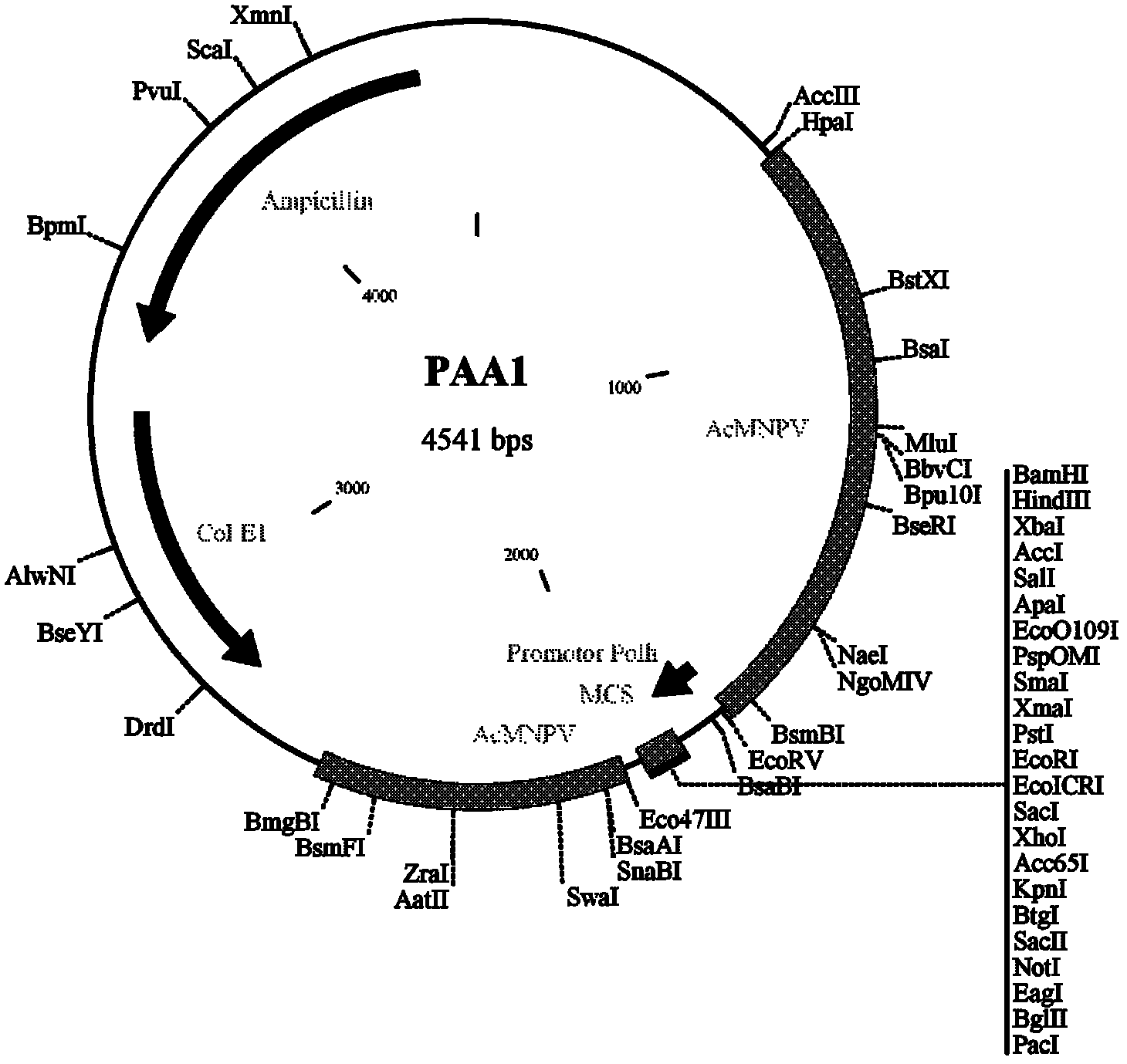
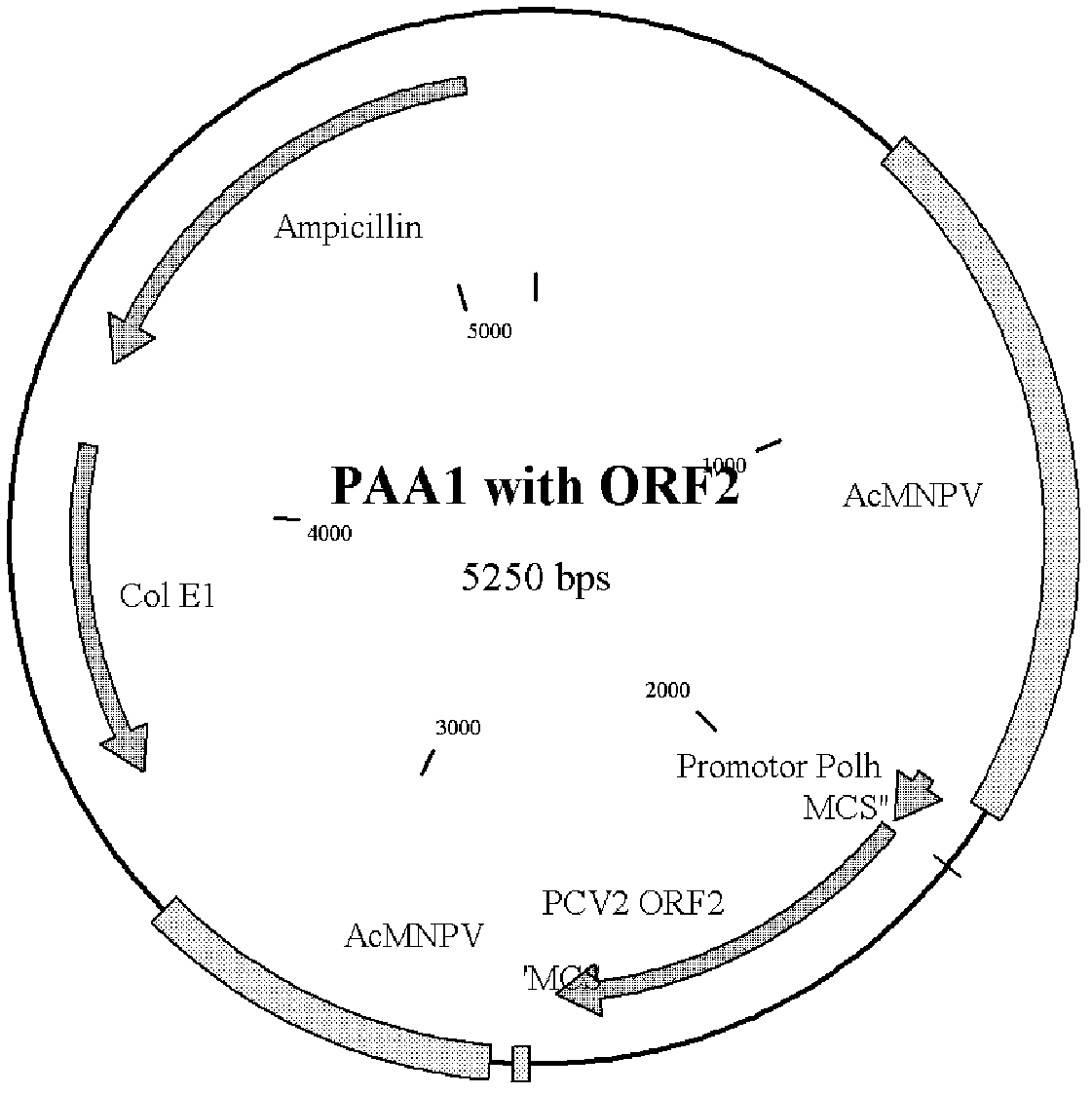
Porcin circovirus type 2 (PCV2) recombinant baculovirus construction method and subunit vaccine preparation method thereof
A subunit vaccine and virus technology, applied in botany equipment and methods, biochemical equipment and methods, antiviral agents, etc., can solve the problems of low virus titer, insufficient antigen content, poor biological safety, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Preparation of subunit vaccines
[0086] The insect cell Sf9-F was cultured with the suspension culture cell technology in a common cell culture device, the culture environment was 27°C 120r / min, and the cells were in the logarithmic growth phase for about 48 hours, and the recombinant baculovirus Bac / CY( CGMCCNo.5267 strain), and then through 72h culture, harvest infected cells, the virus titer can reach 10 8.0 TCID 50 / ml or more, repeated freeze-thawing 3 times, washed several times with PBS to get the supernatant, purified the above-mentioned recombinant protein, added conventional vaccine adjuvant and emulsified to obtain PCV2 recombinant baculovirus subunit vaccine.
Embodiment 2
[0088] Sf9-F cells cultured in the torrent reactor AP20C for the preparation of subunit vaccines
[0089] Sf9-F cells were inoculated into the torrent bag, and after 84 hours of culture, the number of cells reached 2.98 6 pcs / ml (attached Figure 5). After inoculating the virus seed suspension of recombinant baculovirus CGMCC No.5267 strain and continuing to culture for 6 days, the antigen expression test results showed that: in the early stage of virus infection, the antigen protein was mainly concentrated in the cell pellet, and the antigen content in the supernatant was very small; , the antigens were mainly distributed in the supernatant; the highest level of antigen expression appeared at 120 hours after inoculation, and the total antigen content could reach 238.17 μg / ml (attached Figure 6 ). Other processes are with embodiment 1.
Embodiment 3
[0091] Preparation of PCV2 Recombinant Baculovirus Subunit Vaccine
[0092] The insect cell Sf9-F can be cultivated by the spinner bottle culture cell technique to prepare the PCV2 recombinant baculovirus subunit vaccine. Other processes are with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com