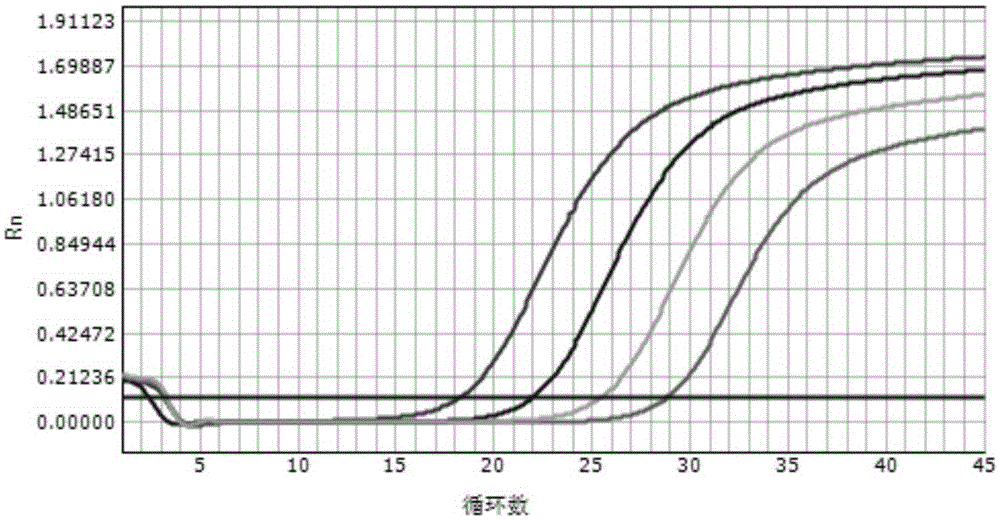
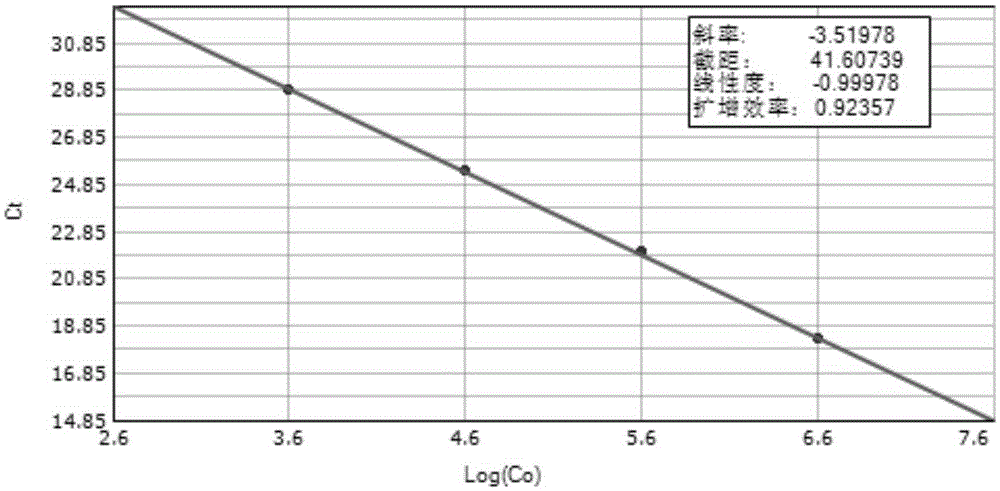
Patents
Literature
142 results about "Hepatitis E virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The hepatitis E virus (HEV) is the causative agent of hepatitis E. It is of the species Orthohepevirus A. The global burden of infections from the two major genotypes (1 and 2) is estimated at 20 million per year, leading to 70,000 deaths and 3,000 stillbirths.
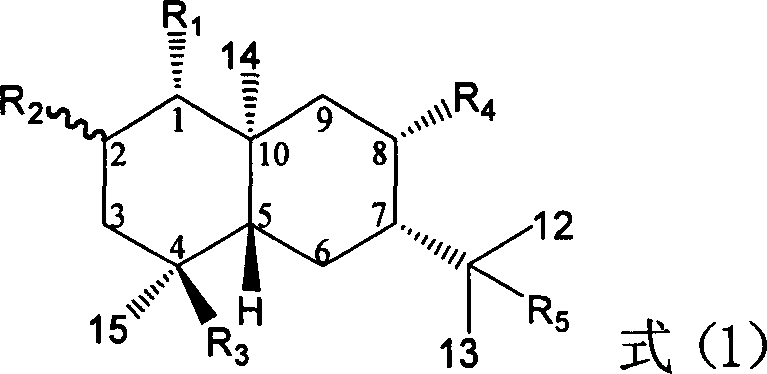
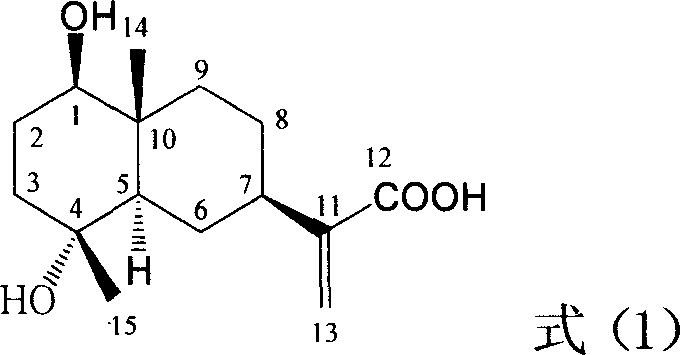
Pharmaceutical use of ent-eudesmane alcohol type sesquiterpene for inhibiting hepatitis virus
InactiveCN1935762APrevention and treatment of viral hepatitis BHBsAg reductionSugar derivativesHydroxy compound active ingredientsDiseaseSolvent
The invention relates to an enantiomorphic amine alkyl sesquiterpene alcohol and glucoside and the medicated salt or solvent thereof, as well as the effect and activity of the composed medicine combination, mainly relating to the medical use in reducing HBV-DNA replication activity. And it has considerably strong inhibiting effect on HBsAG screted by HepG2.2.15 and HBV-DNA replication as compared with positive contrast Lamivudine; and it has obvious inhibition activity to HBV-DNA replication at large dosage (100 mug / mL) and medium dosage(20 mug / mL) as contrasted with Lamivudine, and can be expected to apply to preparing medicines for curing HB virus infection disease.
Owner:赵昱
Pharmaceutical use of 1 beta-hydroxy ilexolic acid for inhibiting hepatitis virus
InactiveCN1935131APrevention and treatment of viral hepatitis BHBsAg reductionOrganic active ingredientsOrganic chemistryChemical structureDisease
The present invention relates to an eudesmane type sesquiterpene derivative 1 beta-hydroxyilicic acid, namely 1 beta-hydroxy-5 alpha H-eudesmane-11 (13)-ethylene-12-acid, its medicineal salt or solvent compound and its medicine composition and medicinal application for preparing medicine capable of curing hepatitis B virus infective disease and resisting hepatitis B virus. Said invention also provides its chemical structure formula.
Owner:WENZHOU MEDICAL UNIV
Sense Antiviral Compound and Method for Treating Ssrna Viral Infection
InactiveUS20080311556A1Disruption of secondary structureInhibition of replicationOrganic active ingredientsBiocideSsRNA virusesViral infection
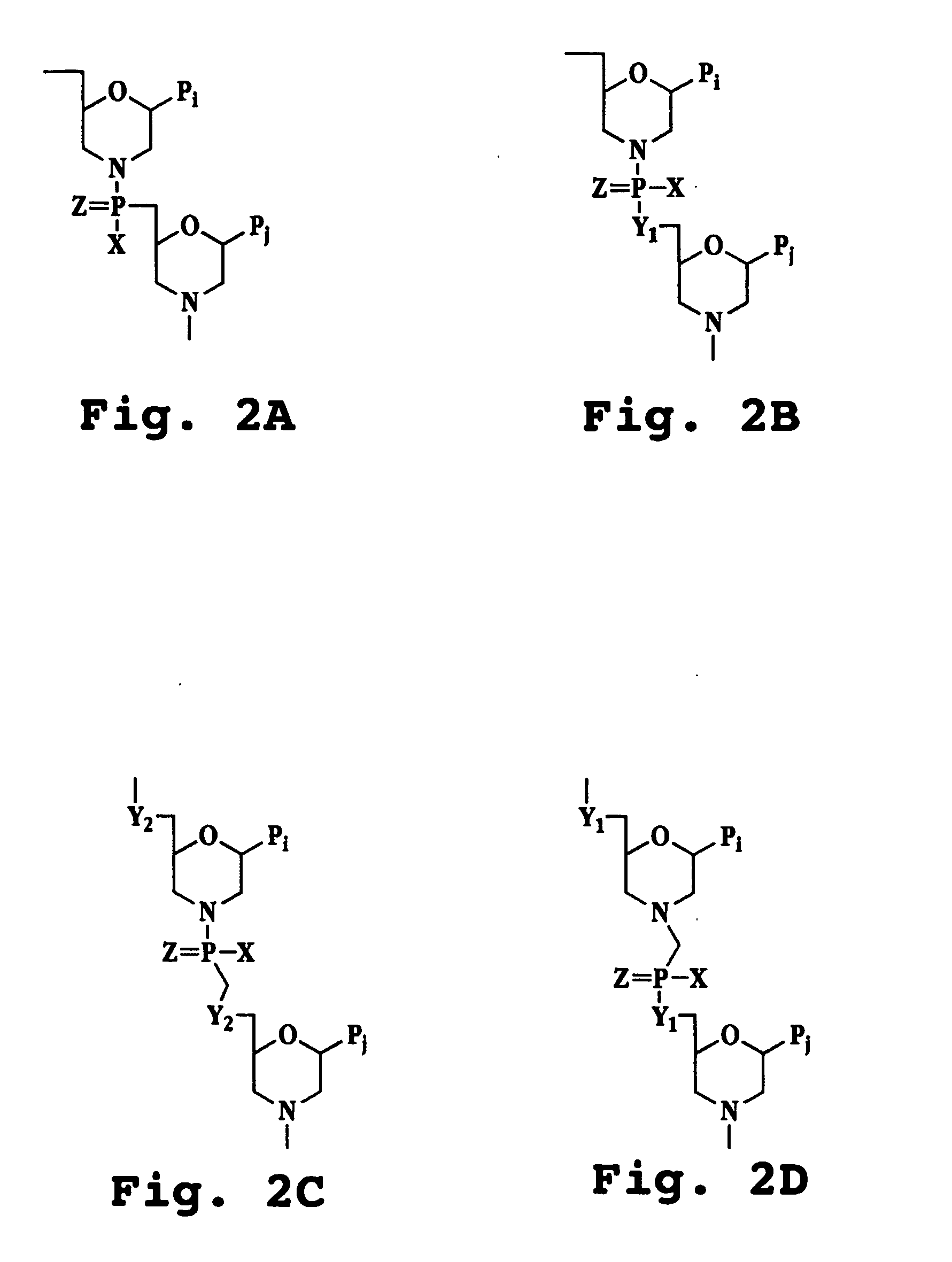
The invention provides sense antiviral compounds and methods of their use in inhibition of growth of viruses of the Flaviviridae, Picornoviridae, Caliciviridae, Togaviridae, Coronaviridae families and hepatitis E virus in the treatment of a viral infection. The sense antiviral compounds are substantially uncharged morpholino oligonucleotides having a sequence of (12-40) subunits, including at least (12) subunits having a targeting sequence that is complementary to a region associated with stem-loop secondary structure within the 3′-terminal end (40) bases of the negative-sense RNA strand of the virus.
Owner:AVI BIOPHARMA
Hepatitis B surface antigen vaccine
InactiveUS6022543AGood antigenicityStimulate immune responseSsRNA viruses positive-senseBacteriaEukaryotic plasmidsHepatitis B virus
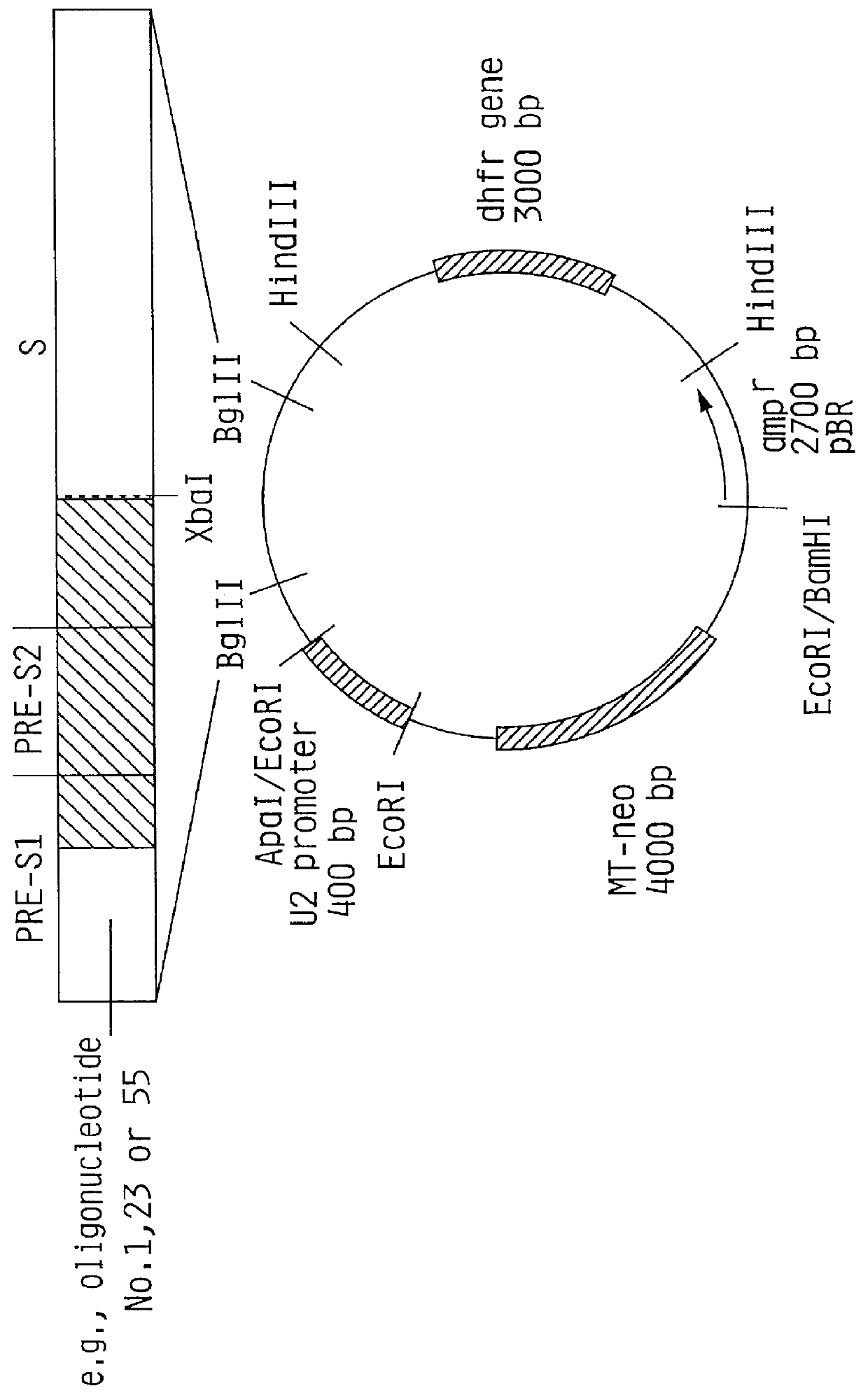
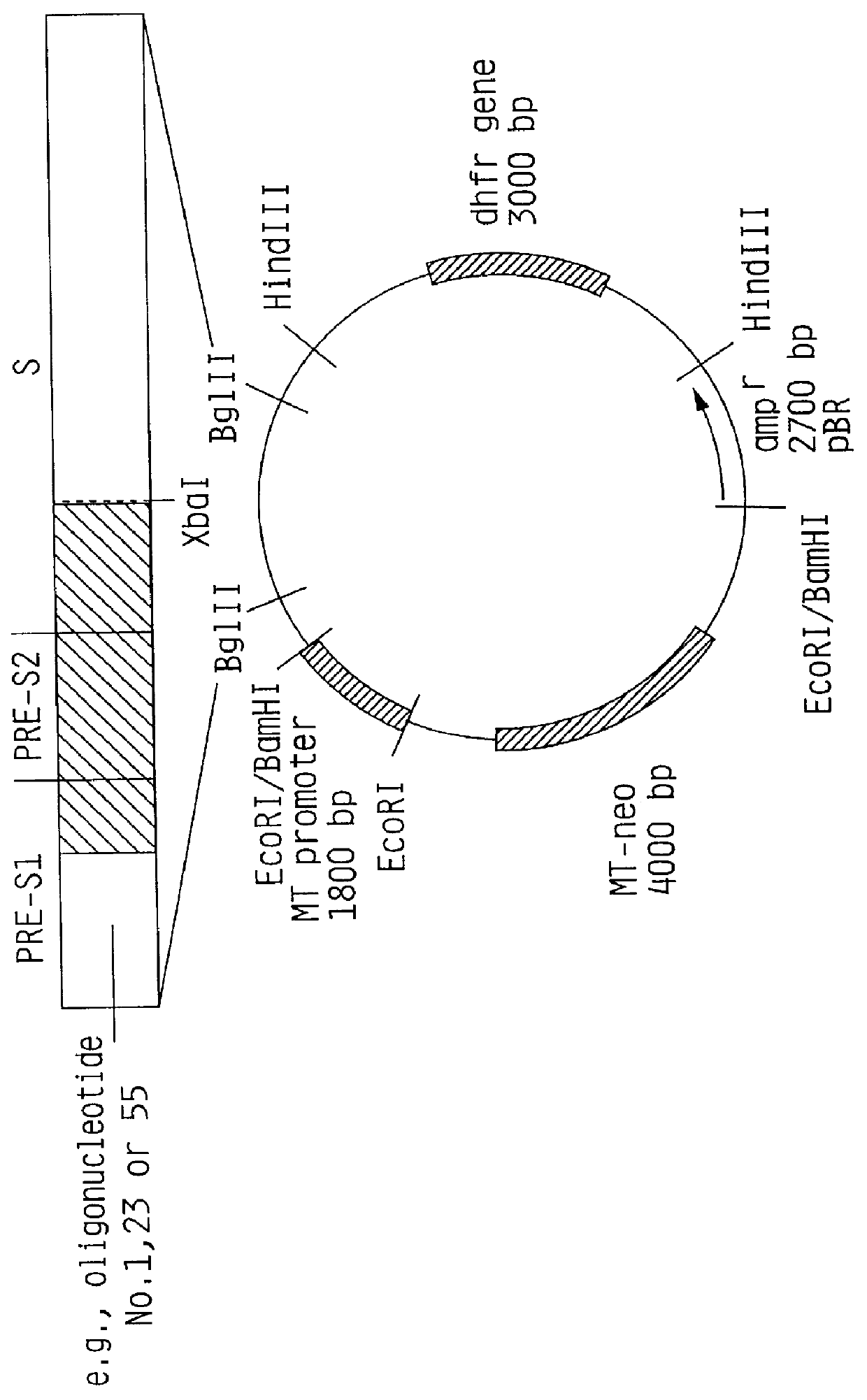
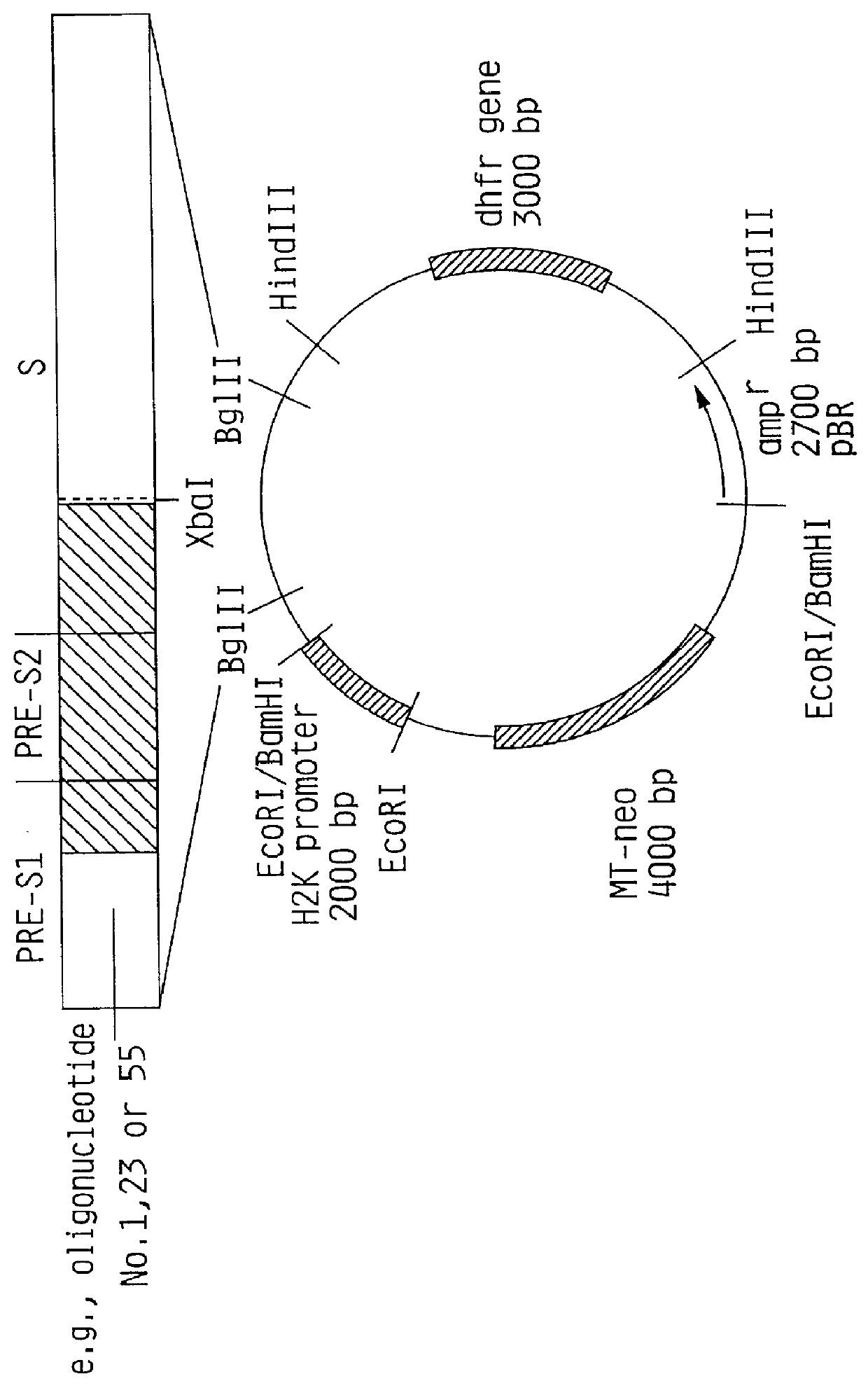
HBV surface antigen particles, prepared by recombinant DNA technology are described, said particles being composed of epitopes from the group of surface peptides and / or core peptide of non-A, non-B hepatitis virus, hepatitis virus A and / or hepatitis virus B. Respective particles are especially characterized by a composition of different epitopes selected from pre-S and S peptides. There are also described DNA-sequences, plasmids and cell lines coding for respective HBV surface antigen particles as well as a new vaccine containing the same.
Owner:MEDEVA HLDG
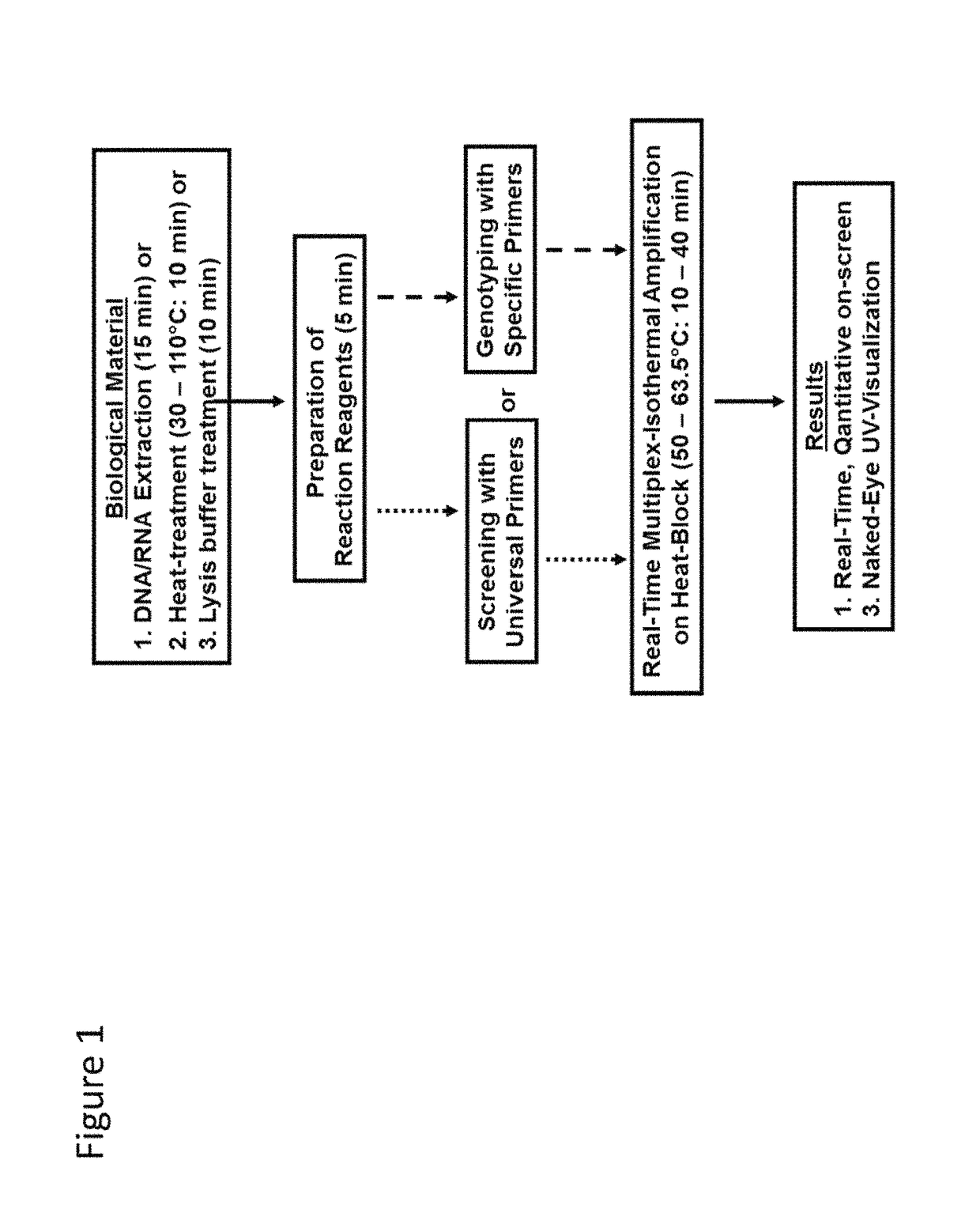
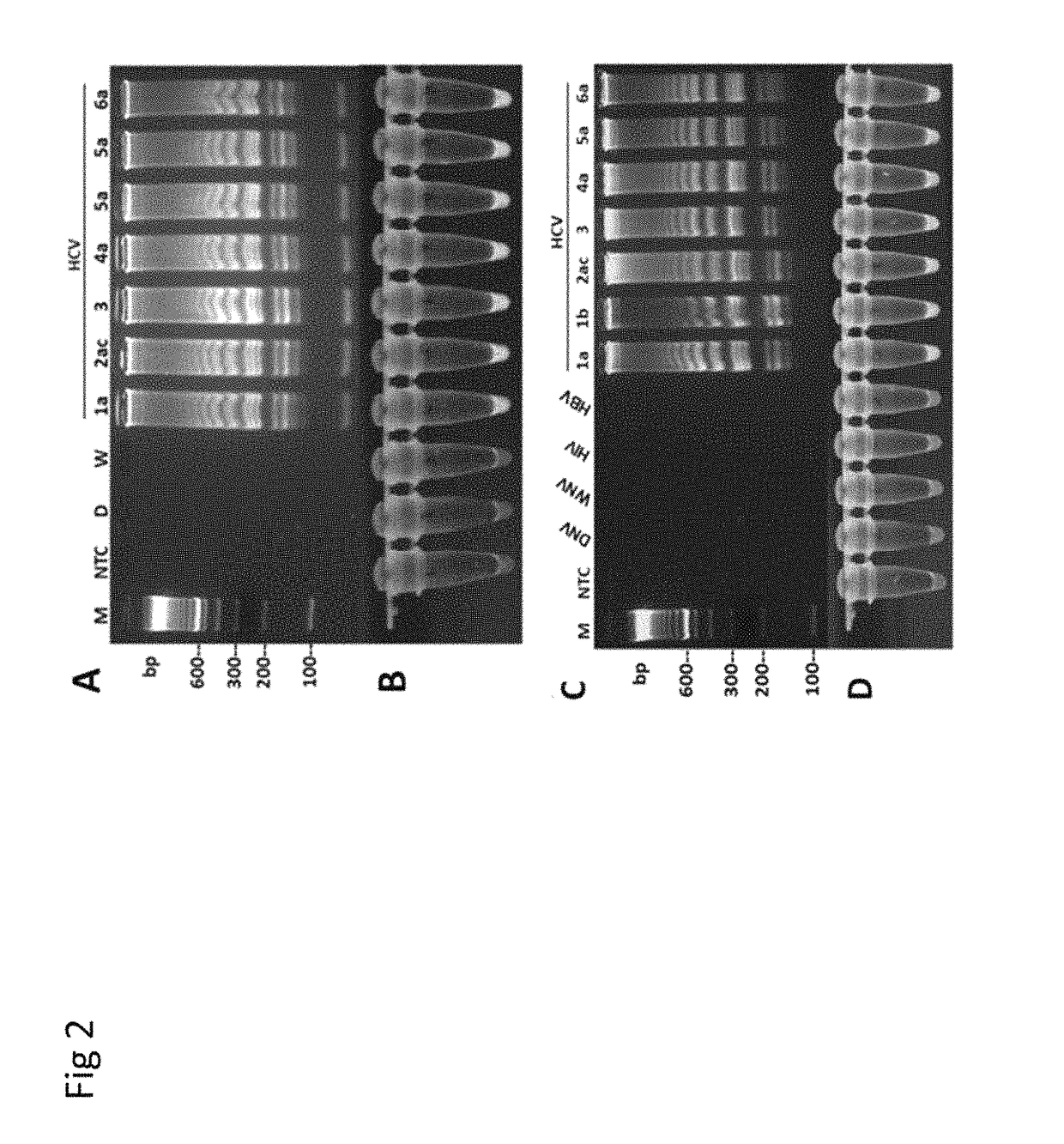
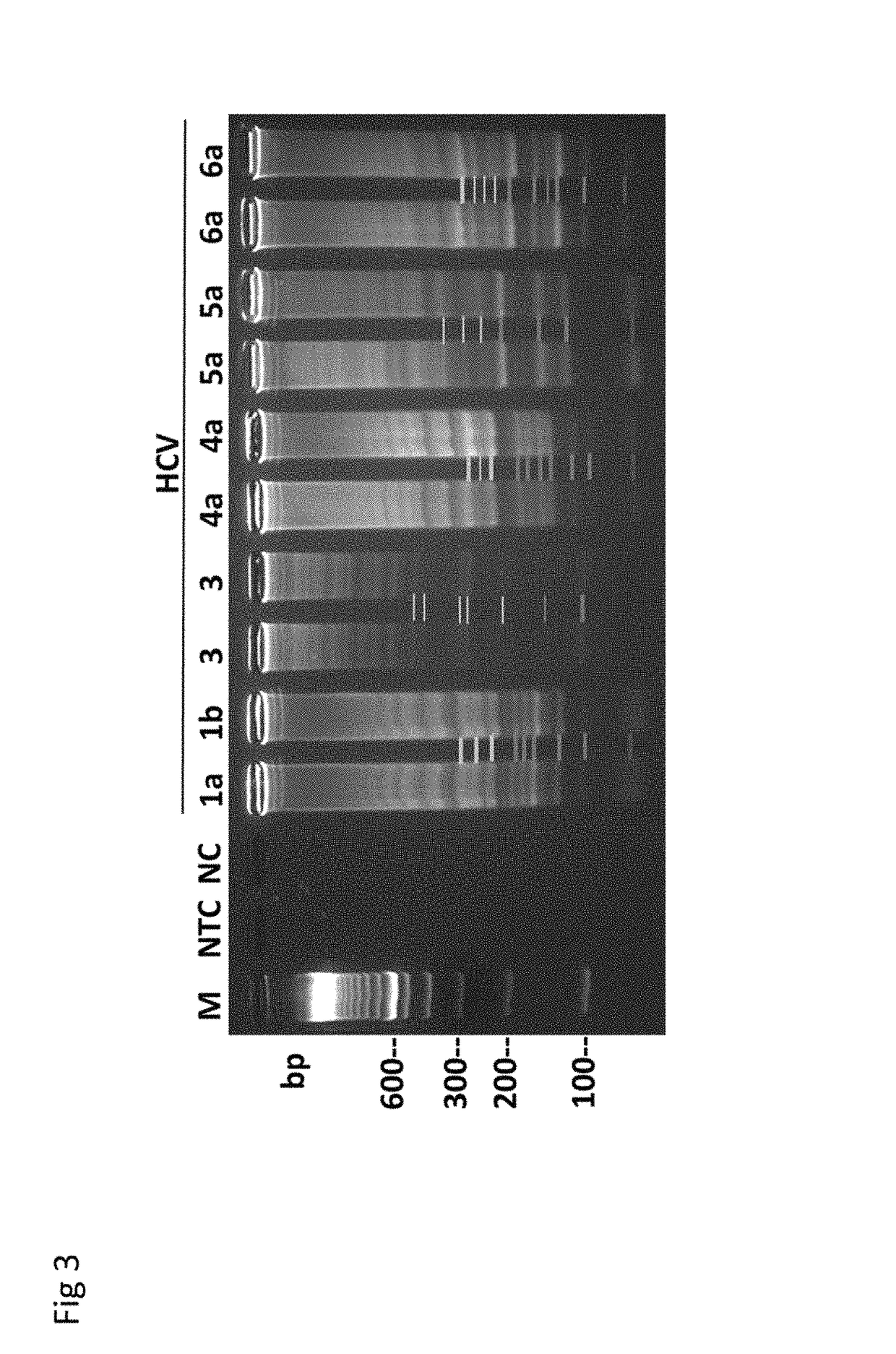
Methods for real-time multiplex isothermal detection and identification of bacterial, viral, and protozoan nucleic acids
ActiveUS10072309B1Flexible processFacilitate strand displacementMicrobiological testing/measurementAgainst vector-borne diseasesProtozoaFluorescence
Herein disclosed are rapid real-time isothermal multiplex methods of detecting, identifying and quantifying bacterial, viral, and protozoan nucleic acids in a sample. These include contacting the sample with two or more sets of pathogen-specific reverse transcription loop-mediated isothermal amplification primers and novel oligofluorophores specific for the target bacterial, viral, and parasitic nucleic acids of interest such as human immunodeficiency virus, Ebola virus, Marburg virus, Yellow fever virus, hepatitis-B virus, Lassa fever virus, Plasmodium, hepatitis-C virus, hepatitis-E virus, dengue virus, Chikungunya virus, Japanese Encephalitis virus, Middle Eastern Respiratory Syndrome Corona virus, Mycobacterium, West Nile virus, Cytomegalovirus, Parvovirus, Leishmania, Trypanosoma, and Zika virus nucleic acids, under conditions sufficient to produce detectable real-time amplification signals in about 10 to 40 minutes. The amplification signals are produced by pathogen-specific fluorogenic labels included in one or more of the primers. Also, novel reaction and sample lysis buffers, primers, and kits for rapid multiplex detection, quantification, and identification of bacterial, viral, and protozoan nucleic acids by real-time isothermal amplification are herein disclosed.
Owner:NYAN DOUGBEH CHRIS
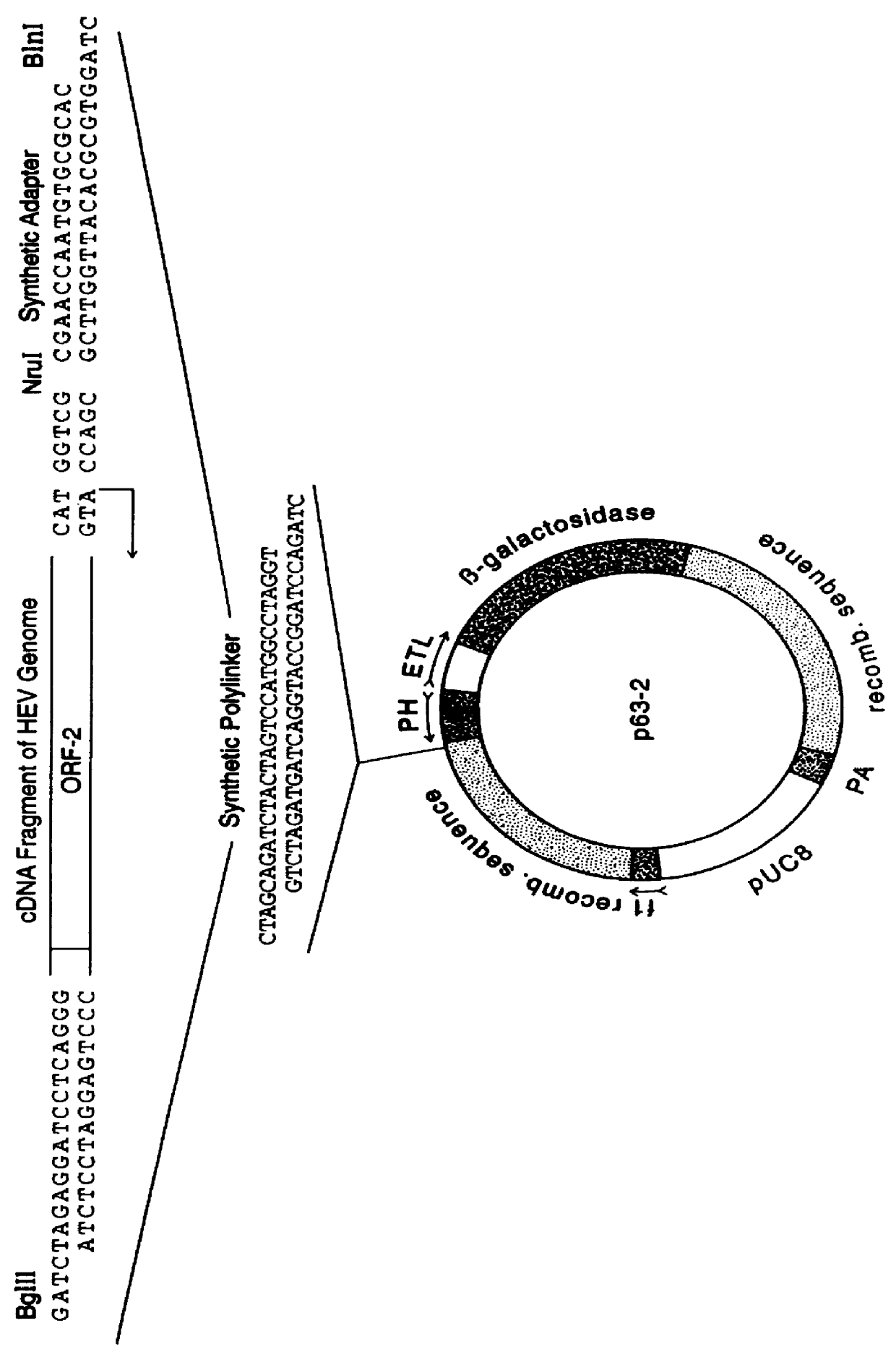
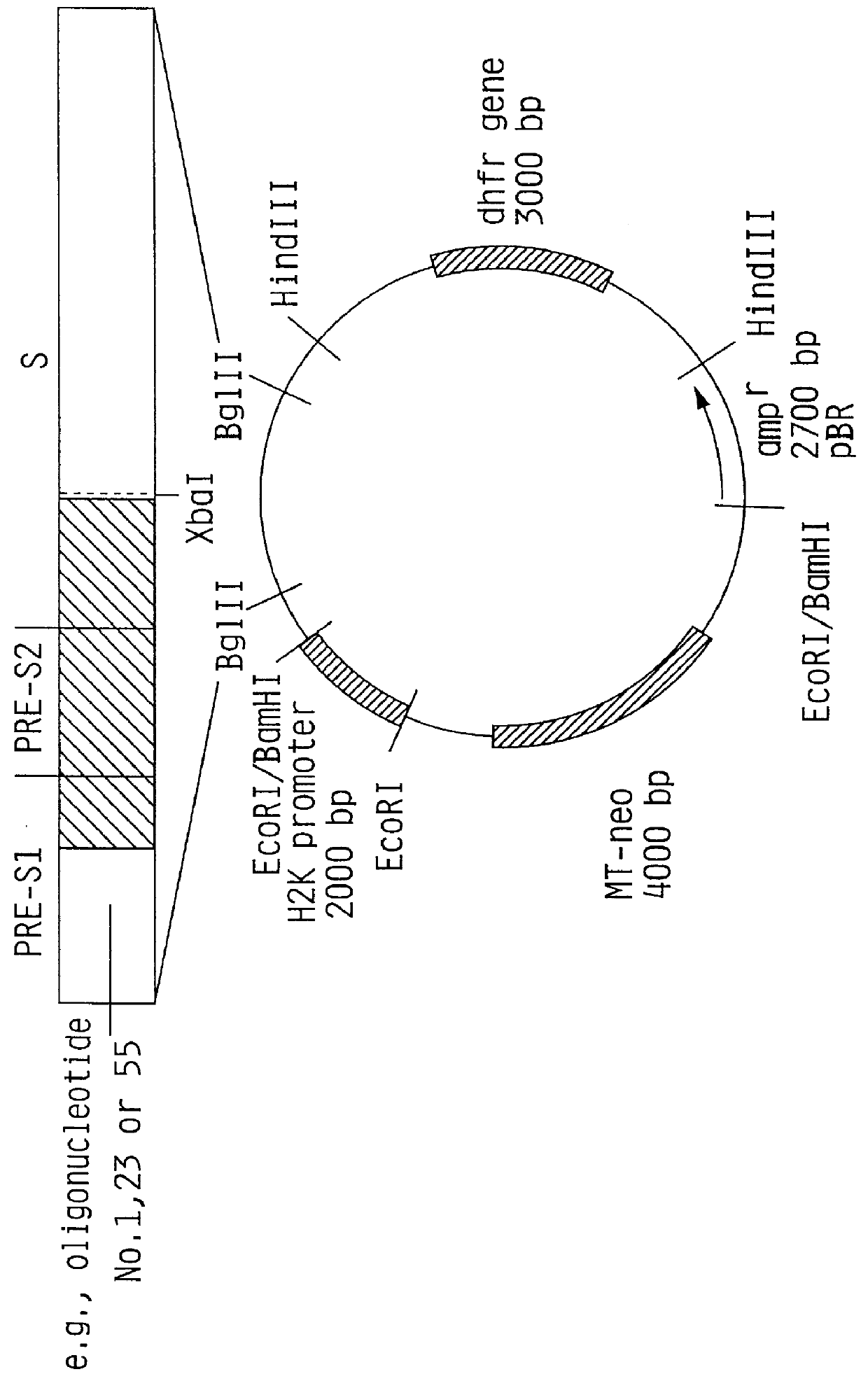
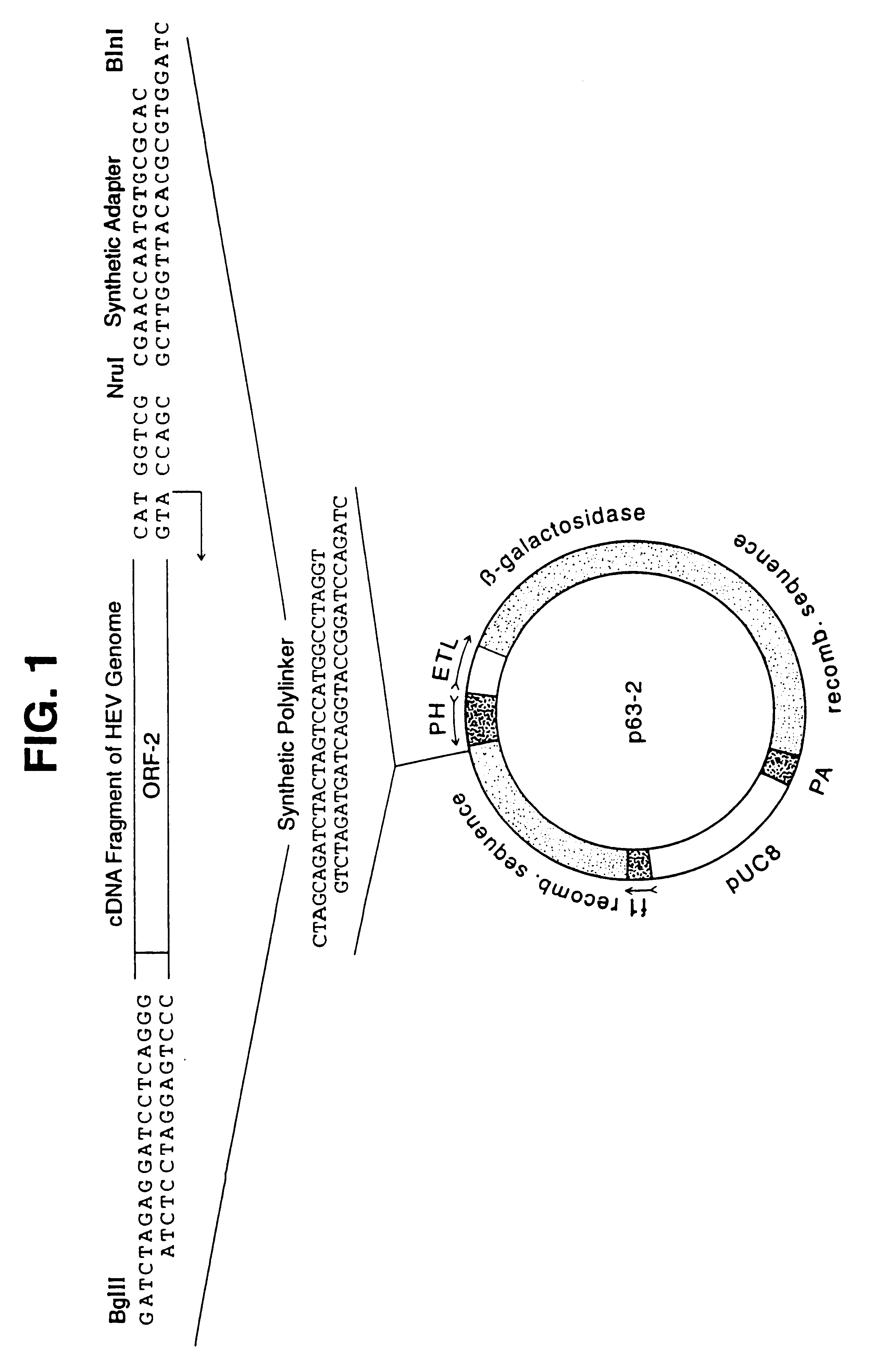
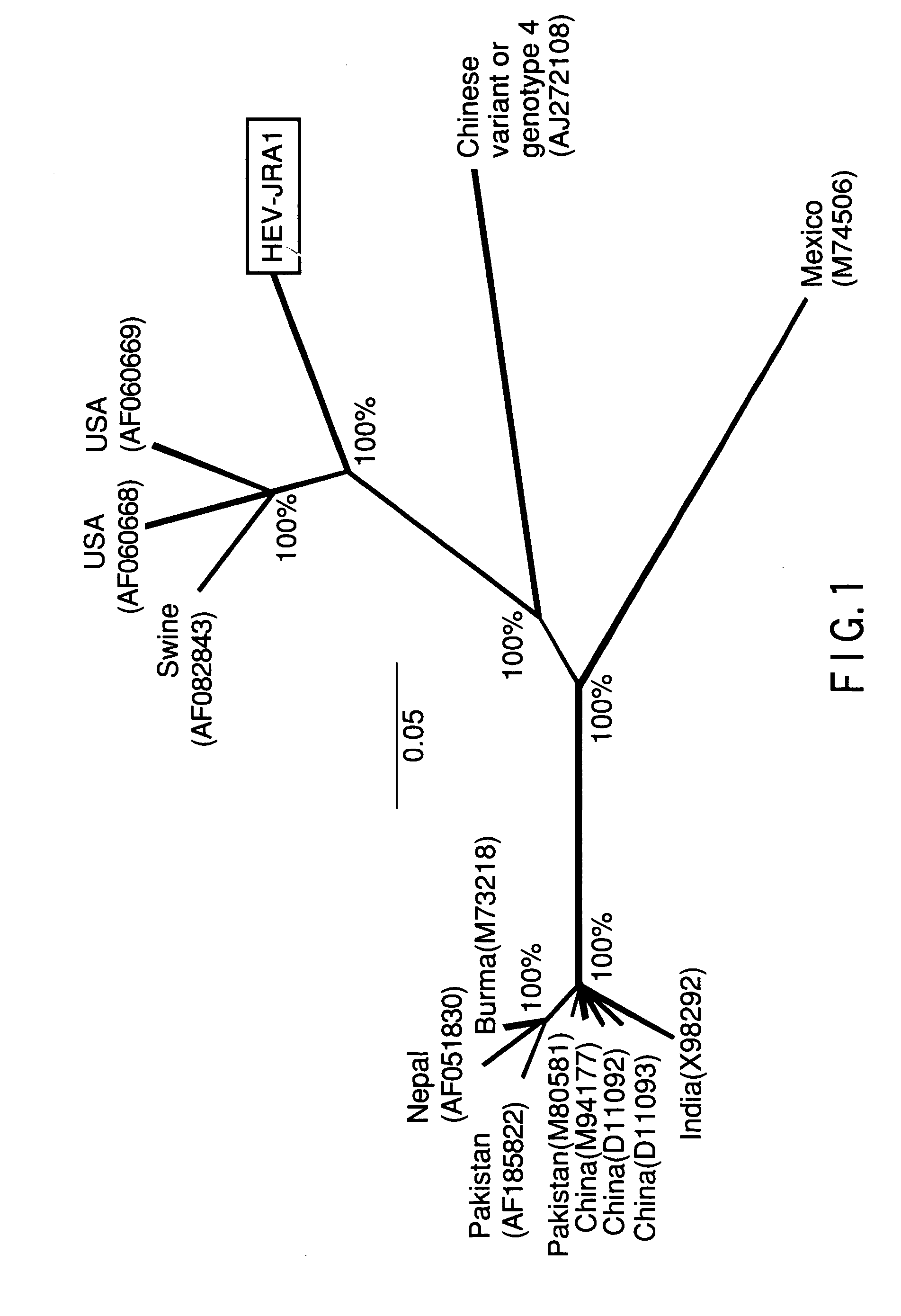
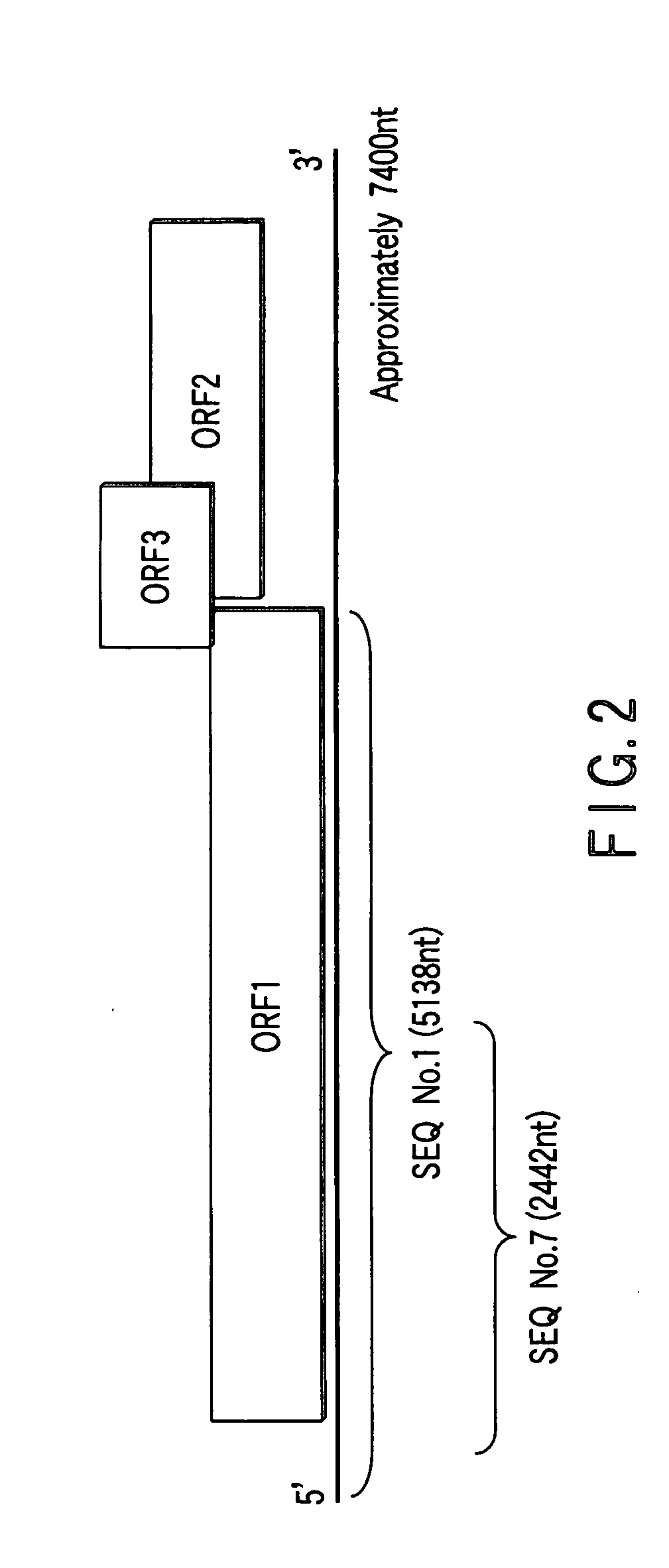
Recombinant proteins of a pakistani strain of hepatitis E and their use in diagnostic methods and vaccines
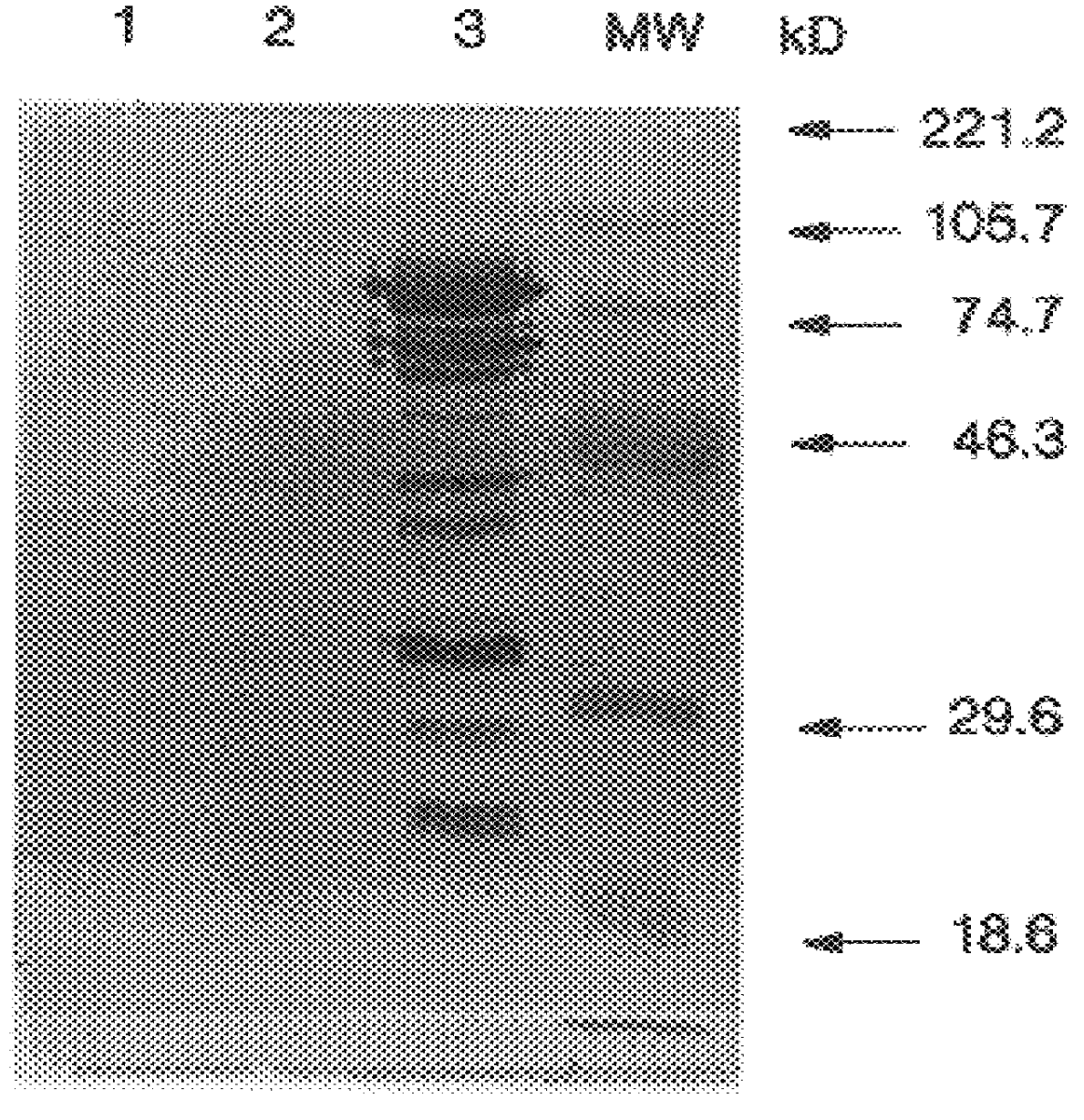
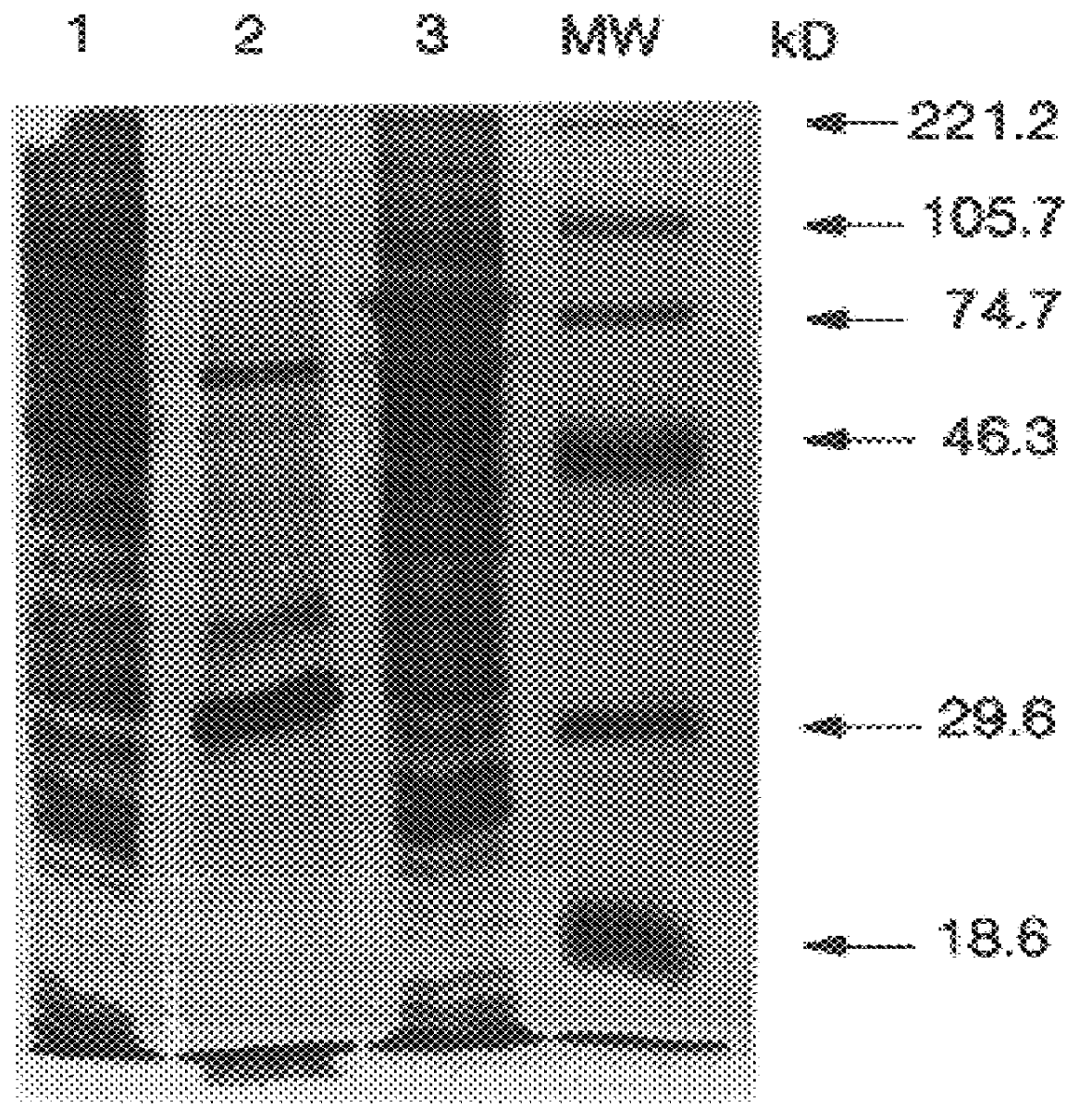
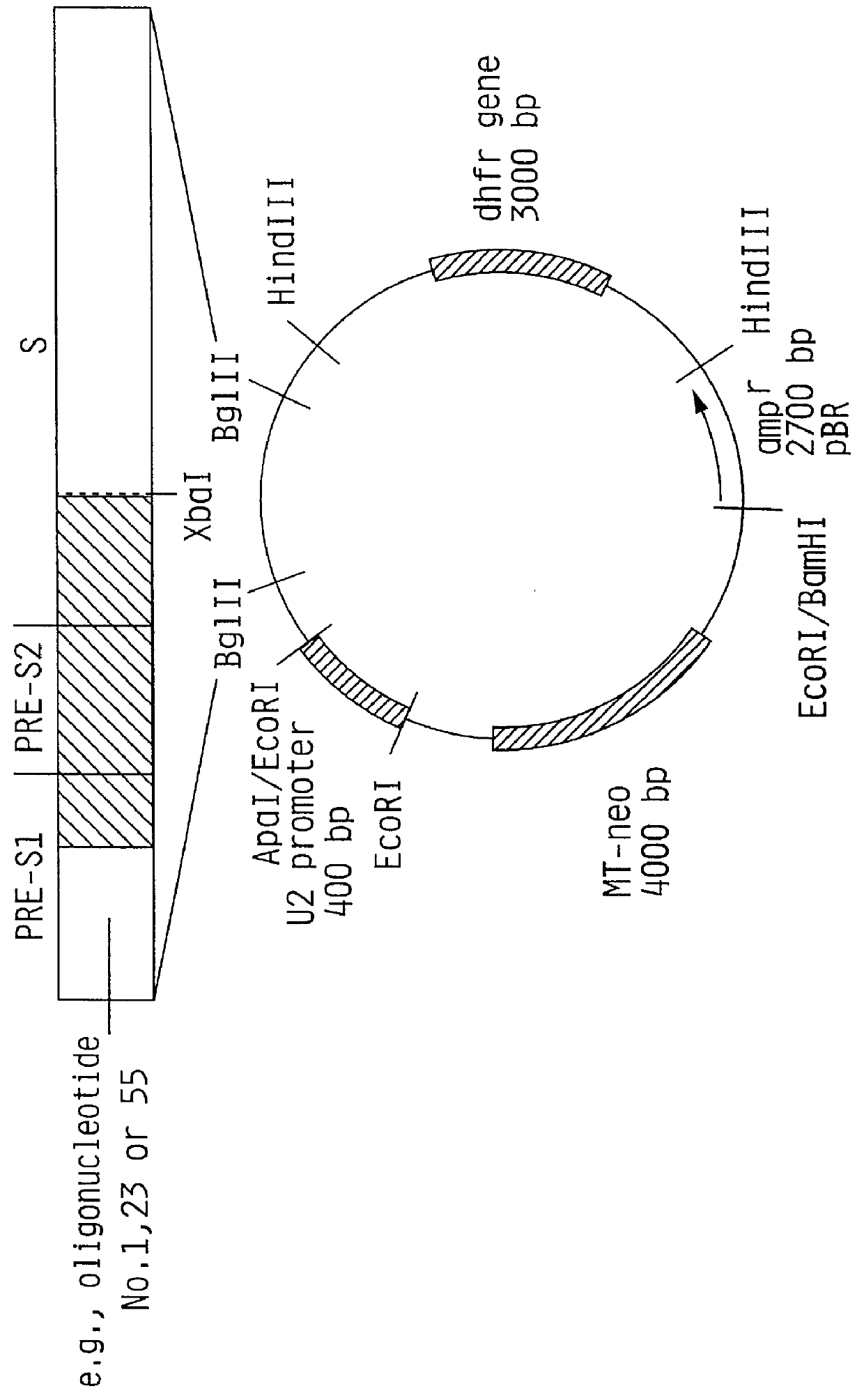
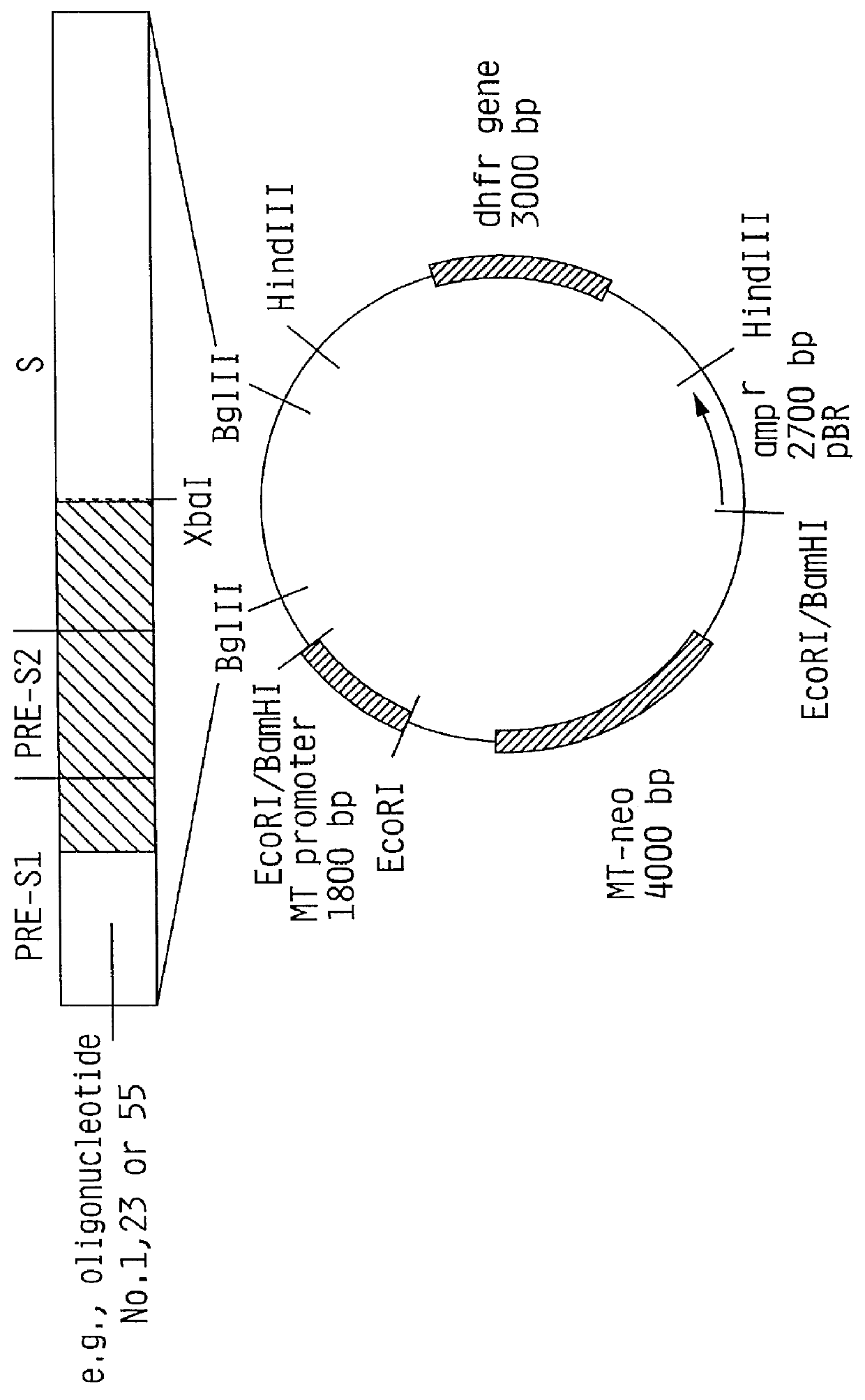
The invention relates to the expression of open reading frame 2 (ORF-2) proteins of a strain of hepatitis E virus from Pakistan (SAR-55) in a eukaryotic expression system. The expressed proteins can serve as an antigen in diagnostic immunoassays and / or as an immunogen or vaccine to protect against infection by hepatitis E.
Owner:NOVAVAX +1
Hepatitis B surface antigen vaccine
InactiveUS6072049AGood antigenicityStimulate immune responseVirusesBacteriaEukaryotic plasmidsHepatitis B virus
HBV surface antigen particles, prepared by recombinant DNA technology are described, said particles being composed of epitopes from the group of surface peptides and / or core peptide of non-A, non-B hepatitis virus, hepatitis virus A and / or hepatitis virus B. Respective particles are especially characterized by a composition of different epitopes selected from pre-S and S peptides. There are also described DNA-sequences, plasmids and cell lines coding for respective HBV surface antigen particles as well as a new vaccine containing the same.
Owner:MEDEVA HLDG
Method and kit for detecting hepatitis E virus (HEV) antibody and method for preparing kit
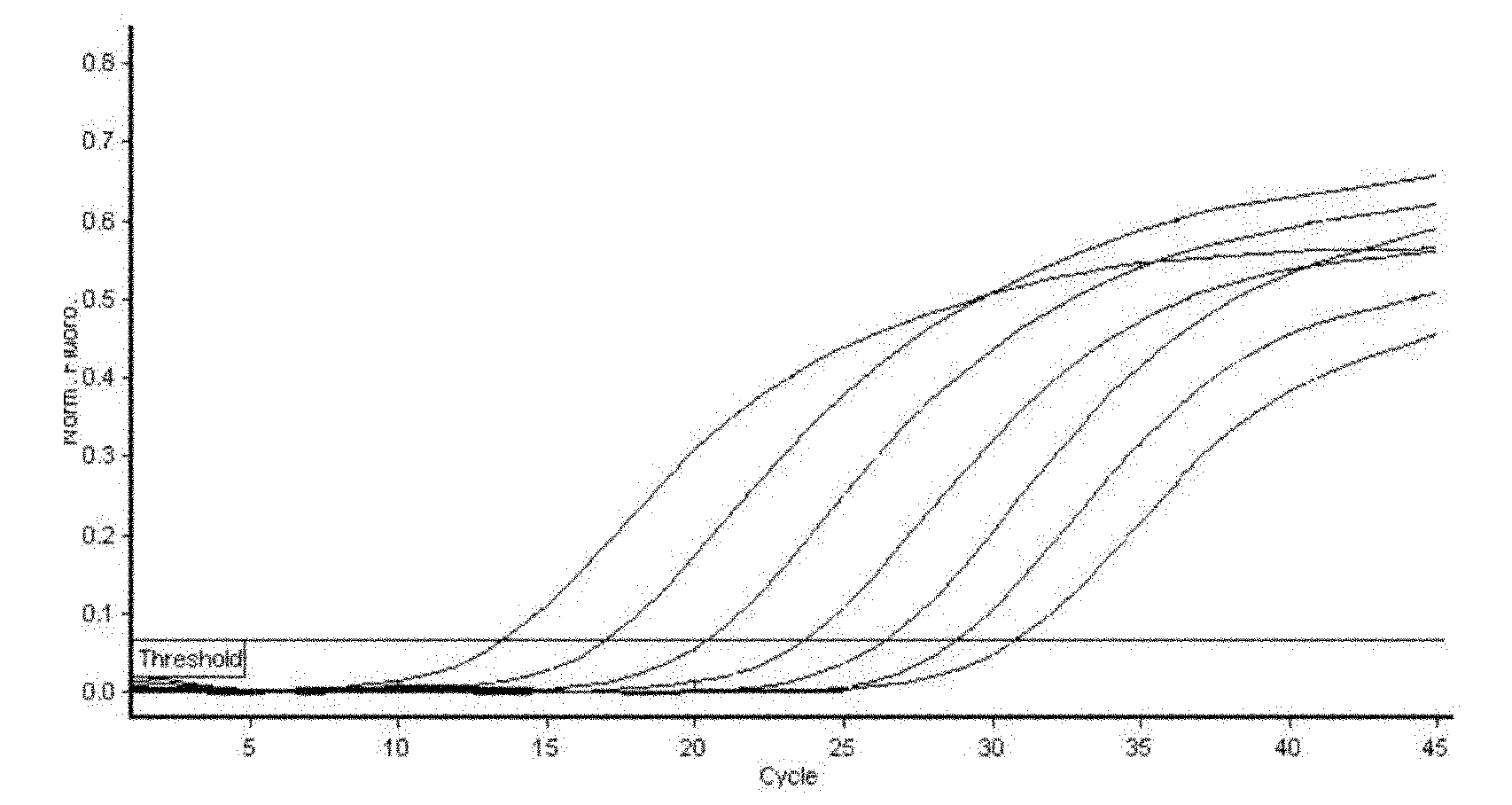
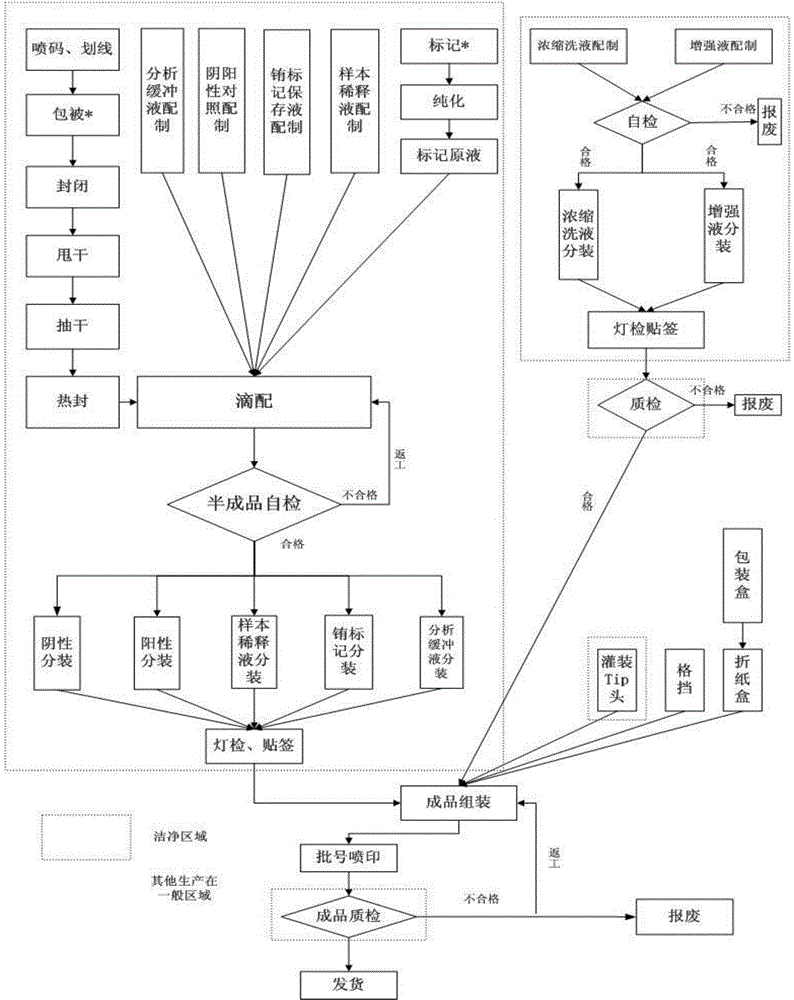
The invention discloses a method and a kit for detecting a hepatitis E virus (HEV) antibody and a method for preparing the kit. With adoption of double-tagging time-resolved fluoroimmunoassay technology, the problem that the previous HEV IgG antibodies and IgM antibodies need to be independently detected or the IgG antibodies and IgM antibodies cannot be distinguished during combined detection is solved; trace detection of the HEV IgG antibodies and IgM antibodies is realized; with adoption of combined detection of HEV IgG antibodies and IgM antibodies in serum of patients, the clinical detection rate of the HEV can be improved, and disease diagnosis and prevention can be promoted; an antigen of the kit comprises multiple main epitopes, and the detection leakage condition can be reduced to the greatest degree. The kit has the characteristics of high detection result accuracy, high sensitivity, high specificity, high stability, simple and convenient detection method, high efficiency and the like.
Owner:GUANGZHOU FENGHUA BIOENG
Porcin circovirus type 2 (PCV2) recombinant baculovirus construction method and subunit vaccine preparation method thereof
InactiveCN102352347AViral antigen ingredientsMicroorganism based processesOpen reading frameViral vector
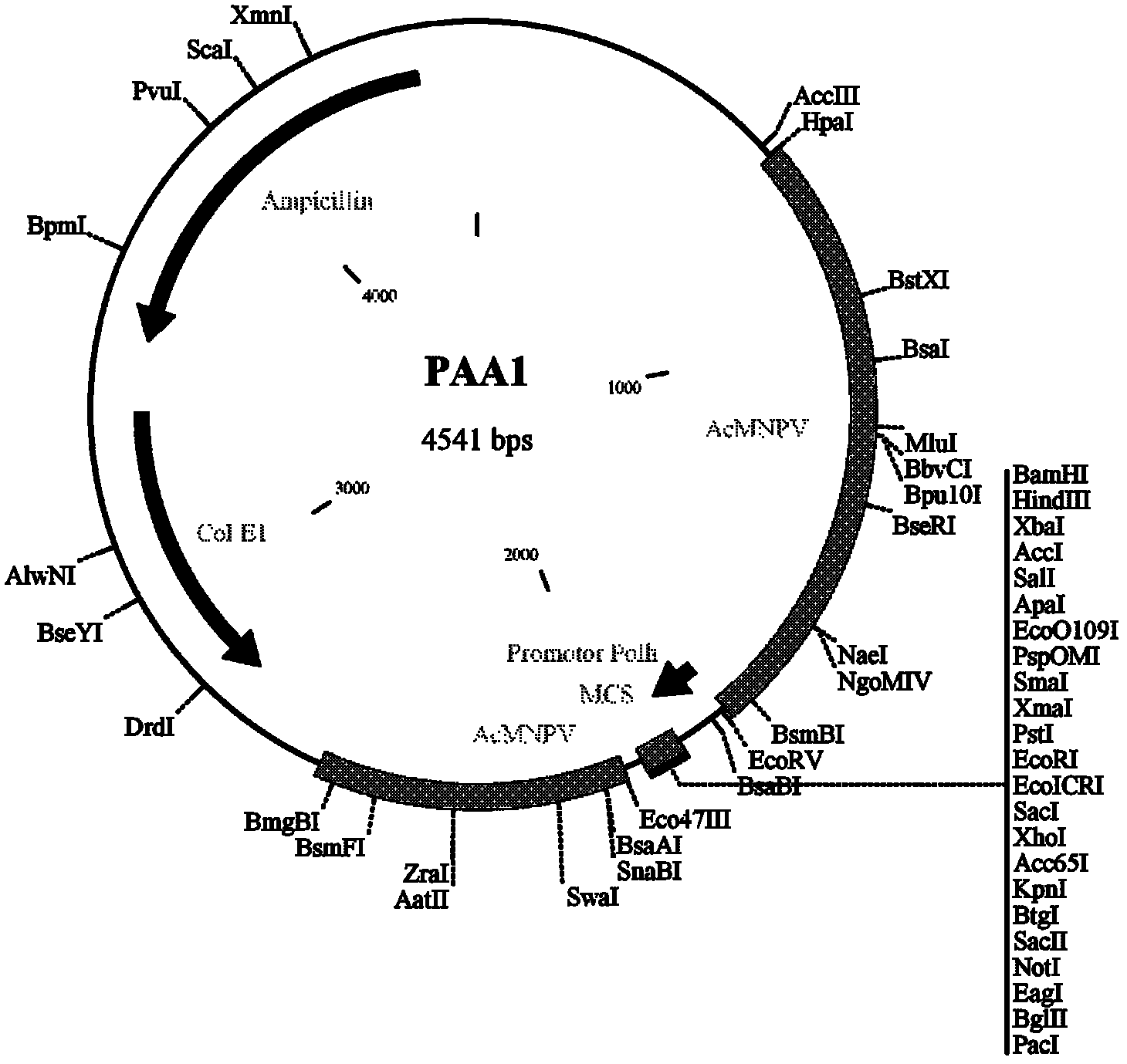
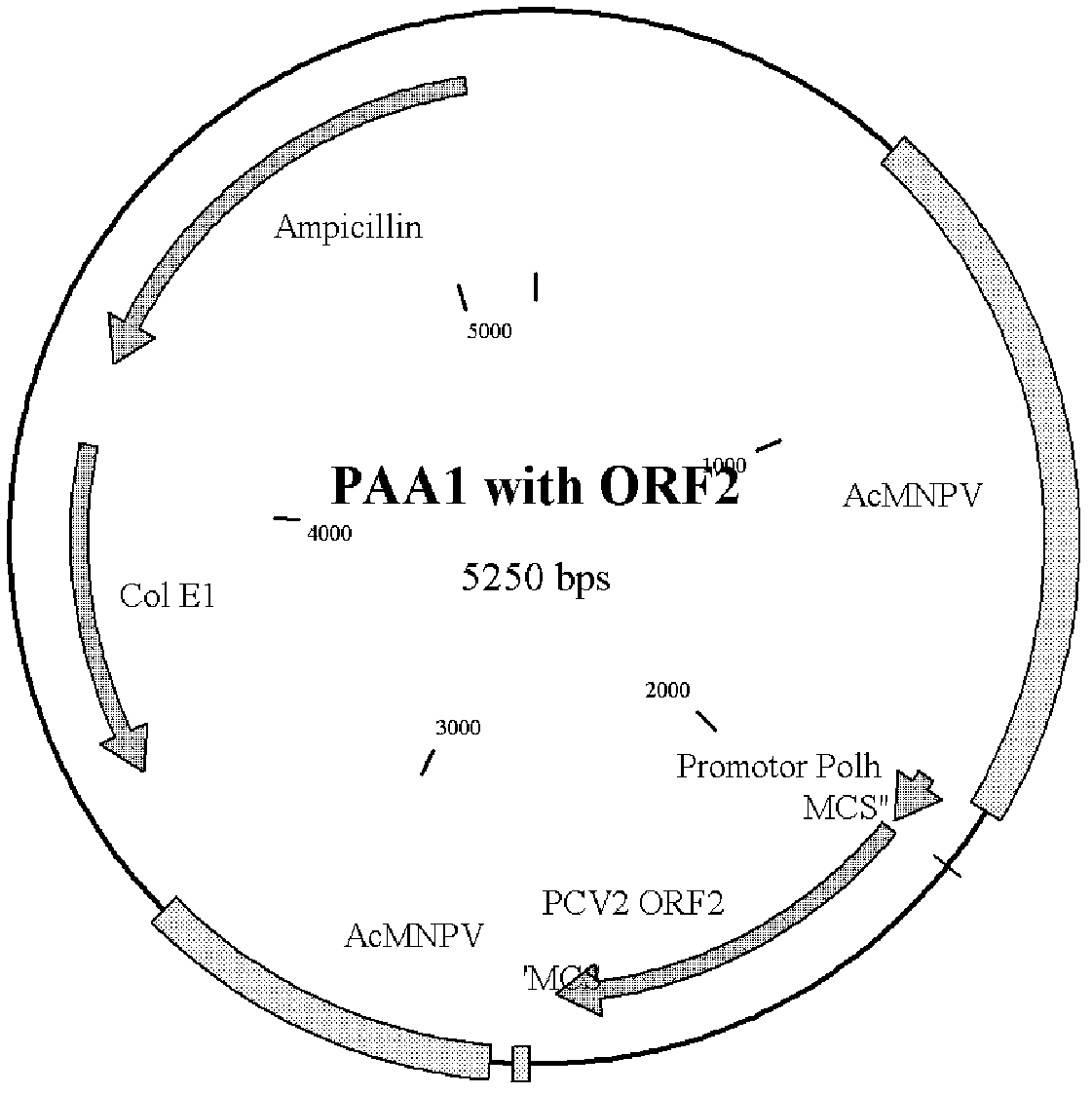
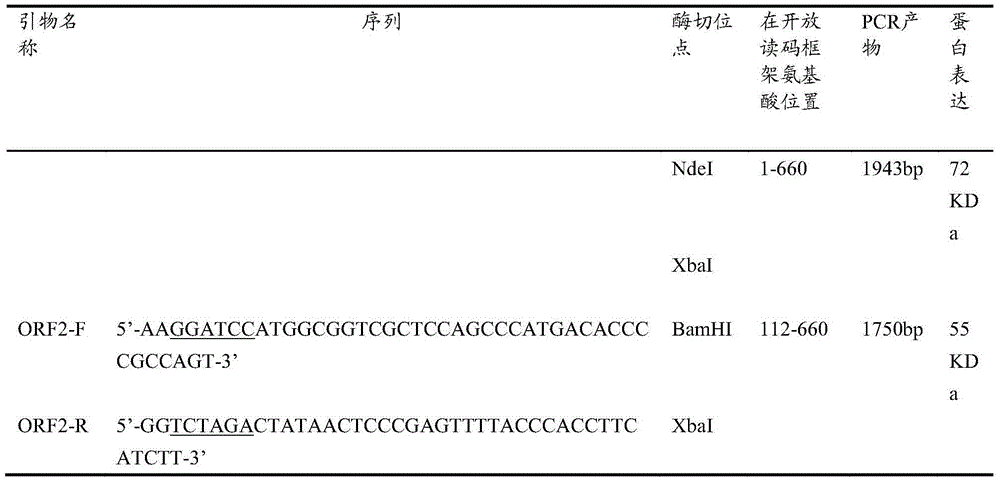
The invention relates to a porcin circovirus type 2 (PCV2) recombinant autographa californica multicapsid nucleopolyhedrovirus construction method and a subunit vaccine preparation method thereof. The novel recombinant lucerne fork vein noctuid nuclear polyhedrosis virus construction method comprises the steps that: the main surface antigen which expresses the PCV2 is used to prepare the subunit vaccine with better immunity. Specifically, in the carrier expression system of the PCV2 recombinant autographa californica multicapsid nucleopolyhedrovirus, a hepatitis E virus open reading frame (ORF2) gene of the PCV2 is directionally inserted, so the gene efficiently expresses in insect cells and has good immunogenicity, the prepared corresponding subunit vaccine can stimulate pigs to generate the protective immunoreaction against the attack of the strong virus of the PCV2.
Owner:ZHEJIANG YEBIO BIOTECH
Combination vaccine with acellular pertussis
The present invention relates to a combination vaccine comprising a mixture of antigens for protection against diseases such as diphtheria, tetanus, acellular pertussis, and infections caused by Haemophilus influenzae and polio viruses. The present invention also relates to inclusion of antigens for protection against infections caused Hepatitis virus and other pathogens, such that administration of the vaccine can simultaneously immunize a subject against more than one pathogen. The invention in particular relates to a fully liquid stable combination vaccine comprising the antigens as indicated above and the methods for manufacturing the same.
Owner:PANACEA BIOTEC
Recombinant proteins of a Pakistani strain of hepatitis E and their use in diagnostic methods and vaccines
A strain of hepatitis E virus from Pakistan (SAR-55) implicated in an epidemic of enterically transmitted non-A, non-B hepatitis, now called hepatitis E, is disclosed. The invention relates to the expression of the whole structural region of SAR-55, designated open reading frame 2 (ORF-2), in a eukaryotic expression system. The expressed protein is capable of forming HEV virus-like particles which can serve as an antigen in diagnostic immunoassays and as an immunogen or vaccine to protect against infection by hepatitis E.
Owner:UNITED STATES OF AMERICA
Protein for preparation of hepatitis E virus-like particles and method
ActiveCN104211784AAvoid damageReduce the cost of trainingSsRNA viruses positive-senseVirus peptidesDiseaseVirus-like particle
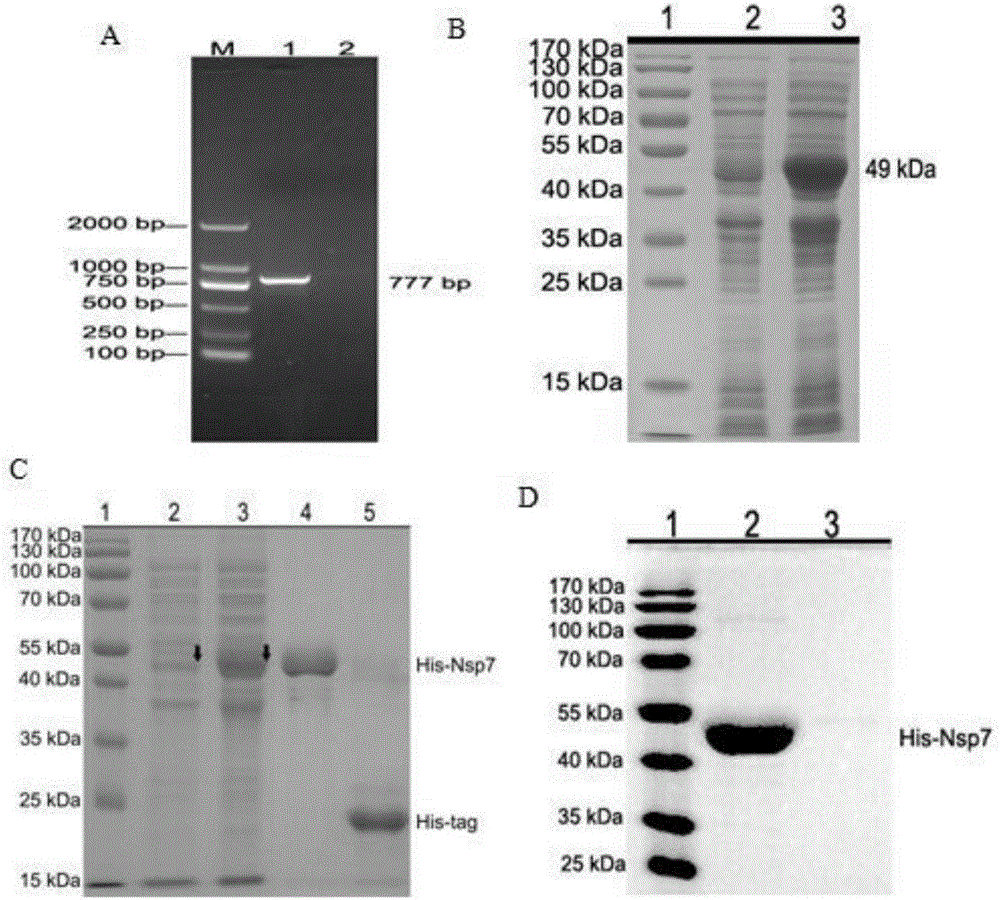
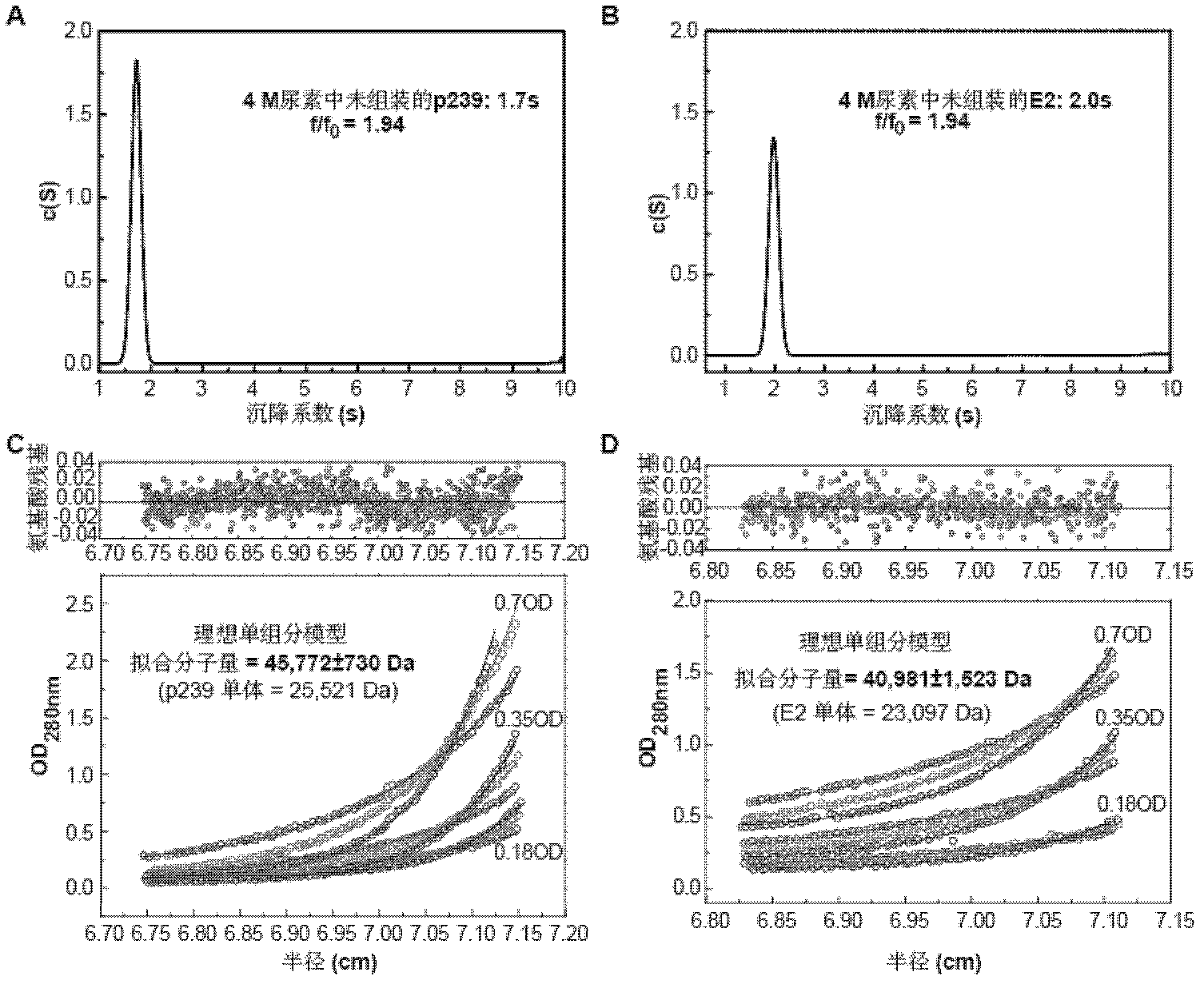
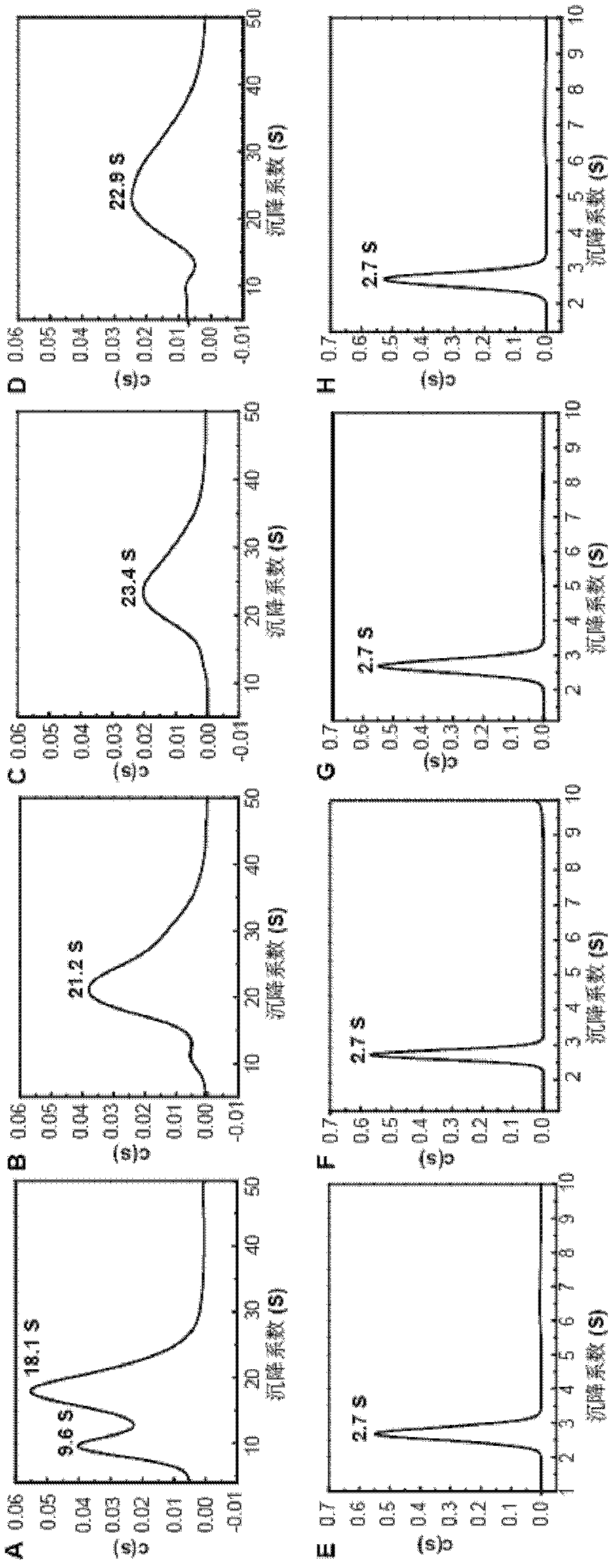
The present invention relates to the field of molecular biology and virology. In particular, the invention relates to a protein capable of being assembled in vitro into hepatitis E virus-like particles, a coding sequence and a preparation method thereof, and the virus-like particles comprising the protein; the protein and the virus like particles can be used for the prevention of or for the treatment of HEV (hepatitis E virus) infection and diseases caused by the HEV, such as hepatitis E and the like. The invention also relates to use of the protein and the virus like particles in the preparation of pharmaceutical compositions and vaccines, the pharmaceutical compositions or the vaccines are used for the prevention or treatment of the HEV infection and diseases caused by the HEV, such as hepatitis E and the like. In addition, the invention also provides a preparation method of the HEV virus like particles.
Owner:XIAMEN UNIV +1
Hepatitis E virus-like particle vaccine preparation method and application
ActiveCN104857510AEasy to prepareEasy to zoom inDigestive systemImmunoglobulins against virusesEscherichia coliDisease
The invention discloses a hepatitis E virus-like particle vaccine preparation method, and belongs to the biological medicine technical field. According to the method, hepatitis E virus truncated capsid protein gene codon optimization is performed, prokaryotic expression system escherichia coli strain B834-pRARE2 is used, by fusion of purification tag MBP expression, different cracking agent screening and combination, bacterial membrane extraction, maltose amylose affinity chromatography, molecular sieve chromatography for removal of MBP and non-virus structural protein, and promotion of virus-like particle assembly, the hepatitis E virus-like particle purity is more than 99.0%, and is free of protein label and bioactive (in the form of non-inclusion body, and soluble). The hepatitis E virus-like particle vaccine produces complete protection to pig, dog and chicken HEV infection, cross protective antigens are between different genotype HEV virus, and the hepatitis E virus-like particle vaccine can be used as an animal vaccine to prevent the diseases and infection caused by pig, dog and poultry HEV.
Owner:张澍 +1
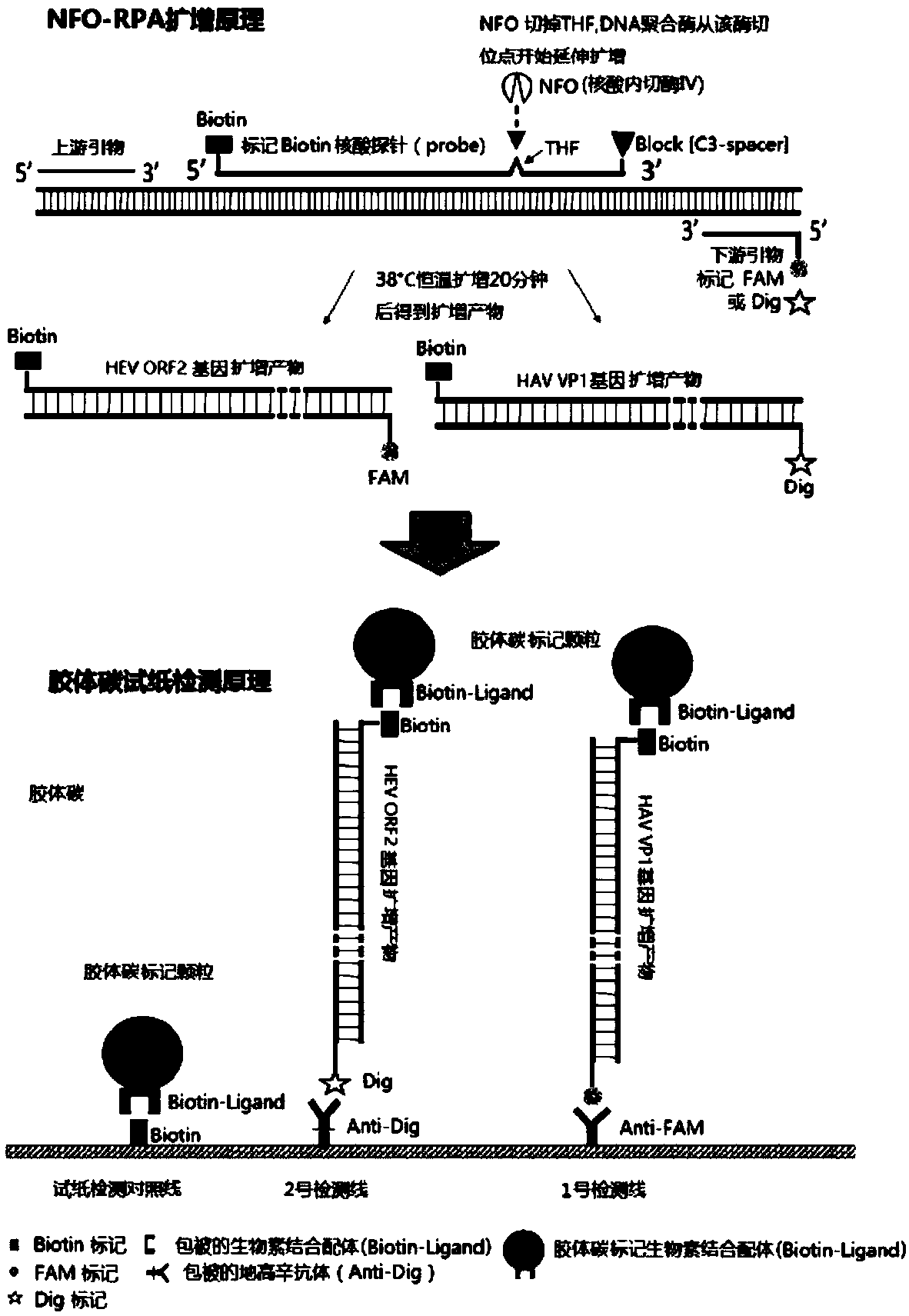
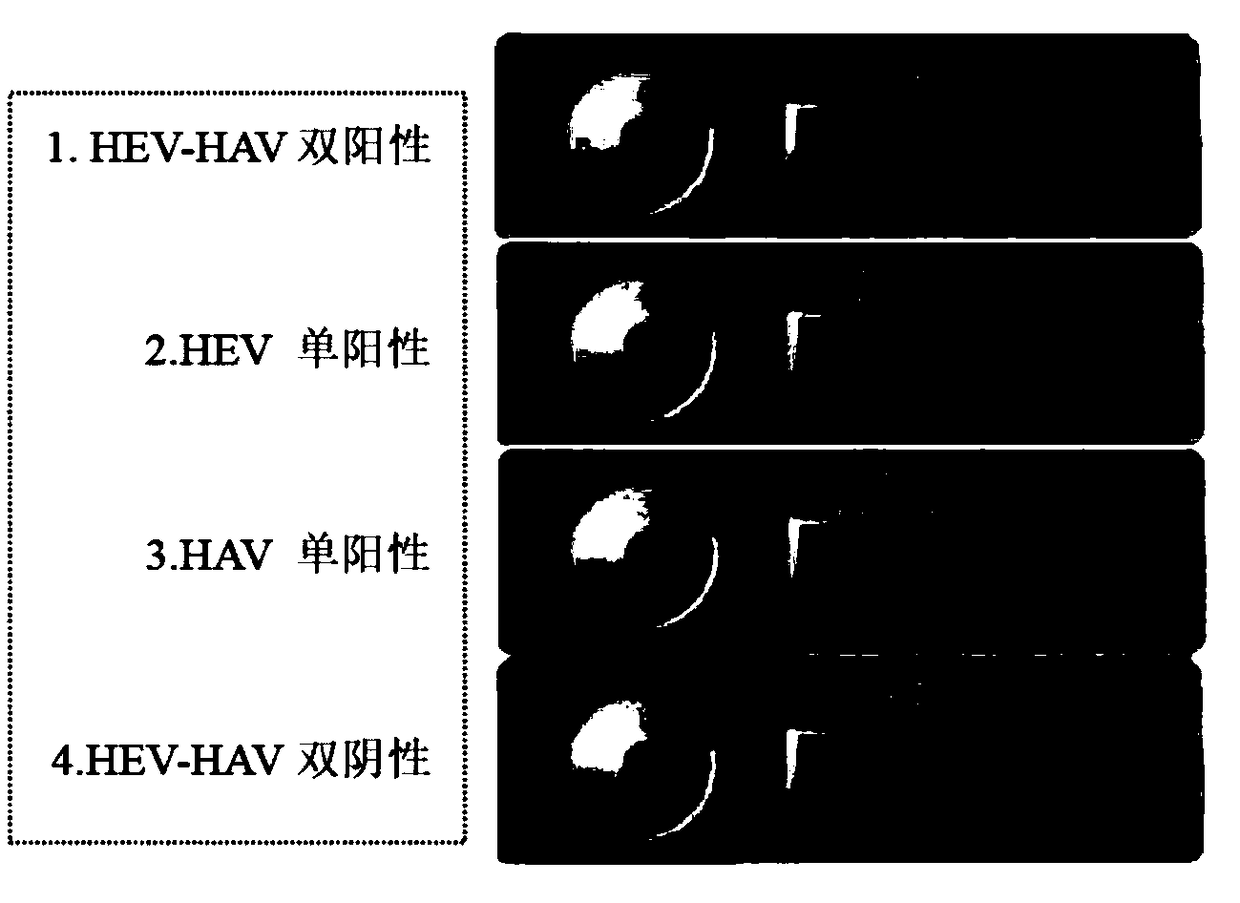
Primer for doubly detecting hepatitis e virus and hepatitis a virus through RT-RPA-lateral flow tomography, probe and kit
ActiveCN108841926AShorten detection timeThe reaction procedure is simpleMicrobiological testing/measurementAgainst vector-borne diseasesControl lineHepacivirus
The invention discloses a RT-RPA-lateral flow tomography kit for doubly detecting hepatitis e virus and hepatitis a virus. The kit comprises an upstream primer, an intermediate probe and a downstreamprimer which are applicable to hepatitis e virus ORF2 gene sequence in the RT-RAP amplification technology, and / or an upstream primer, an intermediate probe and a downstream primer which are applicable to hepatitis a virus VP1 gene sequence, general agents for recombinase polymerase amplification technology, and a lateral flow tomography test strip, wherein the lateral flow tomography test stripcomprises a sample adding pad, a control line, a #1 detecting line and / or a #2 detecting line; the control line, the detecting lines and the primers are combined with a probe label. With the adoptionof the kit, the hepatitis e virus ORF2 gene and / or hepatitis a virus VP1 gene in a sample can be rapidly, sensitively and specifically detected on site in a non-lab environment.
Owner:JINZHOU MEDICAL UNIV
Liquid phase chip multiple detection kit for swine PRRSV (Porcine Reproductive and Respiratory Syndrome Virus), SIV (Swine Influenza Virus) and HEV (Hepatitis E Virus) antibodies
ActiveCN106771178AEffective diagnosisEffective monitoringBiological material analysisBiological testingDiseaseHemagglutinin
The invention provides a liquid phase chip multiple detection kit for swine PRRSV (Porcine Reproductive and Respiratory Syndrome Virus), SIV (Swine Influenza Virus) and HEV (Hepatitis E Virus) antibodies. Different fluorescence labeling microspheres of PRRSV nsp7, SIV HA (Hemagglutinin) and HEV ORF2 (Opening Reading Frame) antibodies are used as carriers; on the basis of a single liquid phase chip detection method of the PRRSV, SIV and HEV antibodies, a multiple liquid phase chip detection method for simultaneously detecting three antibodies is established, provides a more effective and quicker detection method for monitoring the HEV, PRRSV and SIV antibodies, and provides a new clue for establishing a method of differential diagnosis of swine diseases and serum detection of other animal epidemic disease antibodies; aiming at breeding situation of quick development of pig industry in China, complex conditions of the swine diseases, serious mixed infection phenomenon and the like, accurate, quick and effective diagnosis and monitoring of epidemic disease conditions of swine herds are further enhanced.
Owner:SOUTH CHINA AGRI UNIV
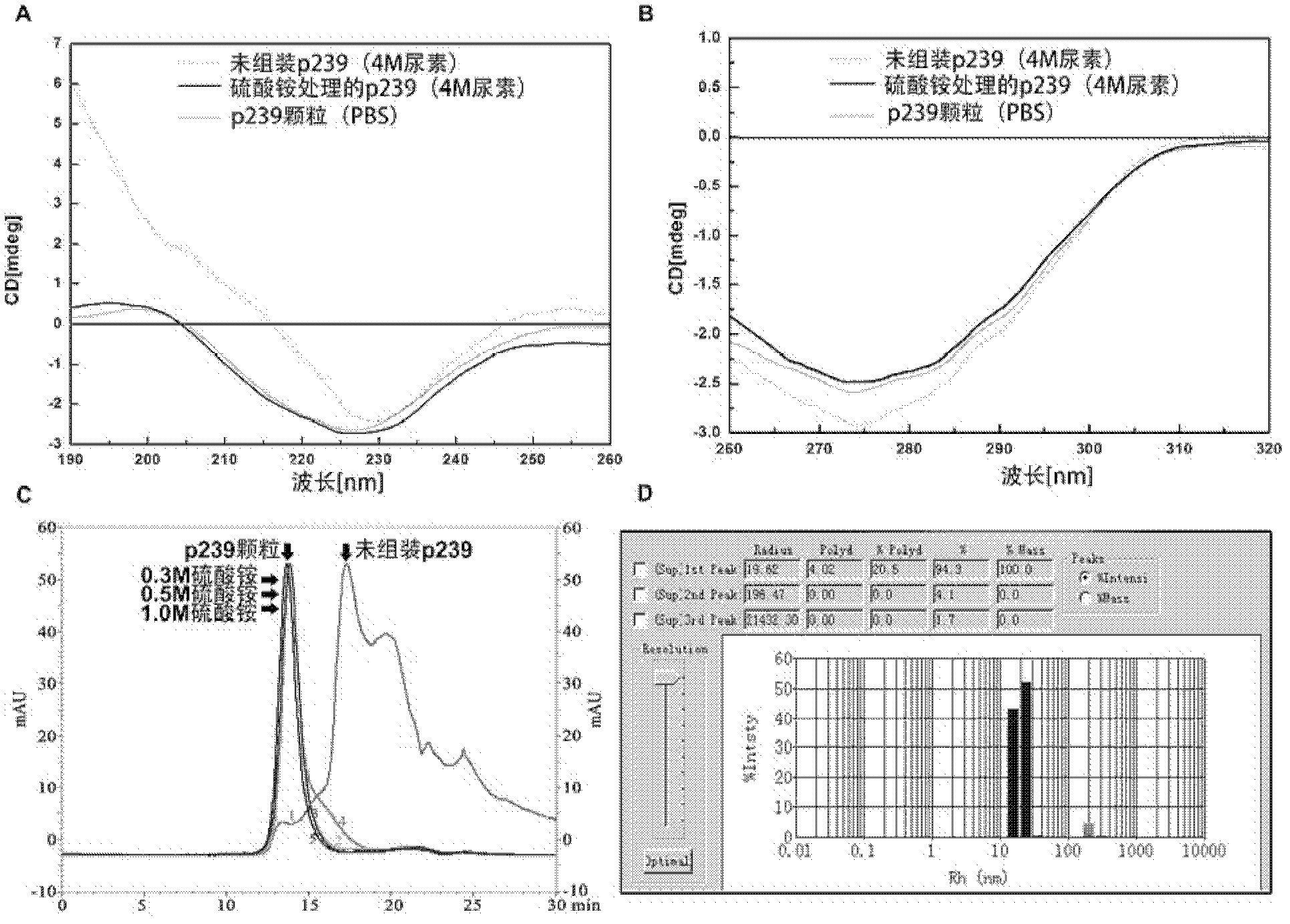
Assembly mechanism of virus-like particle of hepatitis E virus and preparation method thereof
ActiveCN102321591AAvoid damageReduce the cost of trainingViral antigen ingredientsMicrobiological testing/measurementVirus-like particleVirology
The invention illustrates an assembly mechanism of a virus-like particle (VLP) of hepatitis E virus (HEV), and provides a method for assembling the VLP by HEV capsid protein or its variants or fragments. In addition, the invention also provides a method for controlling the assembly of the HEV VLP, which controls the assembly of HEV capsid protein or its variants or fragments to the VLP by controlling the salt concentration and / or temperature.
Owner:XIAMEN UNIV +1
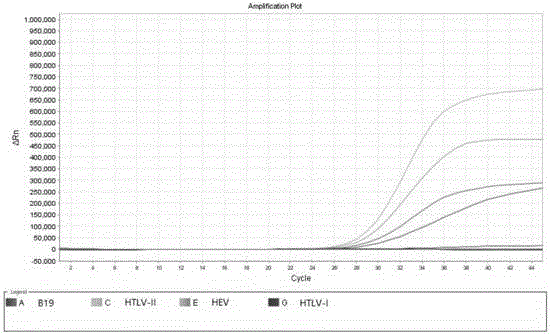
B19 (Human parvovirus B19), HTLV (Human T-cell lymphotropic virus) and HEV (Hepatitis E virus) quadruple fluorescent PCR (polymerase chain reaction) quick hypersensitivity detection kit and application thereof
ActiveCN105886665ANo cross contaminationNo distractionMicrobiological testing/measurementDNA preparationT cellBiology
The invention provides a B19 (Human parvovirus B19), HTLV (Human T-cell lymphotropic virus) and HEV (Hepatitis E virus) quadruple fluorescent PCR (polymerase chain reaction) quick hypersensitivity detection kit and an application thereof, and relates to a real-time PCR method for quadruple detection of target nucleic acid in a nucleic acid extraction liquid in a single PCR reaction container. The method is used for detecting B19, HTLV-I, HTLV-2 and HEV.
Owner:苏州华益美生物科技有限公司
Identification and detection method for hepatitis E virus by utilizing quadruple fluorescence quantitative PCR (Polymerase Chain Reaction)
ActiveCN101962689AHigh sensitivityImprove accuracyMicrobiological testing/measurementFluorescence/phosphorescenceDiseaseFluorescence
The traditional pig source hepatitis E has no effective vaccine for prevention, which adopts a control measure that the finding is carried out as soon as possible and the epidemic condition in epidemic areas is monitored at any time to block the spread of the disease. The invention discloses a rapid detection method for identifying each gene type of hepatitis E virus by utilizing multiple fluorescence quantitative PCR, which is characterized in that the a pair of conservative amplification primers and 4 strip-shaped specificity TaqMan probes are designed aiming at an HEVORF 3 sequence; the four probes are respectively designed aiming at the respective ORF 3 metamorphosis region sequence in a type specificity mode; and the identification and the detection of different gene types can be realized. The invention maintains the characteristics of high sensitivity and high accuracy of a PCR method and has the advantages that the quadruple fluorescence quantitative PCR enhances the detection efficiency and reduces the detection cost; the purposes of identification and diagnosis can be simultaneously realized; and the invention lays a foundation for work of infection source survey, spread environment, virus source tracing, etc.
Owner:THE INSPECTION & QUARANTINE TECH CENT ZHEJIANG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Hepatitis E virus IgG antibody detection kit and preparation method and application thereof
InactiveCN105445463AEliminate distractionsReduce sensitivityMaterial analysisPositive controlHepatitis E Virus IgG Antibody
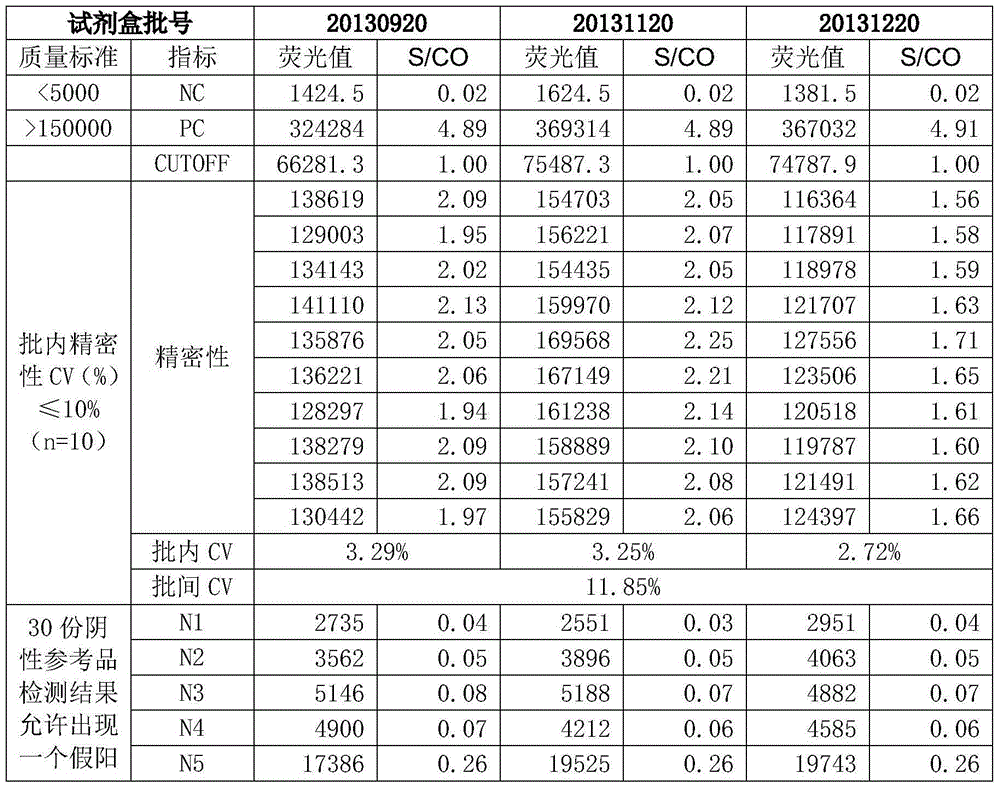
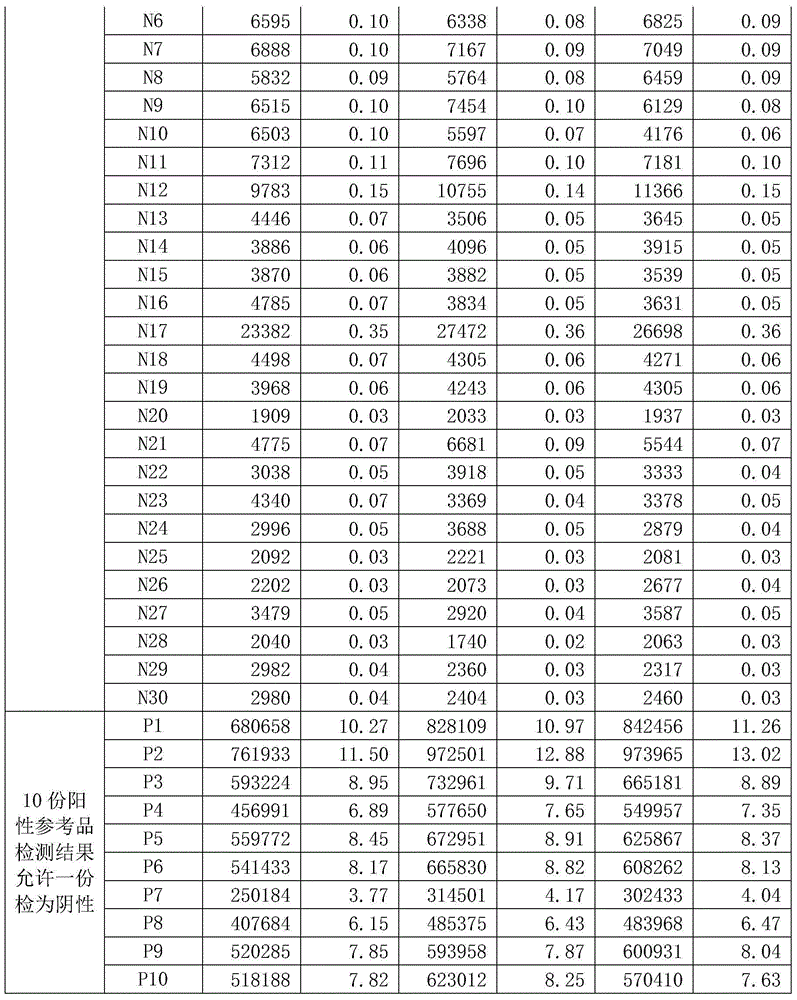
The invention discloses a hepatitis E virus IgG antibody detection kit. The kit includes the following components: a solid material coated with a hepatitis E virus (HEV) gene recombinant antigen, a mouse anti human IgG monoclonal antibody labeled with a signal generation substance, a concentrated washing solution, an enhancing liquid, an analysis buffer liquid, a sample diluent, a positive control and a negative control. In addition, the invention also provides a preparation method of the kit. The preparation method includes the following steps: a first step, preparing the solid material coated with the HEV gene recombinant antigen; and a second step, labeling a mouse anti human IgG monoclonal antibody with the signal generation substance. In addition, the invention also discloses an application of the kit in detection of a hepatitis E virus IgG antibody. The detection kit overcomes the defect of labeling antibodies with macromolecules (such as enzymes), has the advantages of high sensitivity, good stability, low cost and the like, can significantly improve the specificity, sensitivity and stability of detection of the hepatitis E virus IgG antibody, and besides, significantly reduces the cost.
Owner:SUZHOU SYM BIO LIFESCI CO LTD
Preparation method of diagnostic kit for pig hepatitis E virus ELISA
The invention discloses a preparation method of a diagnostic kit for pig hepatitis E virus ELISA, which comprises the following steps: (1) selecting antigen epitopes by a computer; (2) preparing antigens; (3) collecting serum; (4) establishing an indirect ELISA method; (5) verifying the sensibility and the specificity of the indirect ELISA method; (6) verifying the stability of the kit; and (7) carrying out clinical detection on a slaughterhouse. The method has the advantages of simplicity, high speed, high sensitivity, strong specificity, qualified stability and good repeatability. The kit prepared by the invention can effectively detect the hepatitis E virus of infected pigs, is suitable for detection of a large amount of pathogenetic samples, and can be used for quickly diagnosing the pig hepatitis E, preventing and controlling the spread of the pig hepatitis E virus and preventing the hepatitis E virus from spreading to people from pigs.
Owner:SHANGHAI ACAD OF AGRI SCI
A primary micro RNA expression cassette
This invention relates to inhibition of hepatitis gene expression. More specifically, the invention relates to a method of using RNA sequences to inhibit Hepatitis B and C Virus replication. Expression cassettes that include DNA sequences derived from endogenous micro RNAs (miRs) are used in the method and are transcribed by Pol Il promoters, and then processed to generate sequences that are specific to target hepatitis virus sequences (RNAi effecter sequences). The RNAi effecter sequences can target the selected hepatitis virus sequences resulting in gene silencing or transcriptional inhibition of the hepatitis virus gene. The expression cassettes may be delivered in vitro or in vivo to host cells. A pharmaceutical composition containing the expression cassettes is also claimed.
Owner:UNIVERSITY OF THE WITWATERSRAND
Fluorescent quantitative PCR detecting kit for hepatitis E virus and using method of fluorescent quantitative PCR detecting kit
InactiveCN105296676AEasy to operateSimple methodMicrobiological testing/measurementMagnetic beadPolynucleotide
The invention provides a fluorescent quantitative PCR detecting kit for a hepatitis E virus. The detecting kit comprises a PCR reaction solution, wherein the PCR reaction solution comprises a PCR buffering solution, deoxyribonucleoside triphosphate, a DNA polymerase, an upstream primer, a downstream primer and a probe, wherein the upstream primmer and the downstream primer are used for amplifying target polynucleotide, and the probe is used for detecting the target polynucleotide. The fluorescent quantitative PCR detecting kit for the hepatitis E virus is favorable in specificity, high in operation speed, simple and convenient in method and wide in detection range, and meanwhile an interior label additionally arranged in the reaction system can be used for effectively avoiding that the detected result is false negative. When the fluorescent quantitative PCR detecting kit for the hepatitis E virus is used for extracting RNA, a magnetic bead method which is good in adsorption effect and easy to purify is used, and the method can be used for effectively removing PCR inhibitors in complex samples, so that RNA with high purity and high yield is obtained, and detecting sensitivity, accuracy and stability are greatly improved.
Owner:SANSURE BIOTECH INC
Protein complex system for increased immunogenicity and functionality, and methods making and use
ActiveUS20140017269A1Improving immunogenicityImprove functionalitySsRNA viruses negative-senseSsRNA viruses positive-senseEscherichia coliRotavirus RNA
Genes for proteins which spontaneously form dimers and / or oligomers can be recombinantly linked together, which upon expression in E. coli produces stable dimeric fusion proteins that spontaneously self-assemble into enormous, polyvalent complexes having increased immunogenicity and functionality. Linear, network and agglomerate complexes with enormous sizes and polyvalences are constructed using glutathione S-transferase, Norovirus P domains (NoV P− and NoV+), the protruding (P) domain of hepatitis E virus (HEV P), the astrovirus P domain (AstV), a monomeric peptide epitope (M2e of influenza virus), and / or a protein antigen (VP8* of rotavirus) fused in different combinations. The resulting complexes can contain hundreds to thousands NoV P-protein, HEV, AstV, M2e and / or VP8* copies and exhibit higher immunogenicity than the individual proteins alone. The large size and multivalent nature of the complexes are candidates as a bivalent or multivalent vaccines against Norovirus and other pathogens, and for generation of antibodies for diagnosis and research purposes.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
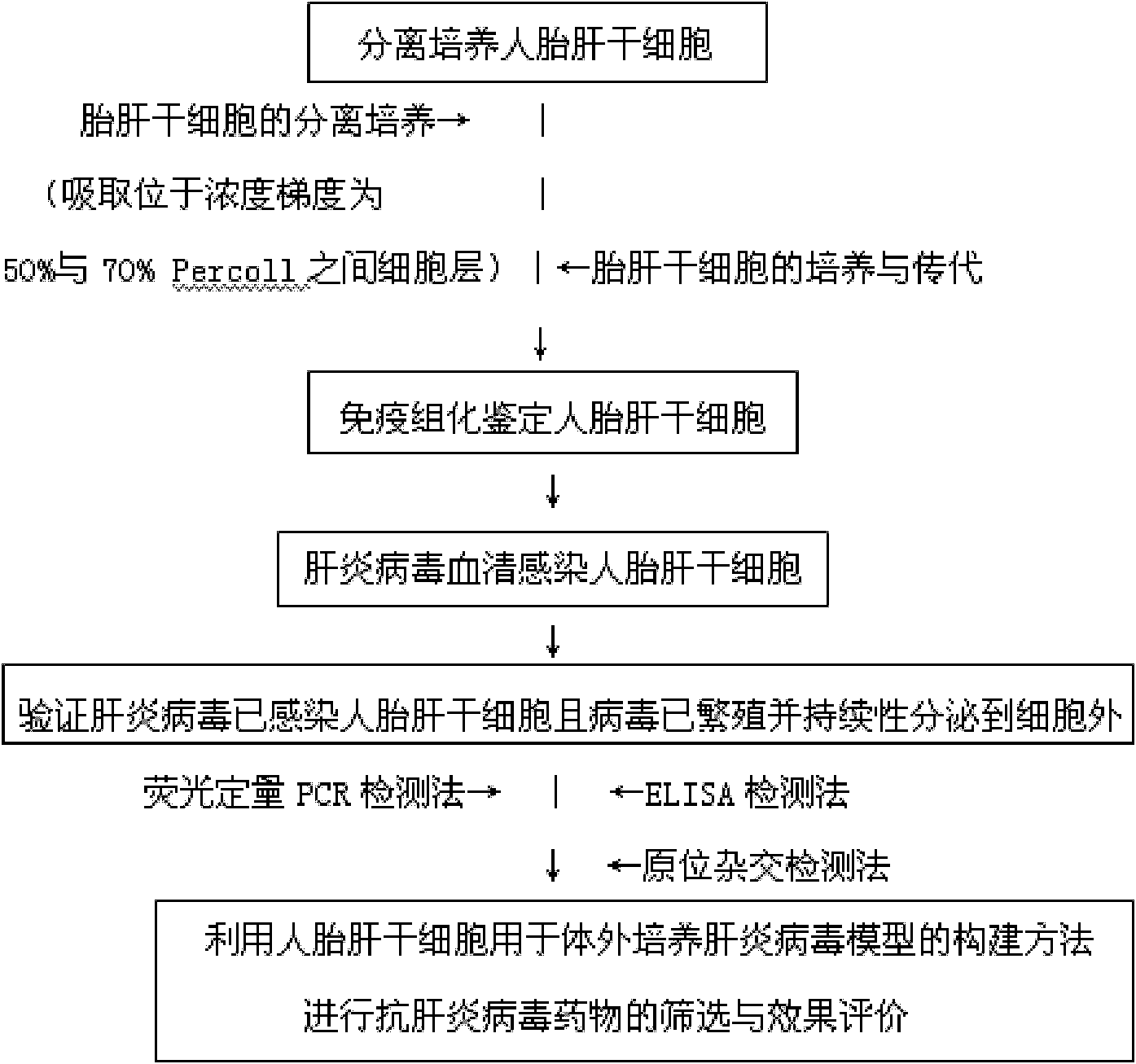
Hepatitis virus in-vitro culture model as well as construction method and application thereof
InactiveCN101914489ASimple methodGood repeatabilitySsRNA viruses positive-senseMicrobiological testing/measurementLiver Stem CellHuman fetal
The invention relates to a hepatitis virus in-vitro culture model as well as a construction method and application thereof. The hepatitis virus in-vitro culture model is constructed by adopting human fetal liver stem cells and comprises the steps of carrying out separate culture on human fetal liver stem cell, immunohistochemically identifying human fetal liver stem cell and infecting human fetal liver stem cell with hepatitis virus by blood serum and verifying whether hepatitis virus infects the human fetal liver stem cells or not and the virus propagate and continuously secrete outside the cell or not; the model can be used for the screening and effect evaluation of an in-vitro hepatitis virus resistant medicament; the human fetal liver stem cells used in the hepatitis virus in-vitro culture model can be infected with hepatitis virus and can continuously secrete hepatitis virus; the human fetal liver stem cells can be subcultured; and the invention has simple and convenient method and high repeatability.
Owner:INST OF HYGIENE & ENVIRONMENTAL MEDICINE PLA ACAD OF MILITARY MEDICAL
Polynucleotide probe and primer originating in hepatitis e virus of japanese, chips having the same, kits having the same and method of detecting hepatits e virus using the same
InactiveUS20040101820A1Efficient methodEasy to detectSugar derivativesMicrobiological testing/measurementPolynucleotideVirus
A polynucleotide probe including a sequence comprising at least eight nucleotides, the polynucleotide probe being used for detecting polynucleotide of hepatitis E virus, is characterized in that the sequence comprising at least eight nucleotides is hybridized with the polynucleotide of the hepatitis E virus, thereby, due to the hybridization, detects the hepatitis E virus.
Owner:TAKAHASHI KAZUAKI +4
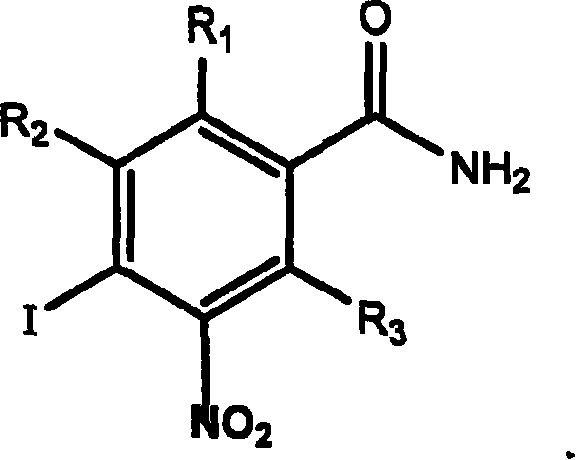
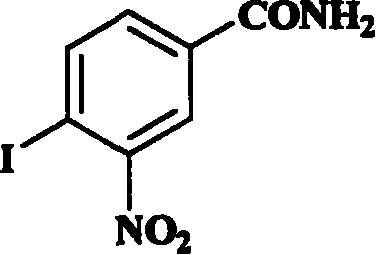
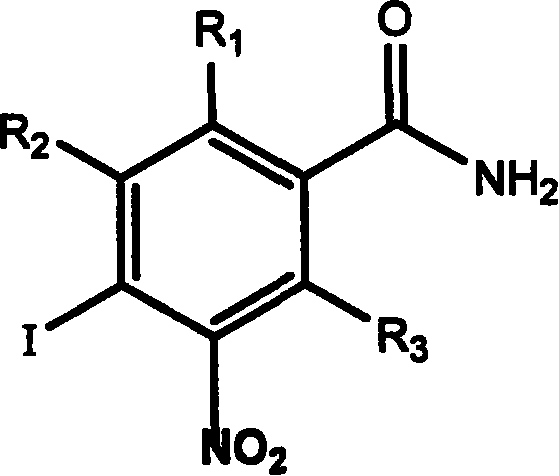
Application of aromatic nitro compound in preparing medicine for treating virus hepatitis
InactiveCN101190211APrevent proliferationNo drug resistanceOrganic active ingredientsDigestive systemNitro compoundDisease
The invention relates to a novel application of aromatic nitro compound in the pharmaceutical field, in particular to the application of aromatic nitro compound in treating viral hepatitis. The aromatic nitro compound can be used alone or can be combined with other commonly used anti- viral hepatitis drugs to effectively restrain the replication and secretion of hepatitis virus. The aromatic nitro compound can remarkably restrain the replication and secretion of hepatitis virus in the bodies of mammals. The aromatic nitro compound is especially suitable for treating the diseases caused by hepatitis B virus and hepatitis C. The invention provides the concrete method for treating the patients with hepatitis B and hepatitis C.
Owner:上海富海科星咨询管理有限公司
Avian and porcine hepatitis e virus shared antigen, monoclonal antibody and preparation method and application
ActiveCN104744572ARapid diagnosisHigh purityVirus peptidesImmunoglobulins against virusesDiseaseShared epitope
The invention relates to two monoclonal antibody hybridoma cell lines which secrete avian and porcine hepatitis e virus (HEV) shared epitope by virtue of a conventional hybridoma technique, wherein the lines are named 3E8 and 1B5 and the cell preservation numbers are respectively CCTCC No: C201395 and CCTCC No: C201396. The amino acid sequences of the shared epitope of the two lines identified by two monoclonal antibodies are identified by using a phage random 7 peptide library kit. The amino acid sequences are respectively V / IPHD (SEQ ID No: 1) and VKLYM (SEQ ID No: 2). Moreover, application of two mimic peptides of epitope in avian and porcine HEV serological diagnosis is analyzed. The two mimic peptides of the shared epitope of avian and porcine HEV capsid proteins disclosed by the invention can be used for rapid diagnosis of avian and porcine HEV and monitor of prevalence of disease. In addition, the two lines disclosed identify the monoclonal antibodies of the shared epitope and can be further used for rapidly diagnosing avian and porcine HEV.
Owner:NORTHWEST A & F UNIV
Hepatitis E virus fluorescent quantitative PCR detection method
InactiveCN101122563AHigh sensitivityStrong specificityMicrobiological testing/measurementMaterial analysis by optical meansFluorescenceTiter
The invention relates to a test method in the bioengineering field, in particular to a fluorescence quantitative PCR test method used for quantitatively test the titer of Hepatitis E virus (HEV) in a clinical specimen. The steps of invention include the design of virus specific quantitative PCR primers and probes, the construction of standard molecular, the extraction of a virus sample RNA, the cDNA synthesis of the sample RNA and the establishment and optimization of the fluorescence quantitative PCR test method. The testing sensitivity of the invention can reach to 10 degrees magnitude virus copy. The invention is equipped with good stability and specificity, which can be used for mass samples test and is capable of precisely measuring the virus load of the sample.
Owner:ZHEJIANG ACAD OF MEDICAL SCI
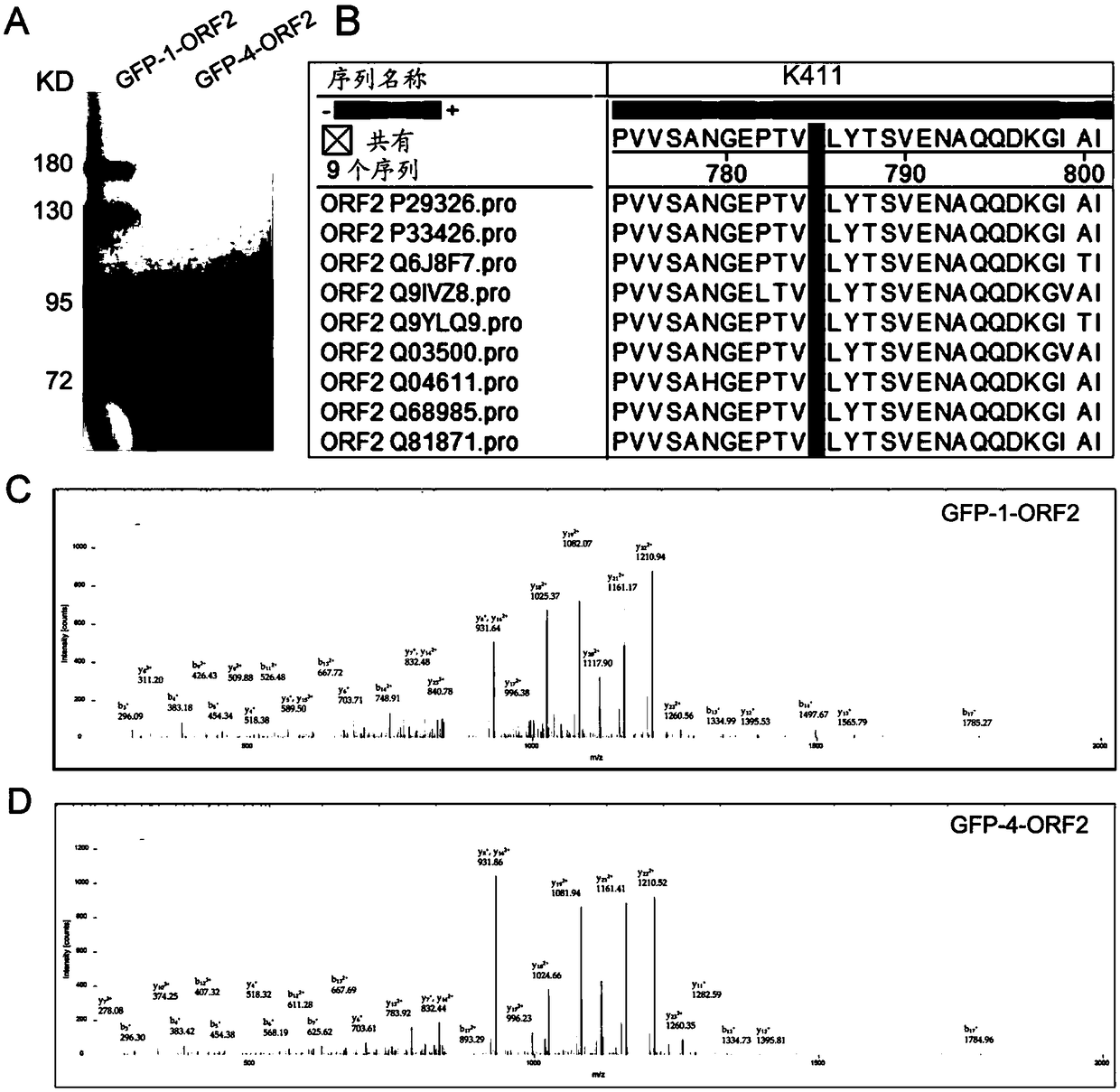
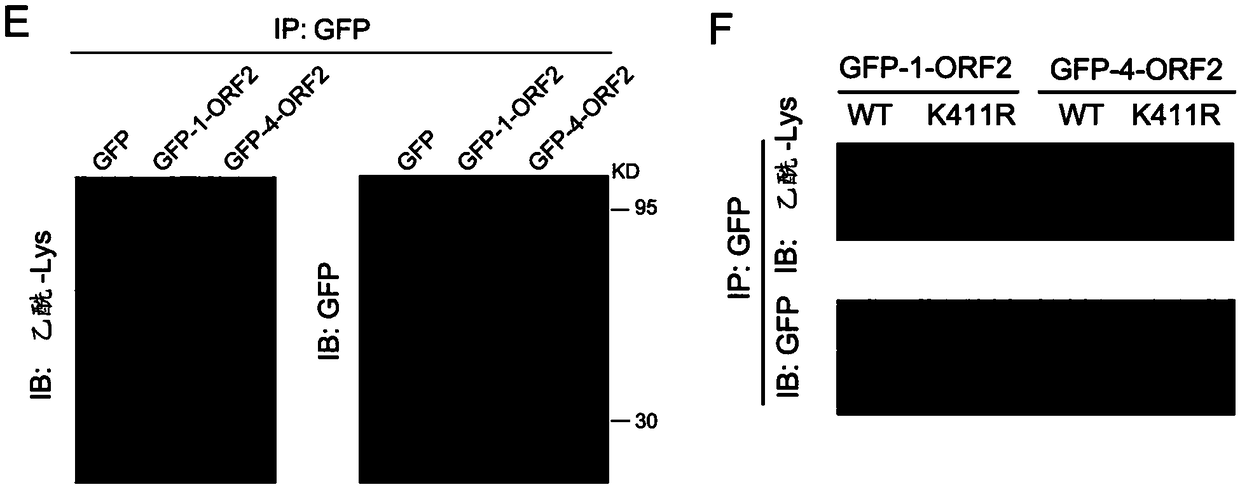
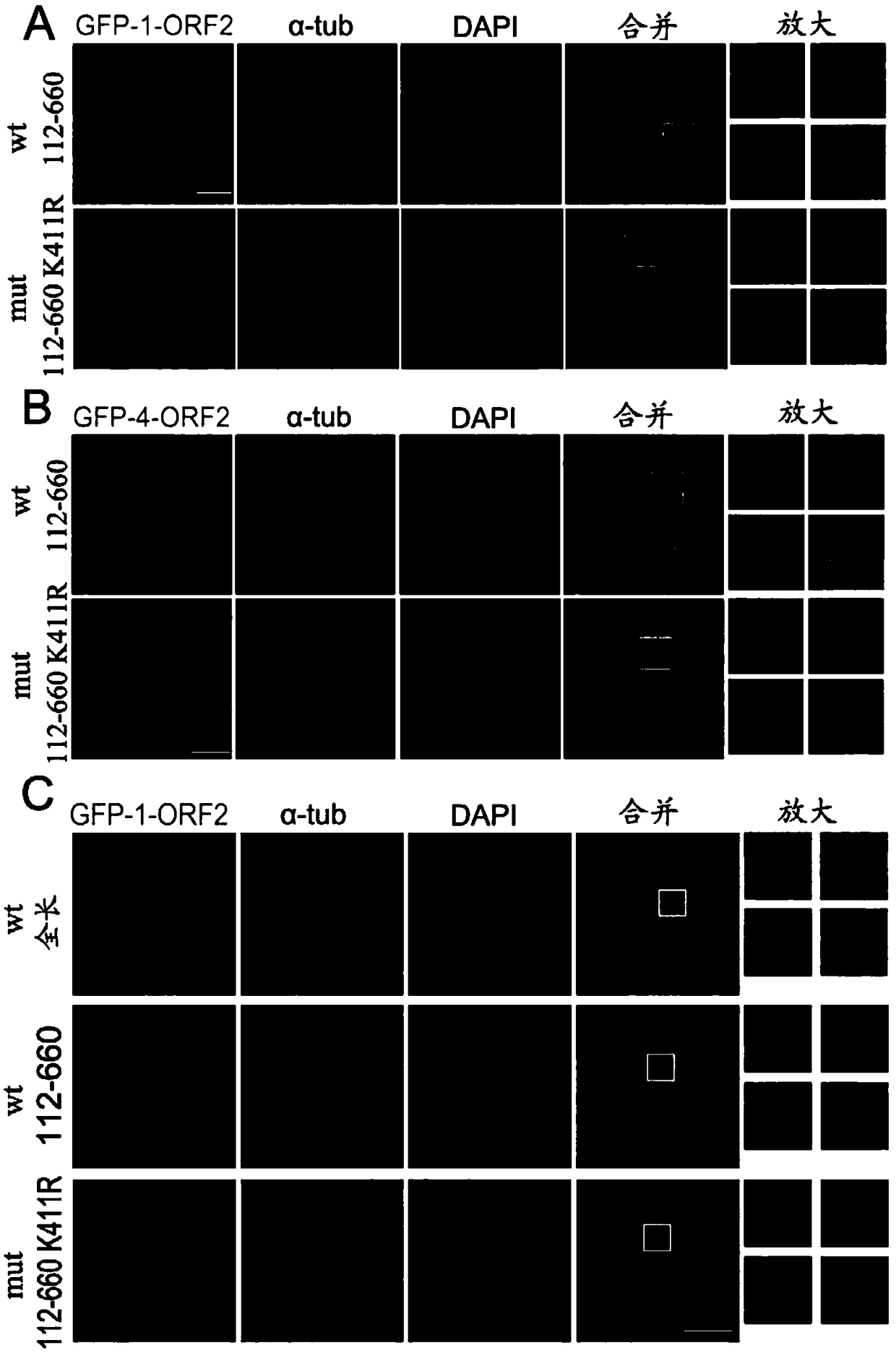
Acetylated HEV (Hepatitis E Virus) capsid protein ORF2 (Open Reading Frame 2) and application thereof
ActiveCN108752435AReduce acetylation levelsHigh expressionSsRNA viruses positive-sensePeptide/protein ingredientsOpen reading frameVirus-like particle
The invention relates to acetylated HEV (Hepatitis E Virus) capsid protein ORF2 (Open Reading Frame 2), a method for increasing the production of the acetylated HEV capsid protein ORF2 and a cell culture. The invention also relates to application of the acetylated HEV capsid protein ORF2 to promotion of ORF2 polymerizaiton and / or virus-like particle assembling.
Owner:NAT INST FOR FOOD & DRUG CONTROL
method for structuring hepatitis e infection model
InactiveCN101991610AEasy to operateEasy to feed and manageDigestive systemViral/bacteriophage medical ingredientsInterspecies transmissionOral medication
The invention discloses a method for structuring a hepatitis E infection model, which comprises the following steps of: infecting Meriones unguiculatus with hepatitis E viruses to obtain the hepatitis E infected model. The infection method is oral administration. The method of the invention has low price, adopts the Meriones unguiculatus infection model which is convenient to feed and test, and avoids the defects of high price, limited sources, high experiment requirement condition, long propagation period and the like of non-human primates used at present. The invention provides the ideal infection model for the study of HEV (hepatitis E virus) pathogenesis, vaccine development, interspecies transmission mechanism and the like.
Owner:CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com