Method and kit for detecting hepatitis E virus (HEV) antibody and method for preparing kit
A technology for detecting antibodies and kits, which is applied in the field of detection of hepatitis E virus antibodies based on double-labeled time-resolved fluorescence immunoassay, which can solve the problems of high environmental and operator influence, low sensitivity, linearity and stability, and difficulty in automation, etc. problem, to achieve the effect of helping diagnosis and prevention, improving clinical detection rate, and reducing missed detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] An embodiment of the kit for detecting hepatitis E virus antibody according to the present invention, the kit includes: a solid phase carrier coated with HEV recombinant antigen, Eu 3+ Labeled mouse anti-human IgG antibody, Sm 3+ Labeled mouse anti-human μ chain antibody, calibrator, sample diluent, experimental buffer, concentrated washing solution and enhancement solution; the HEV recombinant antigen contains ORF2 and ORF3 epitopes.
[0043] The present invention also provides a preparation method of the above-mentioned test kit for detecting hepatitis E virus antibody, said method comprising the following steps:
[0044] (1) Preparation of antigen-coated solid-phase carrier: Dilute HEV recombinant antigen with coating buffer to 0.1-10 μg / mL, coat on the solid-phase carrier, wash the solid-phase carrier once, and then use a blocking The solution was sealed, the solid-phase carrier was shaken dry, dried in the air, vacuum-packed, and stored at 2-8°C for later use. Pr...
Embodiment 2
[0057] The kit experimental method of the present invention is simple and rapid, and can be operated automatically, and the detection system is an open operating system. The using method of the kit for detecting hepatitis E virus antibody described in the present invention is as follows:
[0058] (1) Reagent preparation
[0059] ① Antigen solid-phase carrier: Equilibrate the reagent and the required amount of antigen solid-phase carrier to room temperature (20-25°C). The rest of the solid-phase antigen carrier was put into a ziplock bag in time to be sealed and stored at 2-8°C.
[0060] ②Washing liquid: Mix 40ml of concentrated washing liquid and 960ml of purified water in a clean container, and use it as a working washing liquid for later use. Please prepare purified water by yourself.
[0061] ③ Marker mixed working solution: Prepare within 30 minutes before use, mix Eu 3+ mouse anti-human IgG antibody, Sm 3+ Mouse anti-human μ chain antibody and experimental buffer by v...
Embodiment 3
[0072] Analytical performance evaluation of the test kit for detecting hepatitis E virus antibody of the present invention:
[0073] (1) Detection performance of HEV IgG antibody
[0074] Conformity rate of negative reference products: 30 national negative reference products for detection of HEV IgG antibody, no more than 1 positive reaction;
[0075] Conformity rate of positive reference products: 10 national positive reference products for detection of HEV IgG antibody, no more than 1 negative reaction;
[0076] Minimum detection limit: 6 copies of the national minimum detection limit reference product, no less than 3 positive reactions and negative reaction of matrix serum S1;
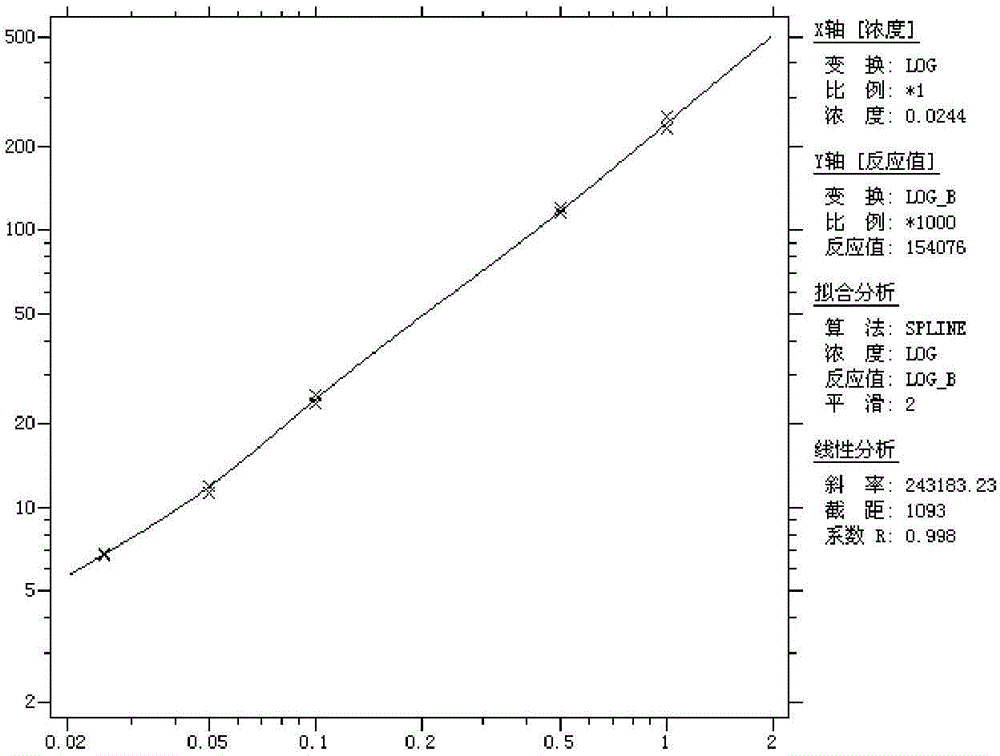
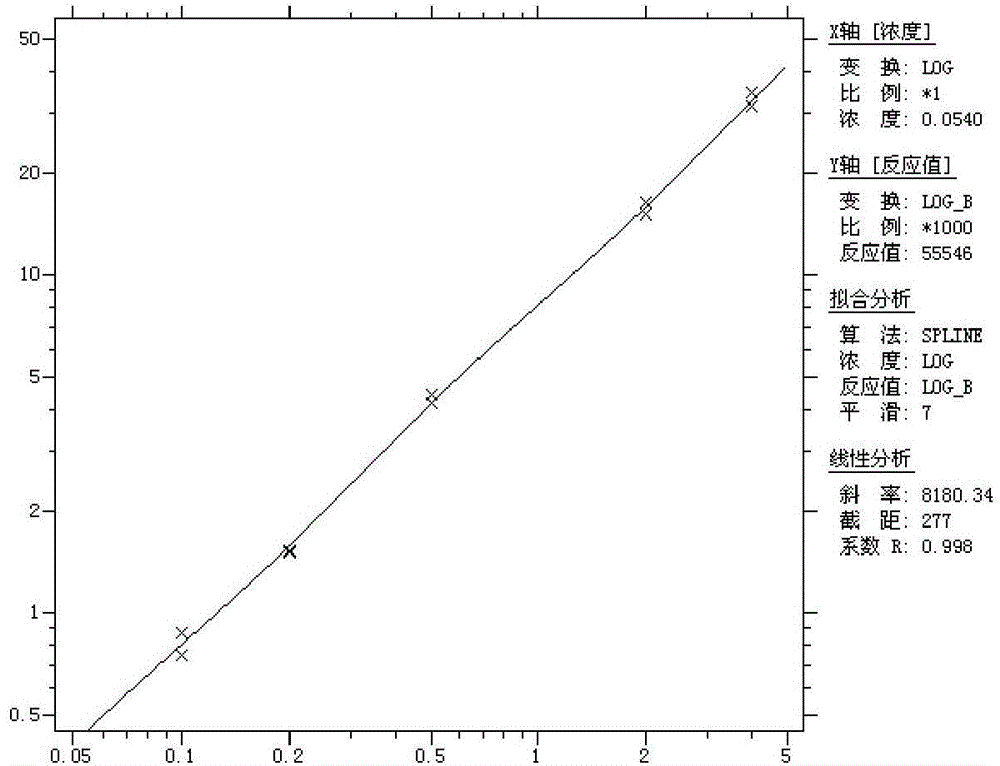
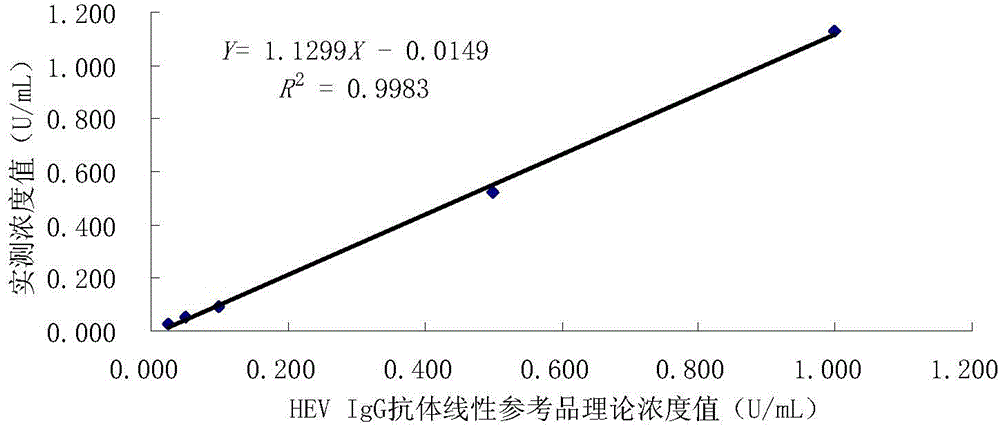
[0077] Linearity: After testing the reference products of the enterprise, after the statistical analysis of the measured values of 5 samples from L1 to L5, the linear correlation coefficient r between the measured value and the theoretical value is greater than 0.98, such as image 3 .
[0078]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com