for identifying hepatitis gene type.
Owner:赵伟 +2
method for structuring hepatitis e infection model
InactiveCN101991610AEasy to operateEasy to feed and manageDigestive systemViral/bacteriophage medical ingredientsInterspecies transmissionOral medication
The invention discloses a method for structuring a hepatitis E infection model, which comprises the following steps of: infecting Meriones unguiculatus with hepatitis E viruses to obtain the hepatitis E infected model. The infection method is oral administration. The method of the invention has low price, adopts the Meriones unguiculatus infection model which is convenient to feed and test, and avoids the defects of high price, limited sources, high experiment requirement condition, long propagation period and the like of non-human primates used at present. The invention provides the ideal infection model for the study of HEV (hepatitis E virus) pathogenesis, vaccine development, interspecies transmission mechanism and the like.
Owner:CHINA AGRI UNIV
Hepatitis A-hepatitis E combined vaccine and preparation method thereof
InactiveCN1883704AImproving immunogenicityLow production costDigestive systemAntiviralsAttenuated Live VaccineHepatitis E
Disclosed are a combined vaccine for hepatitis A and hepatitis E, and preparation method thereof. Said vaccine comprises hepatitis E virus recombined peoteins, hepatitis A attenuated live vaccine and hepatitis A inactivated vaccine. Said method comprises before the paretion of the final combined vaccine, absorbing hepatitis A attenuated live vaccine viruses and / or hepatitis A inactivated vaccine viruses on aluminum hydroxide gel, absorbing hepatitis E virus recombined peoteins on aluminum hydroxide gel, adjusting the pH value of the two compositons, and mixing the two compositons together. The inventiion is provided with convenience, multiple-effect, low cost, and effective prevention of hepatitis A and hepatitis E for human and animals.
Owner:SOUTHEAST UNIV
Virus protein granules of hepatitis E virus-4 ORF2 fragment and prepration method and application thereof
The invention relates to protein granules of hepatitis E virus, in particular to virus protein granules of a hepatitis E virus-4 ORF2 fragment, recombinant expression vectors, host cells and antibodies of hepatitis virus protein granules, application and expression methods of the hepatitis virus protein granules, the antibodies and nucleotide sequences, and preparation methods and application thereof. The sequences of the virus protein granules have at least 477 continuous nucleotide sequences in the sequences expressed as SEQ No: 1 or SEQ No: 3.
Owner:NAT INST FOR THE CONTROL OF PHARMA & BIOLOGICAL PROD
Primer, probe and kit for detecting hepatitis A and hepatitis E viruses
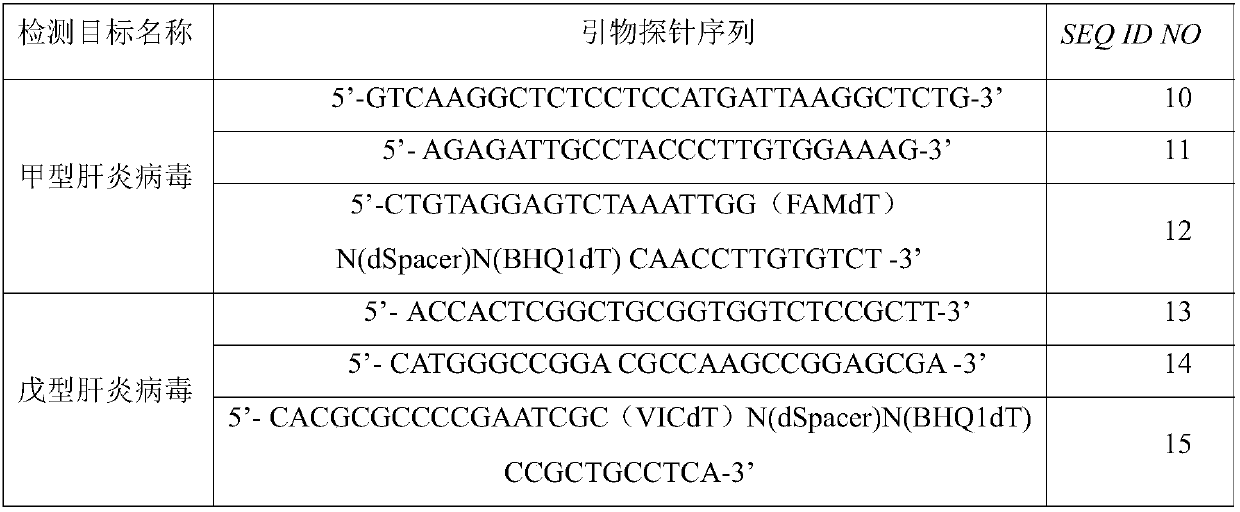
ActiveCN108034763AQuick result determinationComprehensive result determinationMicrobiological testing/measurementMicroorganism based processesNucleotideRecombinase Polymerase Amplification
The invention discloses a primer probe set and a kit and a detection method for detecting hepatitis A and hepatitis E viruses through recombinase polymerase amplification. The primer comprises a nucleotide sequence shown as SEQ ID NO.1-4, and the probe comprises the nucleotide sequence shown as SEQ ID NO.5-6. The invention also provides a kit for detecting hepatitis A and hepatitis E viruses through recombinase polymerase amplification, and the kit comprises the primer probe set provided in the invention. The primer, the probe and the kit obviously increase the sensitivity, specificity, and simpleness of detection of hepatitis A and hepatitis E viruses.
Owner:北京卓诚惠生生物科技股份有限公司
Hepatitis e virus orf2 capsid polypeptides and uses thereof
ActiveUS20190352342A1Improve understandingEasy diagnosisSsRNA viruses positive-senseVirus peptidesSerum igeSubject matter
Hepatitis E virus (HEV) is responsible for over 50% of acute viral hepatitis cases worldwide. The inventors have now identified the precise sequence of infectious particle-associated ORF2 capsid protein. Strikingly, their analyses revealed that in infected patients, HEV produces three forms of the ORF2 capsid protein: ORF2i, ORF2g and ORF2c. The ORF2i protein is associated with infectious particles whereas ORF2g and ORF2c proteins are massively produced glycoproteins that are not associated with infectious particles and are the major antigens present in HEV-infected patient sera. Accordingly, the ORF2i and ORF2g proteins are thus the subject matter of the present invention as well as antibodies specific for the proteins and diagnostic assays (e.g. ELISA) for the diagnosis of Hepatitis E virus infection.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
Hepatitis A, hepatitis B and hepatitis E combination vaccine
ActiveCN101085347ALow costIncrease coverageDigestive systemAntiviralsImmunogenicityHepatitis B virus surface Antigen
A combined vaccine for treating hepatitis a, hepatitis b and hepatitis e is characterized in comprising deactivated hepatitis a virus 200-1000 U / mL, recombined hepatitis b virus surface antigen 10-40 mu g / mL, and recombined hepatitis e virus antigen 10-50 mu g / mL.The inventive combined vaccine uses deactivated HAV and recombined HBsAg with good safety and immunogenicigy. Former research has provn that the recombined HEV protein also has good safety and immunogenicigy; and can be used for development of hepatitis e vaccine. The inventive combined vaccine is technologically feasible. The invention can greatly reduce production costs of viral hepatitis vaccine and market price, and improve coverage ratio of vaccine due to the advantages of convenience, multiple effect and low cost.
Owner:滁州方舟药业有限公司
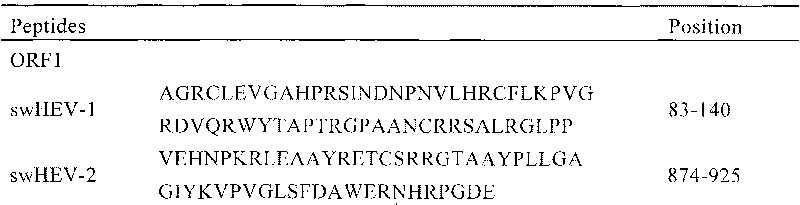
A porcine hepatitis E virus antigenic epitope and its application
InactiveCN102276700APopular controlEpidemic interruptionVirus peptidesAntiviralsEpitopeInapparent Infection
The invention discloses a swine hepatitis E virus antigen epitope and applications thereof, and the sequence of the swine hepatitis E virus antigen epitope is shown in SEQ ID No:8. The swine hepatitis E virus antigen epitope obtained by computer software screening and experiment validation, on one hand, is applicable to rapid specific swine hepatitis E diagnostic kits, and can be used for rapid diagnosis of swine hepatitis E, and especially for the development of differential diagnosis technology of vaccine immune antibody and natural inapparent infection antibody for the monitoring of swine hepatitis E prevalence; on the other hand, can also be used to design specific, safe and high-efficient swine hepatitis E vaccines for the prevention of swine hepatitis E, the control of swine hepatitis E prevalence, and the obstruction of hepatitis E spreading from swine to human body.
Owner:SHANGHAI ACAD OF AGRI SCI
Recombinant proteins of a Pakistani strain of hepatitis E and their use in diagnostic methods and vaccines
A strain of hepatitis E virus from Pakistan (SAR-55) implicated in an epidemic of enterically transmitted non-A, non-B hepatitis, now called hepatitis E, is disclosed. The invention relates to the expression of the whole structural region of SAR-55, designated open reading frame 2 (ORF-2), in a eukaryotic expression system. The expressed protein is capable of forming HEV virus-like particles which can serve as an antigen in diagnostic immunoassays and as an immunogen or vaccine to protect against infection by hepatitis E.
Owner:HEALTH & HUMAN SERVICES DEPT OF
Real-time fluorescent reverse transcription PCR detection primer, probe, detection kit and detection method for hepatitis e viruses
InactiveCN107287347ATime-consuming to solveResolve SensitivityMicrobiological testing/measurementDNA/RNA fragmentationBiologyHepatitis E
The invention discloses a real-time fluorescent reverse transcription PCR detection primer, a probe, a detection kit and a detection method for hepatitis e viruses. Aiming at hepatitis e viruses, the invention providesa specific primer, a probe and a detection kit containing the primer and the probe, and provides a detection method which utilizes the detection kit for confirming that whether the sample to be detected contains the hepatitis e viruses through real-time fluorescent reverse transcription PCR amplification. The detection kit and the detection method can realize quick detection on all genotypes of the hepatitis e viruses, have the advantages of strong specificity, high sensitivity, wide application range, simple, convenient and quick operation, accurate and reliable detection result and short detection time, can enable the sensitivity to reach 1*10<3>copies / mL, can solve the problems of long time consumption, low sensitivity and the like of the traditional detection method, are especially suitable for quick detection of large-scale samples, and can lay the foundation for infection source survey, propagation environments, virus sources and the like.
Owner:广州白云机场海关综合技术服务中心
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com