Hepatitis E virus-like particle vaccine preparation method and application
A technology of hepatitis E virus and particles, which is applied in the field of biomedicine and can solve problems such as unclear functions and complex manufacturing processes of virus-like particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
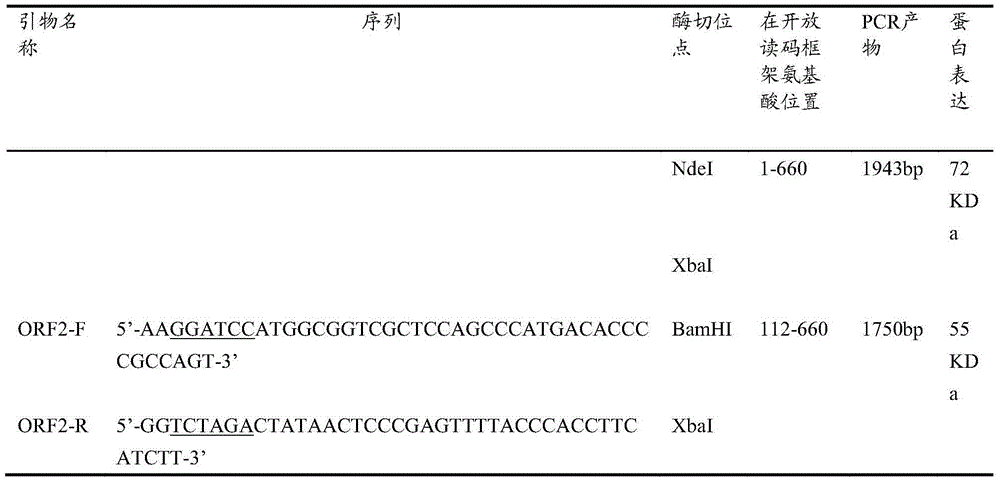
[0066] Example 1 Hepatitis E virus capsid protein gene codon optimization
[0067] Reagents and enzymes used: T4 DNA ligase and restriction endonuclease were purchased from NEB Company in the United States, Isopropyl-β-D-thiogalactopyranoside (IPTG), polymerase, dNTP and protein markers were purchased from Roche Company in Germany, and the bioreactor was purchased from Lithuania Company, all chemicals were purchased from German Merck (Germany) and American Sigma Company.
[0068] The genome sequence of HEV gene ORF2 was obtained from the GenBank China HEV isolate (accession no.AF444002.1). The wild-type truncated ORF2.1 encodes the 1-660 amino acids of the HEV capsid protein. GenScript software was used to analyze the rare codons of the wild-type ORF2 genome sequence, and two restriction enzymes NdeI and XbaI were placed at both ends of the ORF2 gene . The pGEM-T plasmid was digested according to the restriction sites shown in Table 1 to synthesize the full-length ORF2 gene ...
Embodiment 2
[0074] Soluble, massive expression and extraction of the capsid protein HEV-ΔORF2 protein of embodiment 2 hepatitis E virus truncated
[0075] 1 Test method
[0076] 1.1 Construction and screening of soluble expression plasmids
[0077] The ΔORF2 gene product synthesized in Example 1 was ligated with the pMAL-p4x expression plasmid fragment digested with the same restriction enzymes. In order to increase the solubility of the expression product, select a suitable fusion partner and purification tag, especially the optimized fusion partner is the N-terminal maltose-binding protein, and no fusion partner or purification tag is added to the C-terminus to ensure that the recombinant expression product can be used as a vaccine antigen. The MBP (maltose binding protein) tag protein is 40kDa in size and is encoded by the malE gene on the pMAL-p4x expression plasmid. MBP can increase the solubility of fusion proteins overexpressed in bacteria, especially eukaryotic proteins. The MB...
Embodiment 3
[0099] Example 3 Hepatitis E virus truncated capsid protein HEV-ΔORF2 proteolytic digestion and affinity chromatography purification
[0100] 1 Test method
[0101] 1.1 Digestion of MBP-ΔORF2 fusion protein
[0102] Factor Xa was prepared in buffer (20mM Tris, pH 8.0, 0.2M NaCl, 5mM CaCl 2 ), adding 1 unit of factor Xa to 100 μg of protein, adding factor Xa to the HEV-ΔORF2 protein prepared in Example 2, acting at room temperature for 36-48 hours, and digesting 95% of the protease.
[0103] 1.2 Purification of anti-maltose antibody on Sepharose 4 affinity medium
[0104] Enzyme digestion reaction solution and 5mL anti-maltose antibody-coupled Sepharose 4 affinity medium were mixed and incubated at 4°C for 18-24 hours, and 0.5mg MBP was adsorbed per 1mL medium, which could be covalently bound to specific sites on MBP for purification and MBP fusion expressed protein. The supernatant was harvested after centrifugation at 4000 rpm at room temperature.
[0105] The protein in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com