Chemiluminescence immunoassay kit of hepatitis E virus IgM antibody and preparation method thereof
A hepatitis E virus and chemiluminescence immunological technology, applied in the field of clinical in vitro diagnostic immunoassay medicine, can solve the problems of inability to widely apply clinical diagnosis and scientific research work, limited promotion and use, and high use cost, and ensure high sensitivity and high sensitivity. , the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1 Preparation of hepatitis E virus IgM antibody chemiluminescence immunoassay assay kit of the present invention
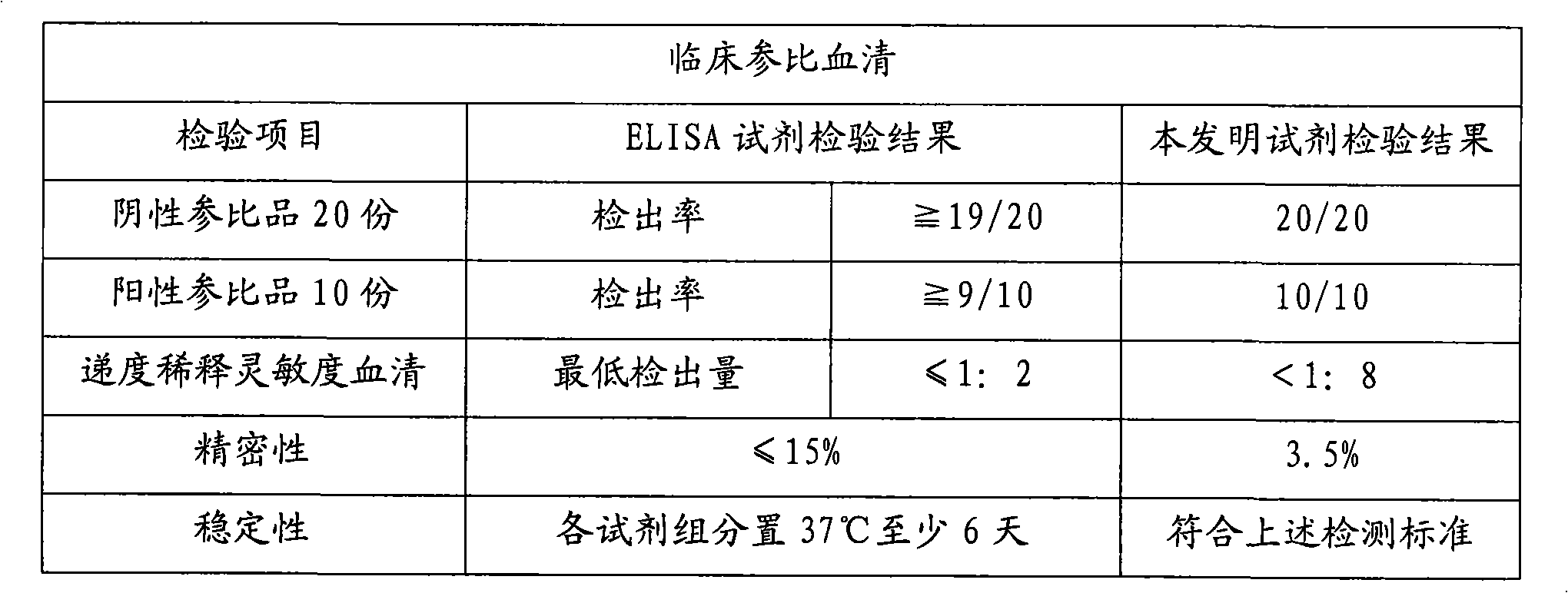
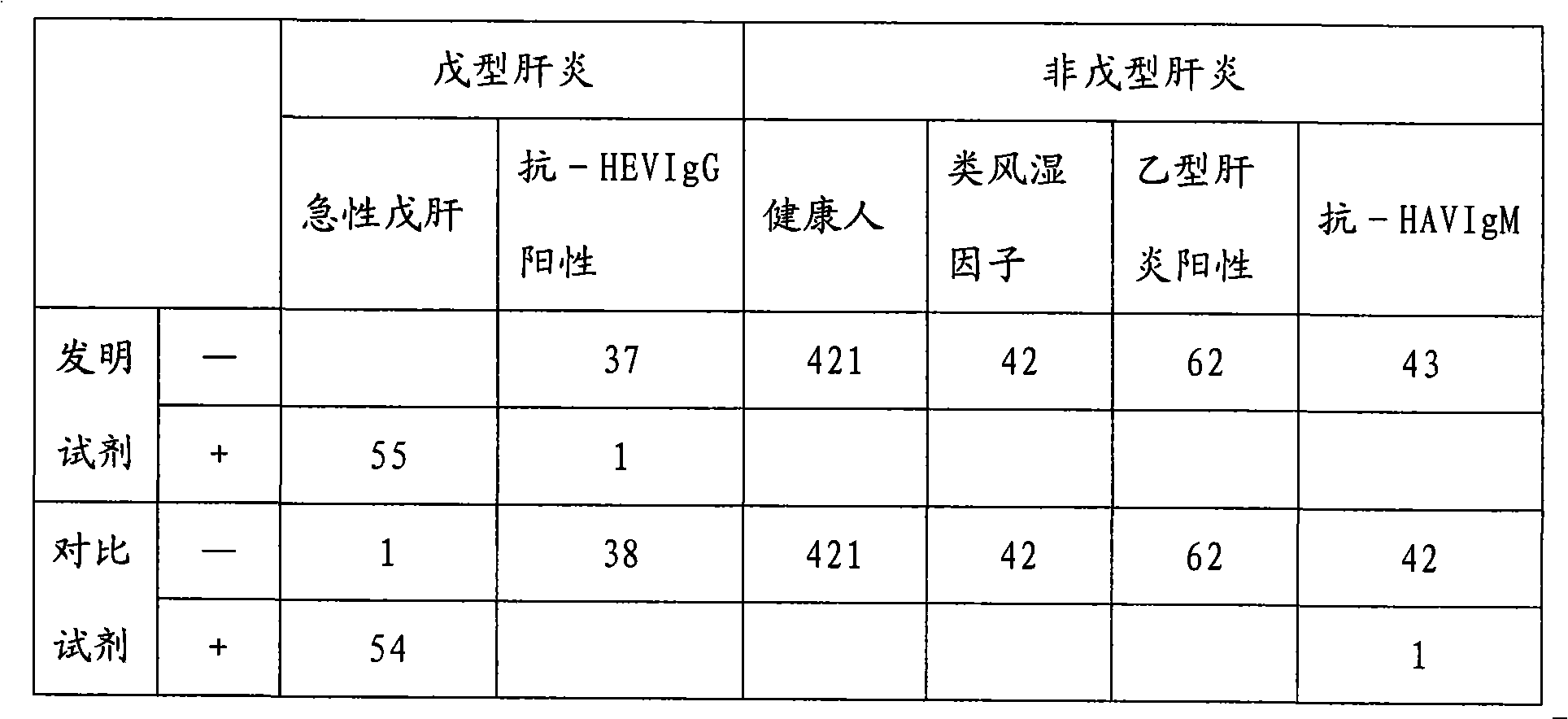
[0020] In the research process of the present invention, the inventor of the present invention has carried out screening experiment and quality identification to the raw material used at first, comprise the activity of antigen marker and coated antibody, carrier (such as opaque white microwell plate) Adsorption performance and variability, HRP activity, luminescence intensity and luminescence duration of chemiluminescent substrates, etc. Then the coating method was studied, experiments were carried out with different coating buffers and protection solutions, the most suitable coating buffer and protection solutions were selected, and the best concentration conditions were found through experiments with different coating concentrations of antibodies. There are different methods for HRP labeling. Through repeated exploration and comparative experim...
Embodiment 2~3
[0043] Embodiment 2~3 preparation hepatitis E virus IgM antibody chemiluminescence immunoassay assay kit of the present invention
[0044] Except that the hepatitis E virus recombinant antigen was labeled with alkaline phosphatase, AMPPD was used as a chemiluminescent substrate, plastic beads and plastic tubes were used as carriers, the pH value of the coating solution was 4.5, and the pH value of the blocking solution was 7.5. Hepatitis E virus IgM antibody chemiluminescent immunoassay assay kits were prepared in the same manner as in Example 1.
Embodiment 4
[0045] Example 4 Preparation of Hepatitis E virus IgM antibody chemiluminescence immunoassay assay kit of the present invention
[0046] Except that magnetic particles are used as the carrier, and the magnetic particle anti-human-μ chain antibody solid-phase carrier is prepared by the glutaraldehyde coupling method, all the others are prepared by the same method as in Example 1. Reagent test kit.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com