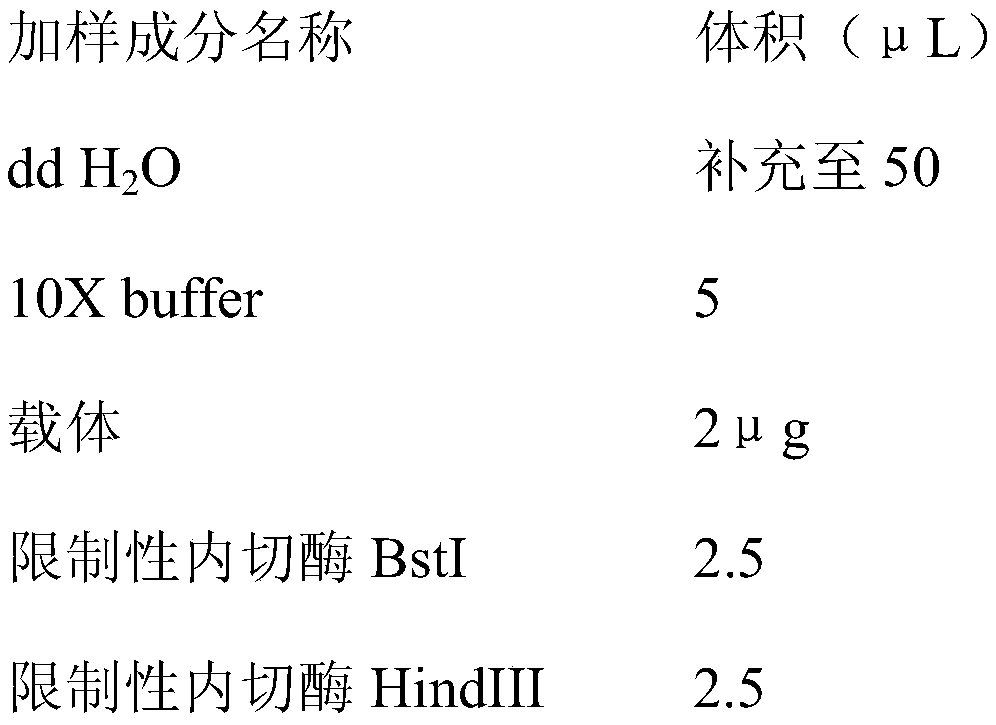
Patents
Literature
880 results about "Subunit vaccines" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A subunit vaccine is a vaccine that contains isolated proteins from a virus, but lacks viral nucleic acid.
Method for differentially quantifying naturally processed hla-restricted peptides for cancer, autoimmune and infectious diseases immunotherapy development
ActiveUS20130096016A1Efficient use ofBiological material analysisLibrary member identificationDiseaseAntigen
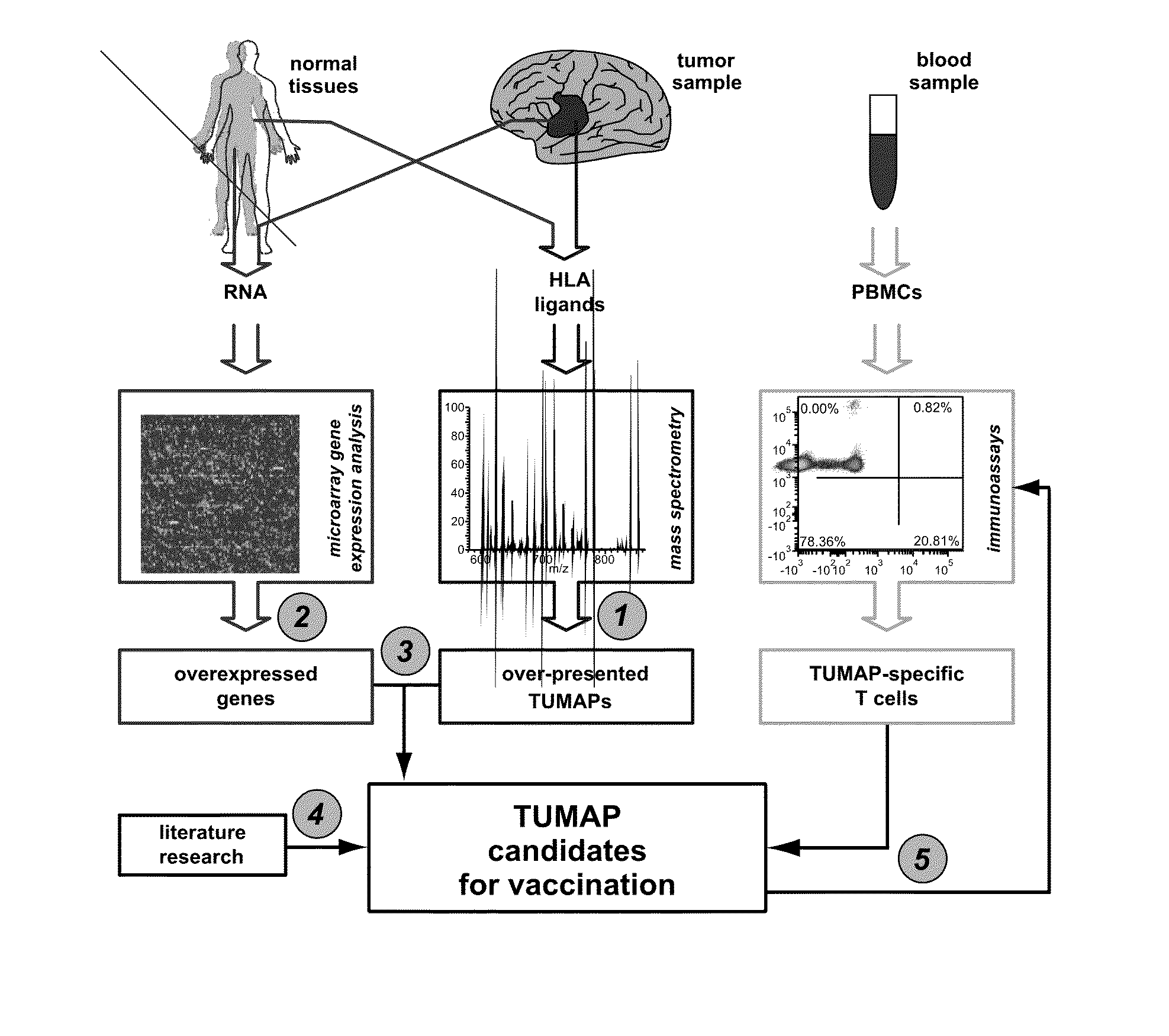
The invention relates to a method for quantitatively identifying relevant HLA-bound peptide antigens from primary tissue specimens on a large scale without labeling approaches. This method can not only be used for the development of peptide vaccines, but is also highly valuable for a molecularly defined immunomonitoring and the identification of new antigens for any immunotherapeutic strategy in which HLA-restricted antigenic determinants function as targets, such as a variety of subunit vaccines or adoptive T-cell transfer approaches in cancer, or infectious and autoimmune diseases.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
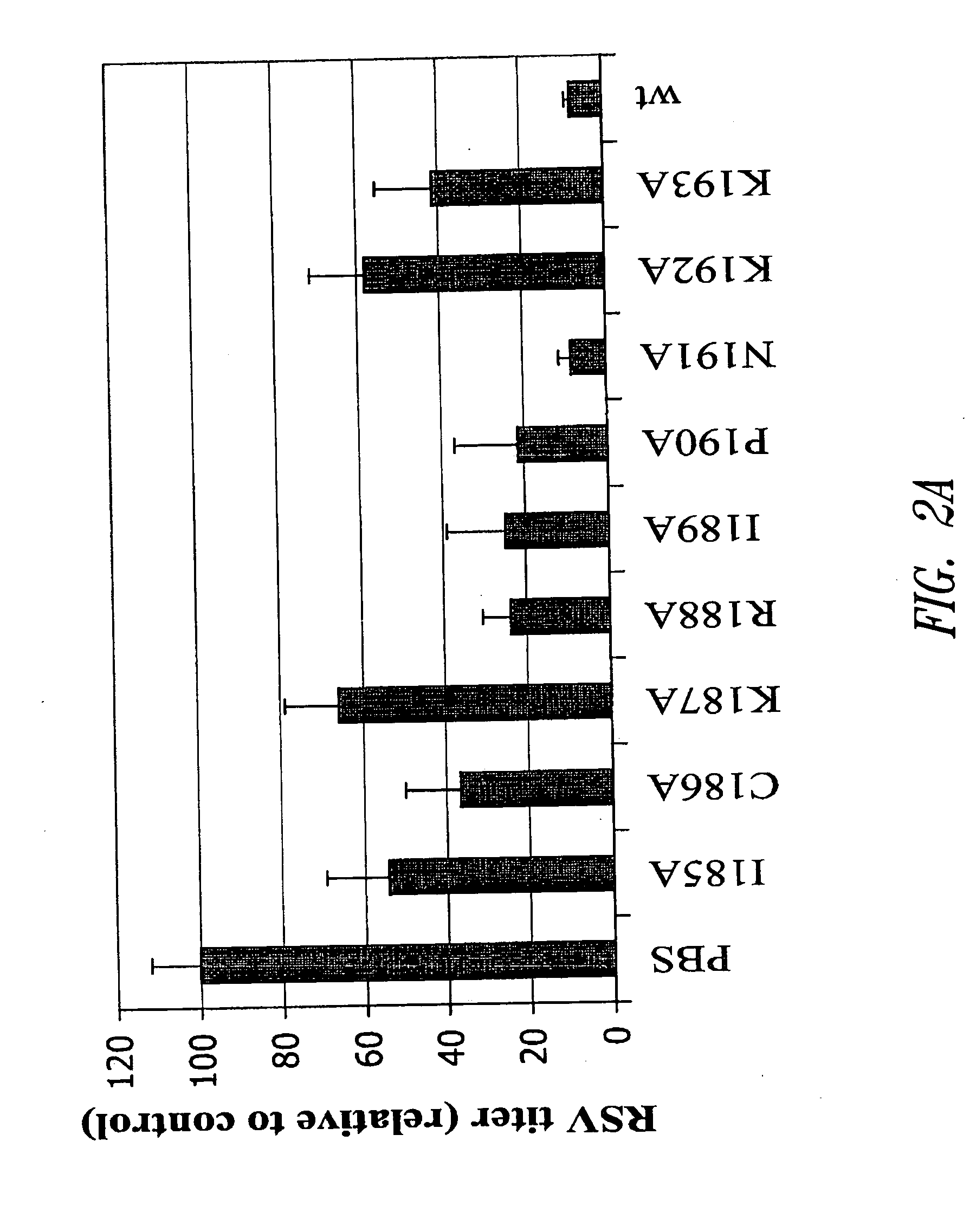
Subunit vaccine against respiratory syncytial virus infection
InactiveUS20050042230A1Reduced responseSsRNA viruses negative-senseVirus peptidesPrevention infectionProtection sex
The present invention relates generally to methods of treating or preventing RSV infections, and more specifically, to compositions, and the use thereof, comprising one or more RSV G protein immunogen or fragment thereof capable of eliciting protective immunity without eliciting an immunopathological response or eliciting a reduced immunopathological response.
Owner:ID BIOMEDICAL CORP LAVAL
One dose vaccination with Mycoplasma hyopneumoniae
InactiveUS6846477B2Preventing and reducing lung lesionMaintaining immunityAntibacterial agentsBiocideDiseaseVaccines Administered
The present invention relates to methods for treating or preventing a disease or disorder in an animal caused by infection by Mycoplasma hyopneumoniae (M. hyo) by administering to the animal at approximately three (3) to ten (10) days of age, a single dose of an effective amount of a M. hyo vaccine. The M. hyo vaccine can be a whole or partial cell inactivated or modified live preparation, a subunit vaccine, or a nucleic acid or DNA vaccine. The M. hyo vaccine administered in accordance with the present invention can be synthesized or recombinantly produced.
Owner:ZOETIS SERVICE LLC +1
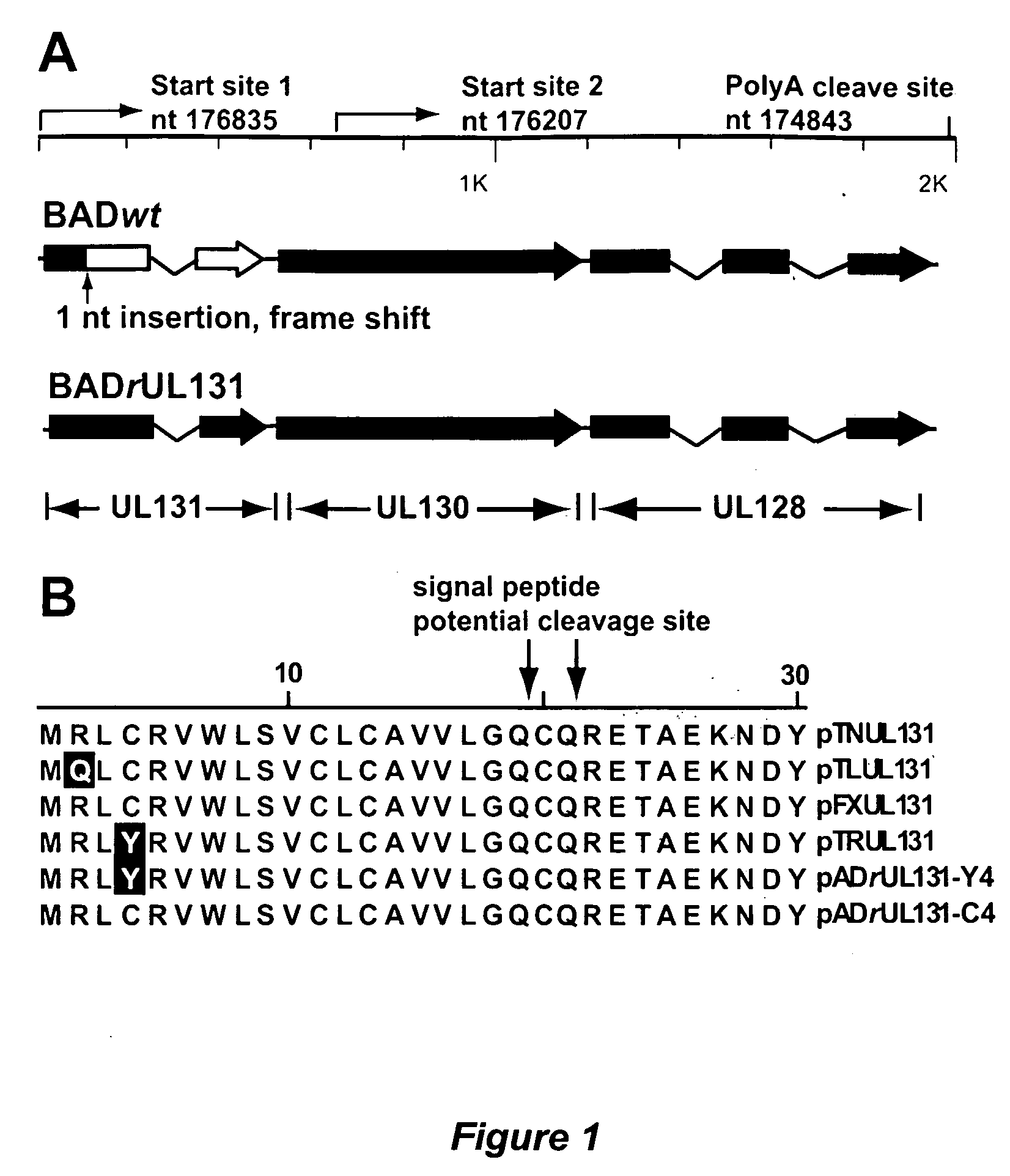
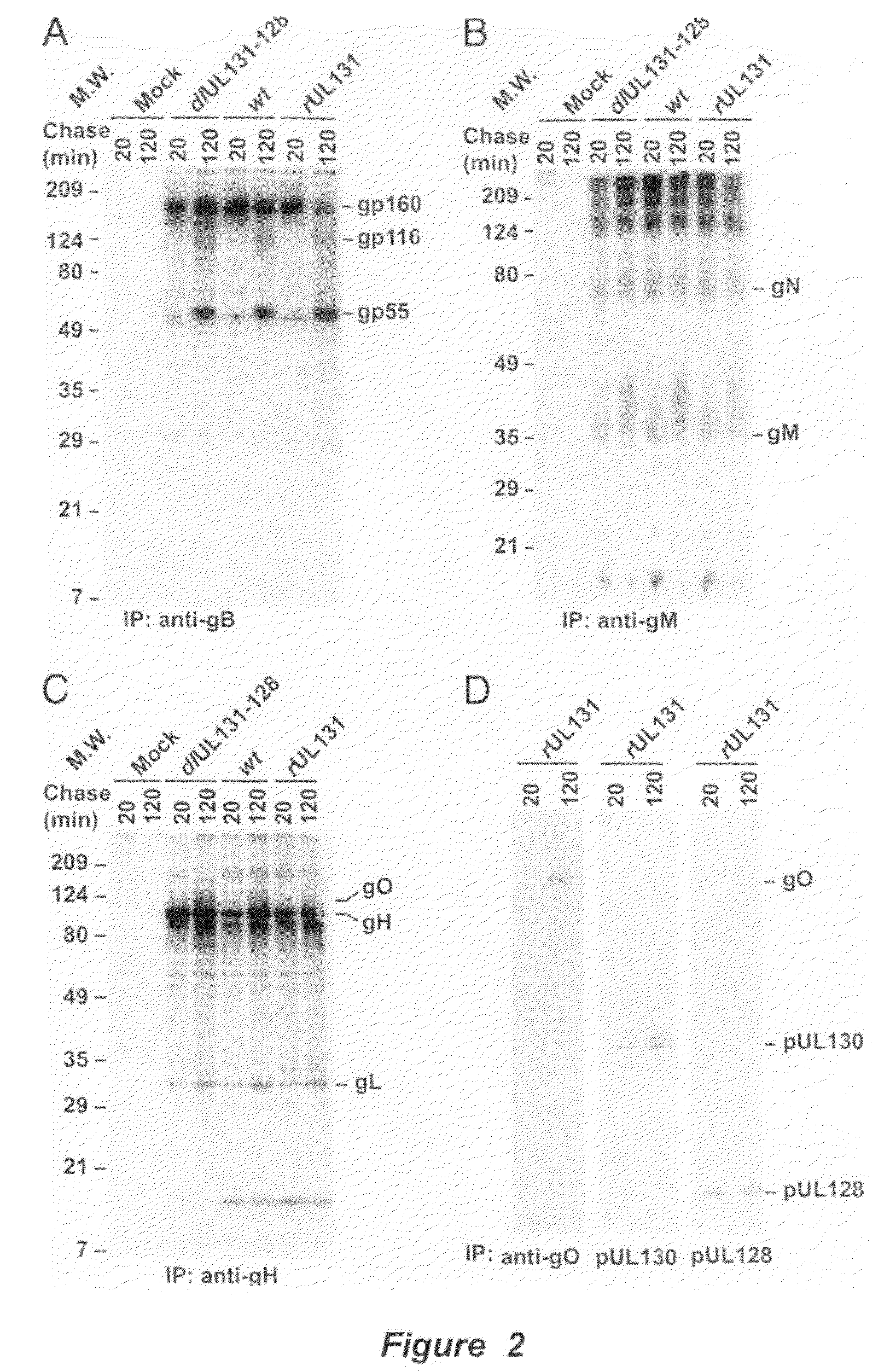
Cytomegalovirus surface protein complex for use in vaccines and as a drug target
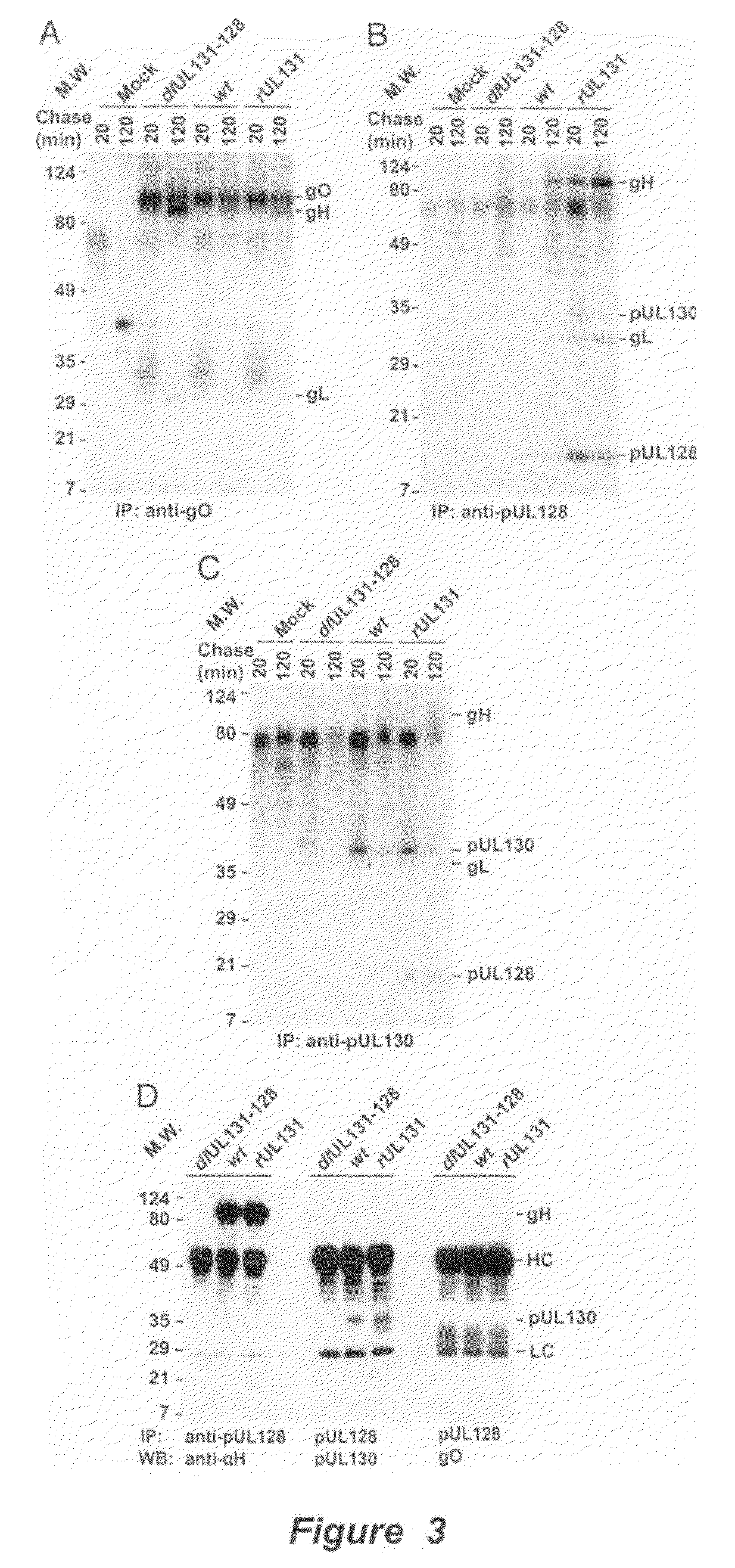
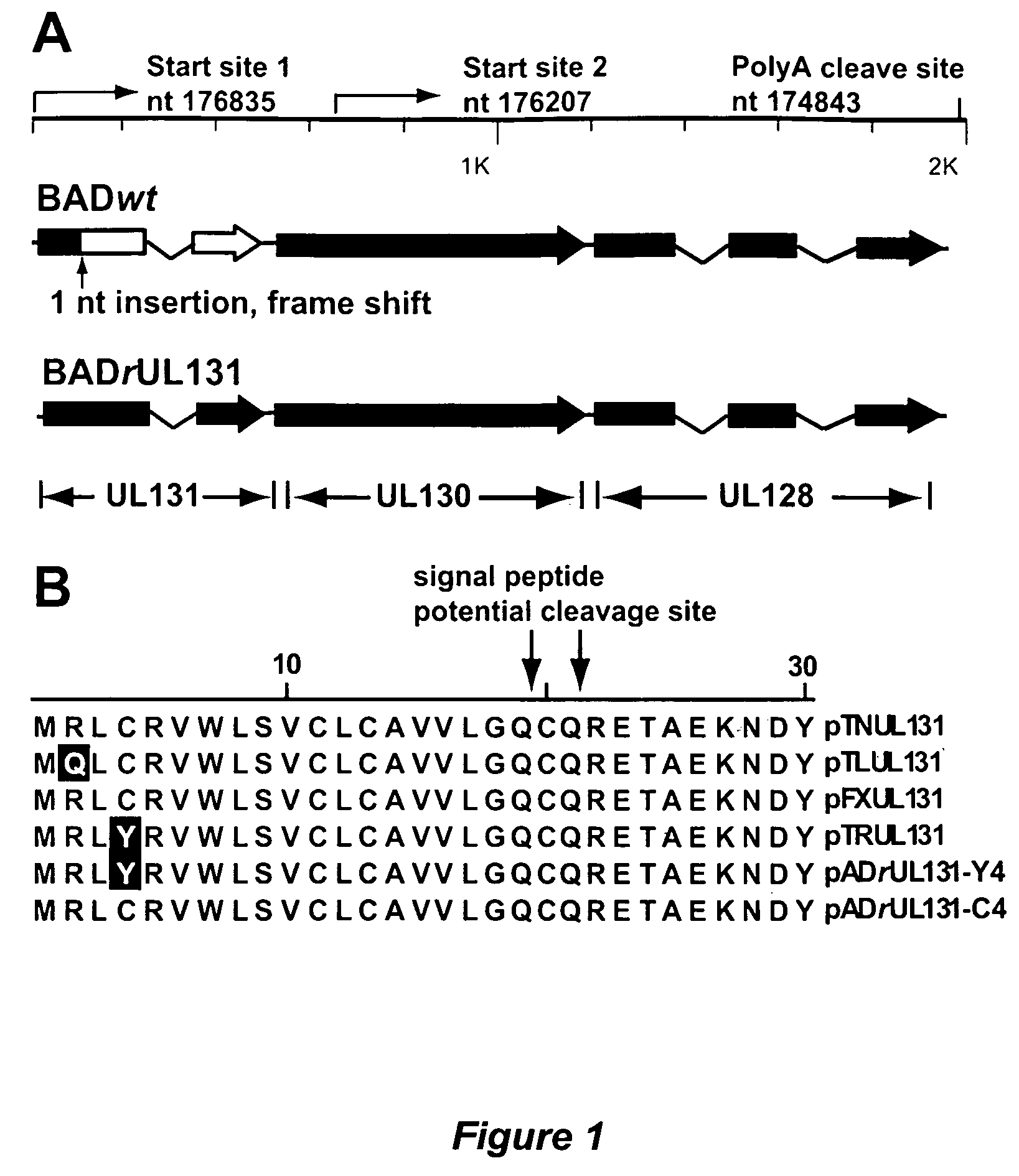
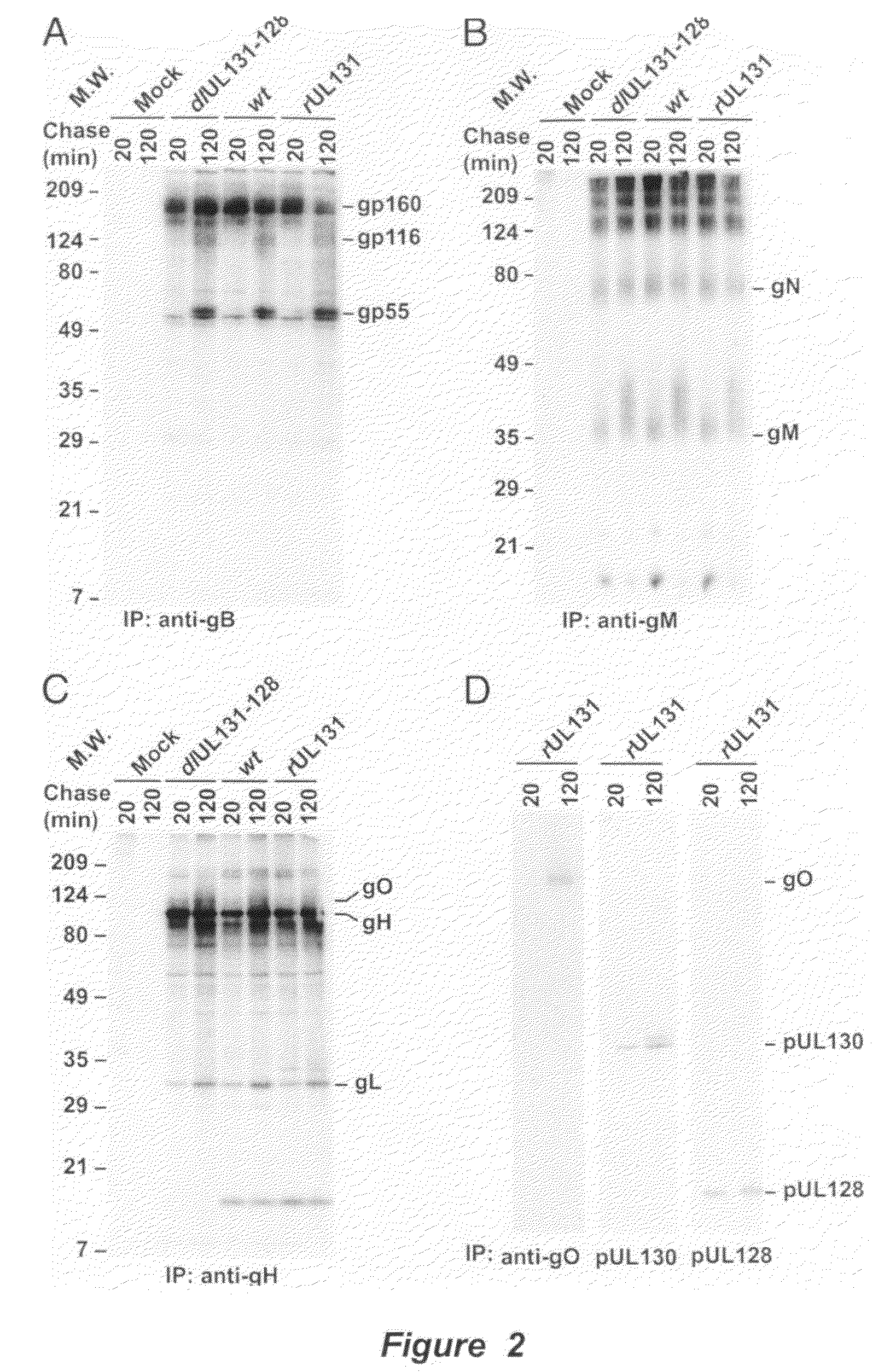
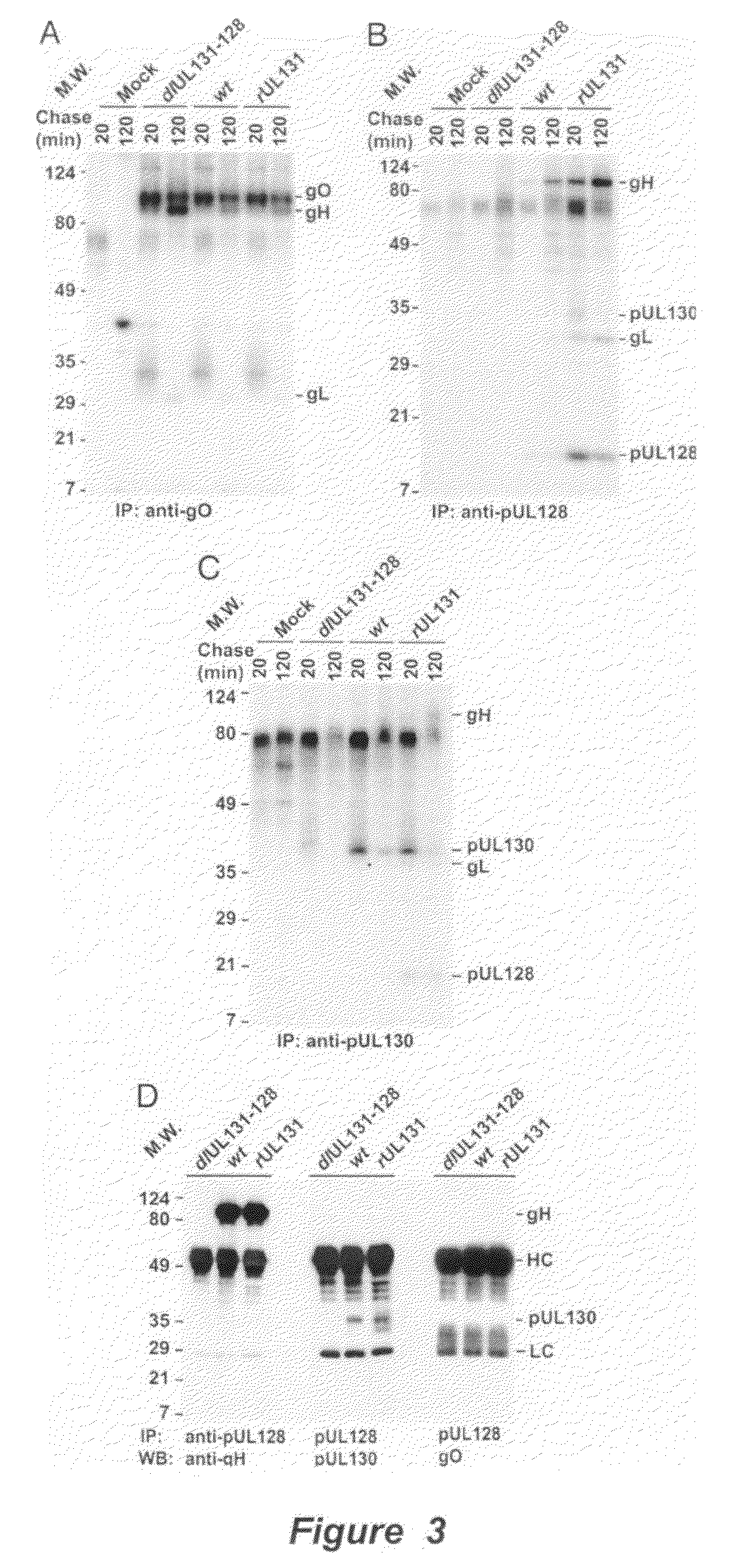
Immunogenic compositions and prophylactic or therapeutic vaccines for use in protecting and treating against human cytomegalovirus (CMV) are disclosed. Subunit vaccines comprising a human CMV protein complex comprising pUL128 or pUL130, and nucleic acid vaccines comprising at least one nucleic acid encoding a CMV protein complex comprising pUL128 or pUL130 are described. Also disclosed are therapeutic antibodies reactive against a CMV protein complex comprising pUL128 or pUL130, as well as methods for screening compounds that inhibit CMV infection of epithelial and endothelial cells, methods for immunizing a subject against CMV infection, methods for determining the capability of neutralizing antibodies to inhibit human CMV infection of cell types other than fibroblasts, and methods of diminishing an CMV infection.
Owner:THE TRUSTEES FOR PRINCETON UNIV
HIV envelope polypeptides and vaccine
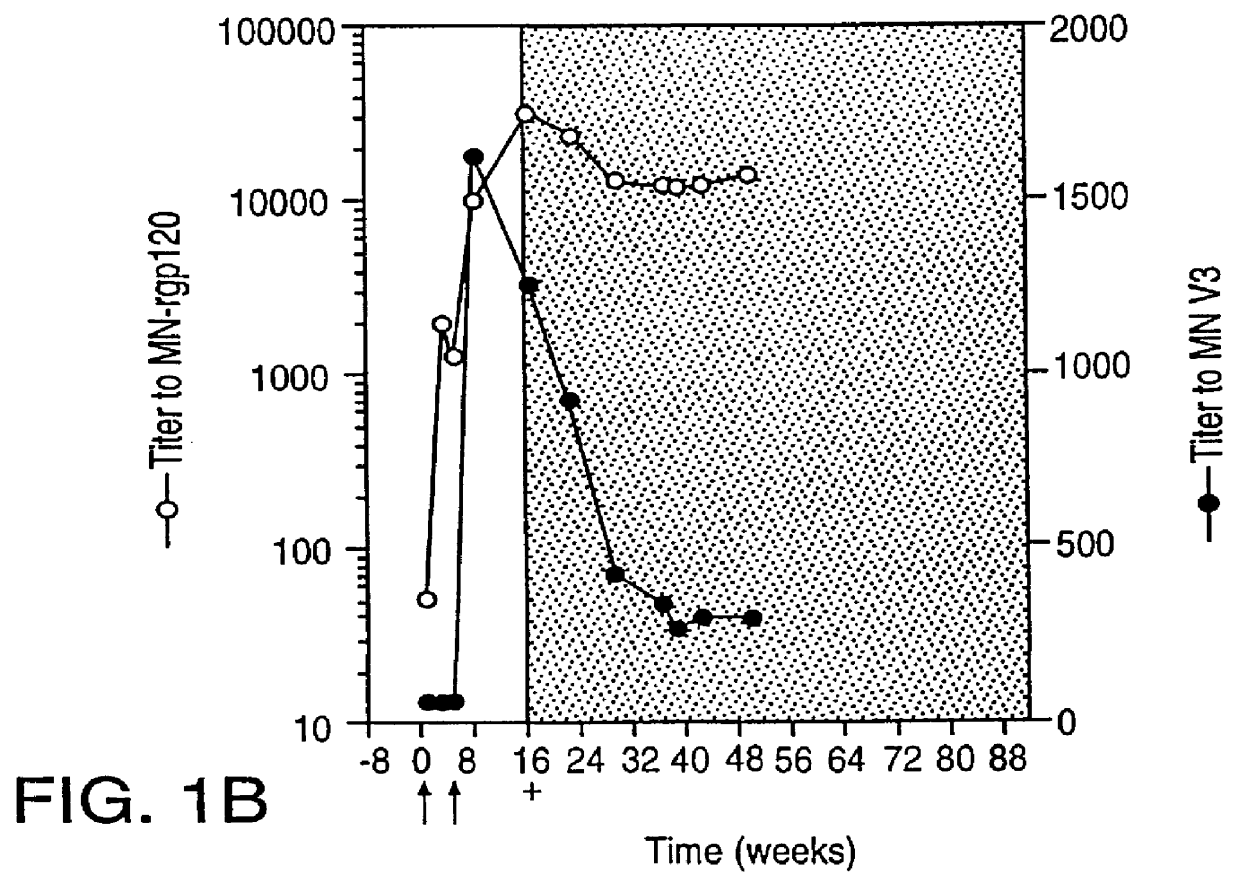
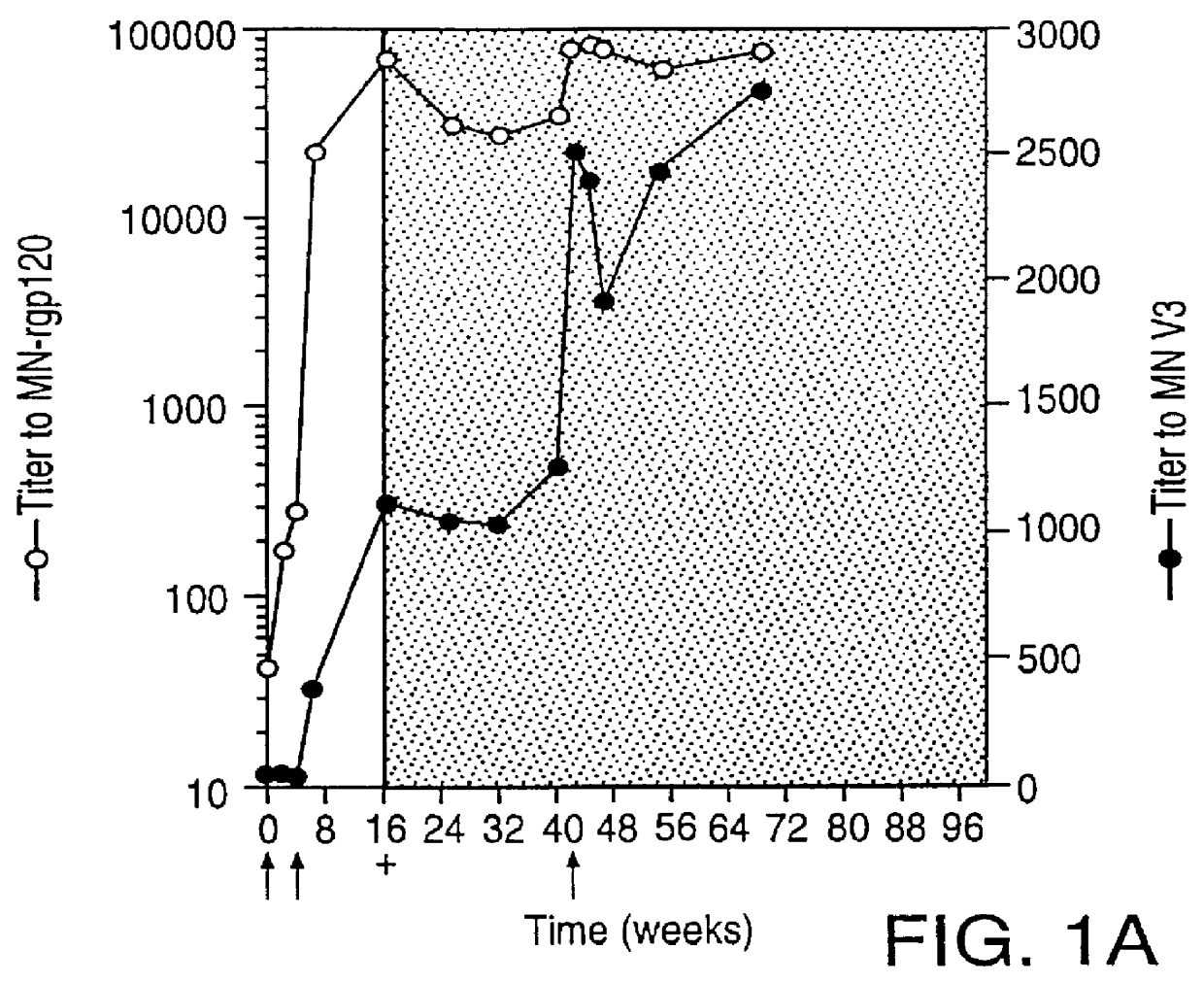
Oligonucleotide sequences encoding gp120 polypeptides from breakthrough isolates of vaccine trials using MN-rgp120 and the encoded gp120 polypeptides are provided. Use of the gp120 polypeptides from one or more of the isolates in a subunit vaccine, usually together with MN-rgp120, can provide protection against HIV strains that are sufficiently different from the vaccine strain (e.g.; MN-rgp120) that the vaccine does not confer protection against those strains. Antibodies induced by the polypeptides are also provided.
Owner:GENENTECH INC
Influenza recombinant subunit vaccine
InactiveUS20070042002A1Improving immunogenicityImprove efficacySsRNA viruses negative-senseVirus peptidesAdjuvantEnation
The invention provides influenza proteins, including subunit proteins and immunogenic compositions that can be utilized, with or without adjuvants, as vaccines to protect against influenza infection in animal models and humans. The recombinant proteins are expressed from transformed insect cells that contain integrated copies of the appropriate expression cassettes in their genome. The invention uses a Drosophila melanogaster expression system to provide high yields of recombinant subunit proteins with native-like conformation.
Owner:HAWAII BIOTECH INC
Methods for the production of non-covalently complexed and multivalent proteosome sub-unit vaccines
InactiveUS6476201B1Shorten the timeIncrease temperatureAntibacterial agentsOrganic active ingredientsContinuous monitoringContamination
A continuous method for preparing proteosome-amphiphilic determinant vaccines for parenteral or mucosal administration using diafiltration or ultrafiltration technology. The amphiphilic determinants include lipopolysaccharides from gram negative bacteria, e.g. S. flexneri, P. shigelloides and S. sonnei. Proteosomes are obtained from group B type 2b meningococci. The active proteosome-amphiphilic determinant complexes (non-covalent complexes) of the vaccine are formed using diafiltration or ultrafiltration to remove the detergent under non-static conditions. The use of diafiltration or ultrafiltration decreases processing time and the opportunity for contamination and further permits the use of ambient temperature and efficient scale-up. In addition, the process permits the reliable and continuous monitoring of the dializate which enhances the efficiency of the entire process. The time of dialysis for the production of a lot of vaccine is reduced from 7-10 days to less than 72 hours and usually less than 48 or 24 hours. The use of the process optimizes the presence of each antigenic component in the preparation of multivalent vaccines.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY +1
Cytomegalovirus surface protein complex for use in vaccines and as a drug target
Owner:THE TRUSTEES FOR PRINCETON UNIV
Method for Differentially Quantifying Naturally Processed HLA-Restricted Peptides for Cancer, Autoimmune and Infectious Diseases Immunotherapy Development
The invention relates to a method for quantitatively identifying relevant HLA-bound peptide antigens from primary tissue specimens on a large scale without labeling approaches. This method can not only be used for the development of peptide vaccines, but is also highly valuable for a molecularly defined immunomonitoring and the identification of new antigens for any immunotherapeutic strategy in which HLA-restricted antigenic determinants function as targets, such as a variety of subunit vaccines or adoptive T-cell transfer approaches in cancer, or infectious and autoimmune diseases.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Subunit vaccine for novel coronavirus and application of subunit vaccine
InactiveCN111533809AImproving immunogenicityImprove stabilitySsRNA viruses positive-senseViral antigen ingredientsAntibody fragmentsTGE VACCINE
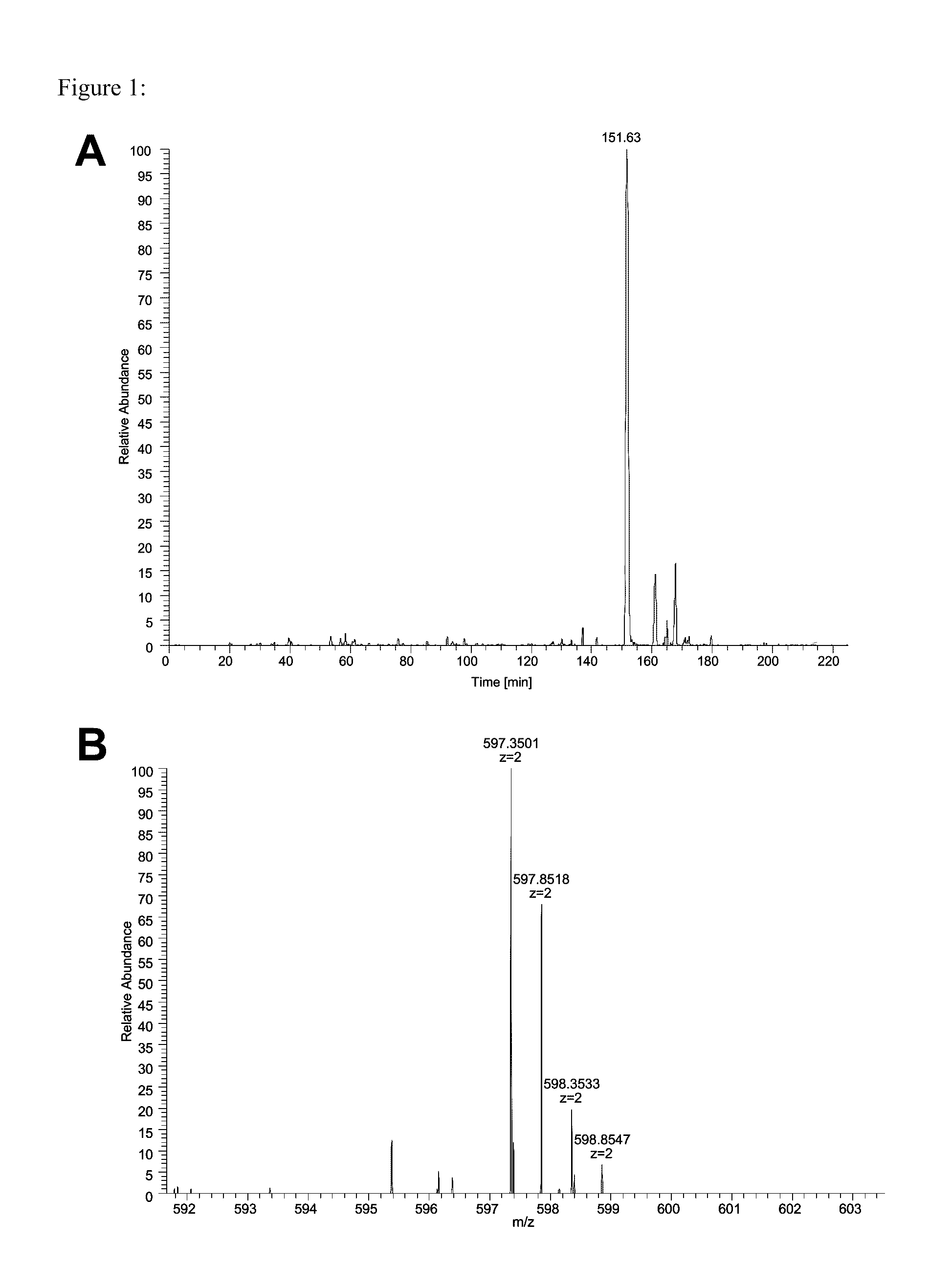
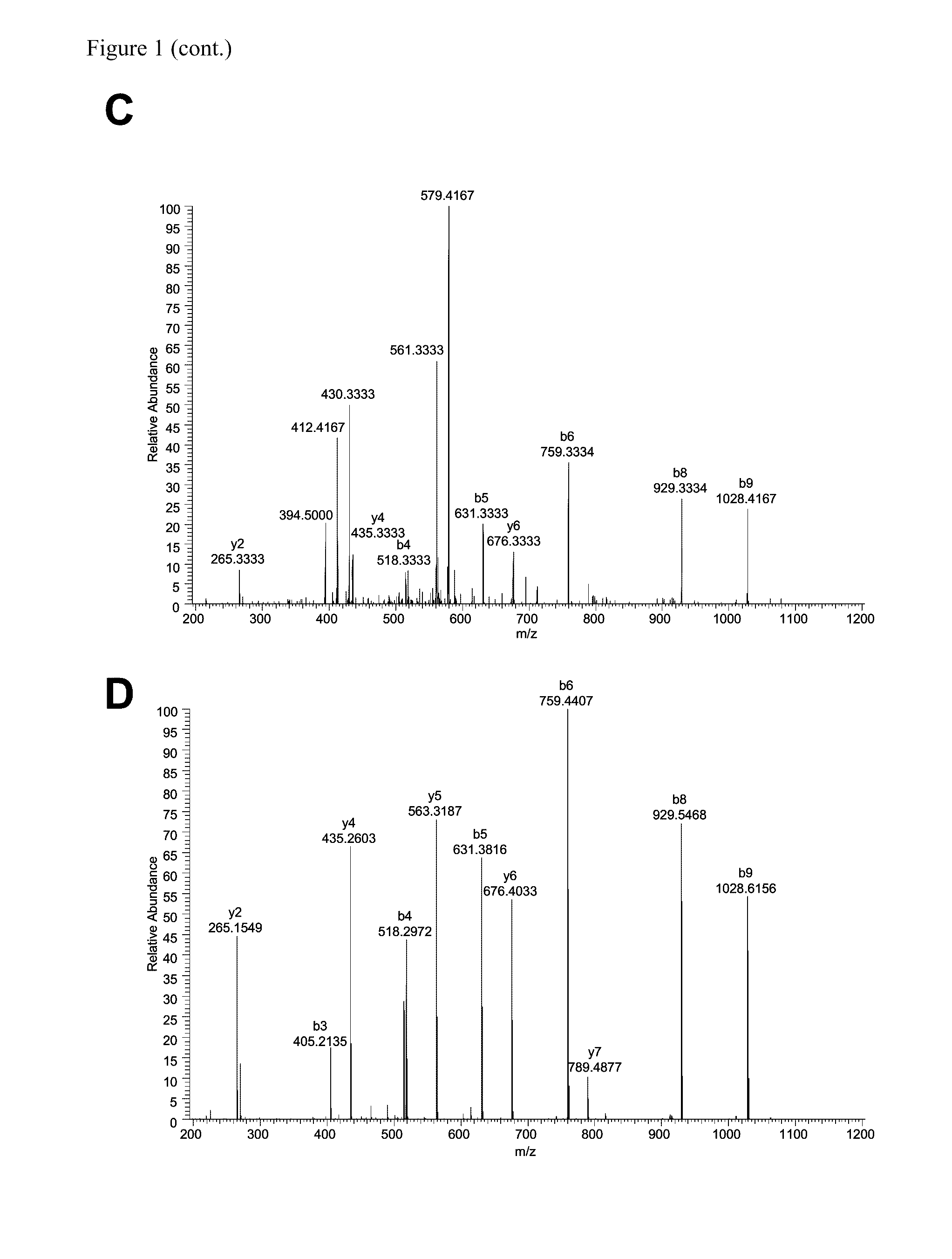
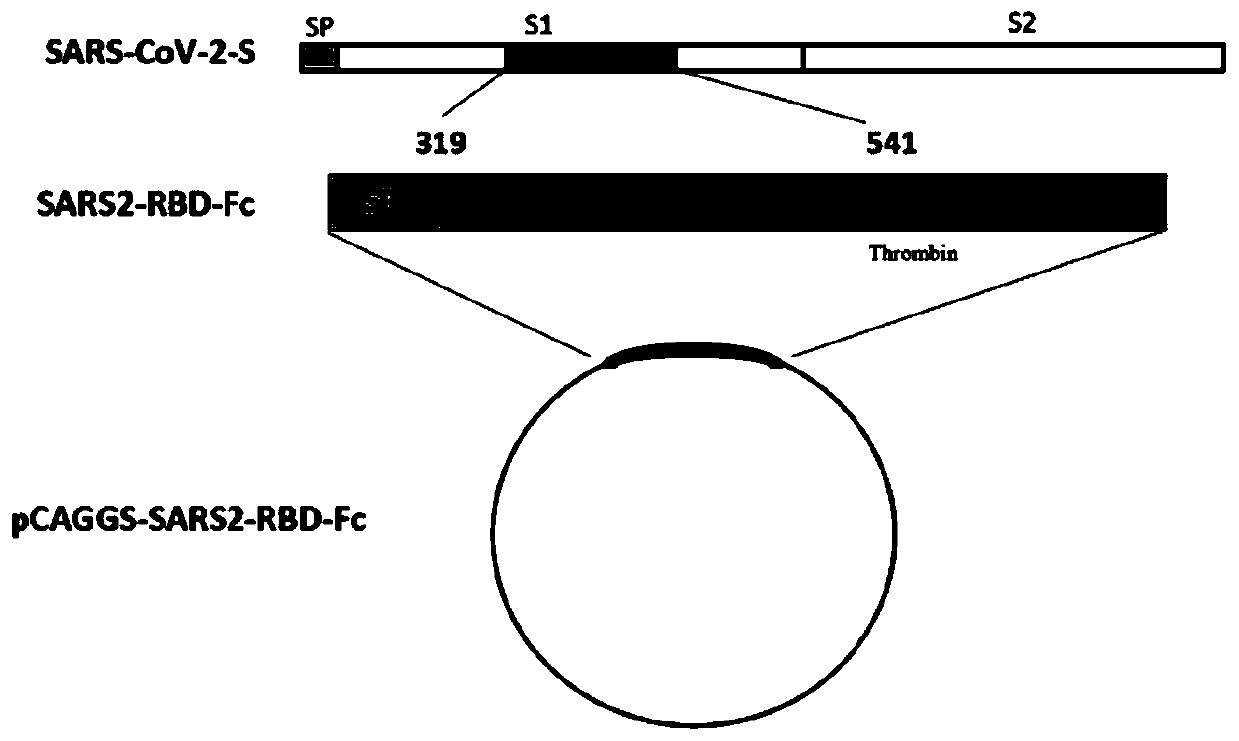
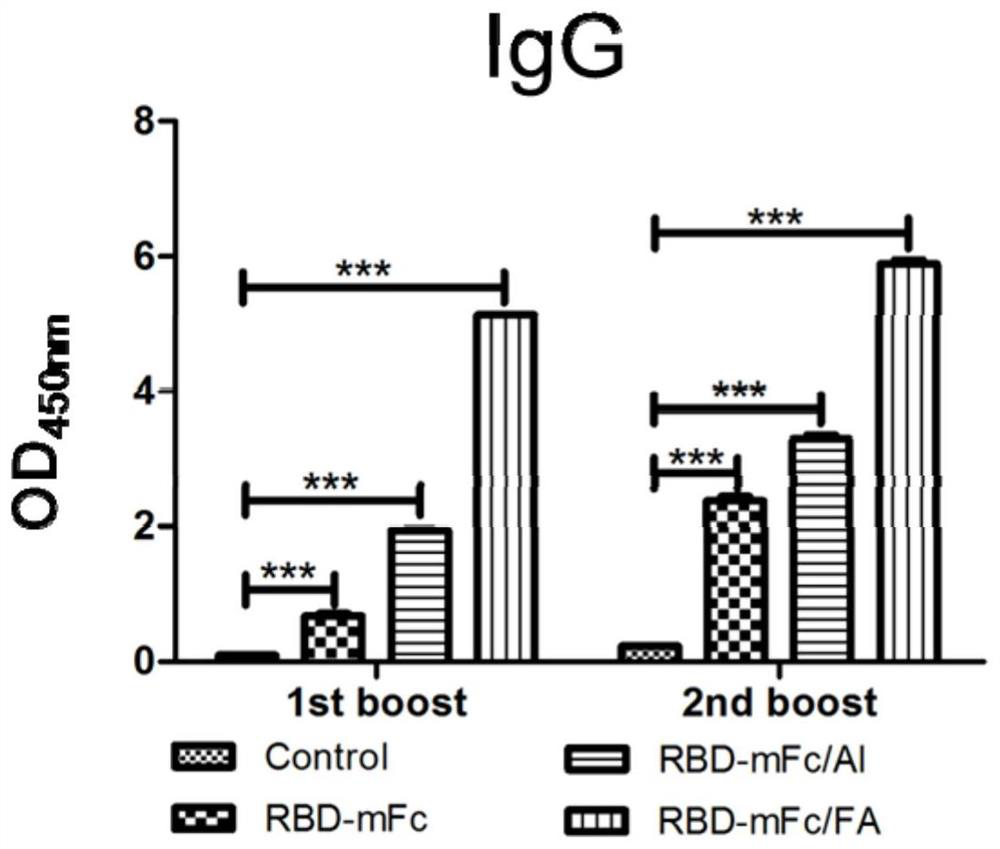
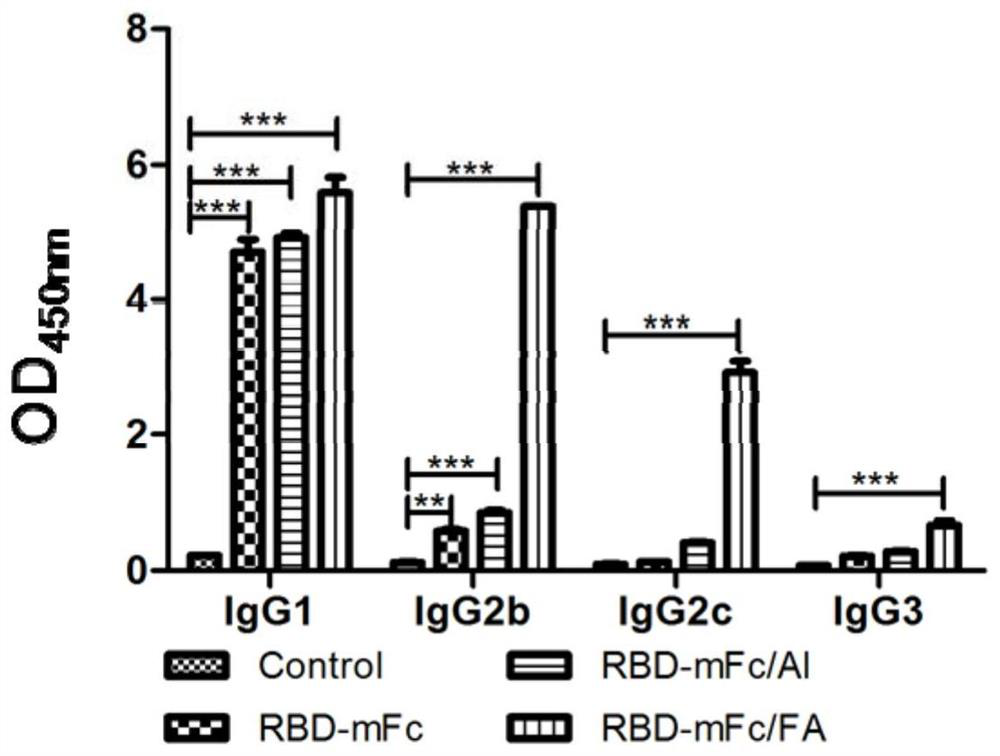
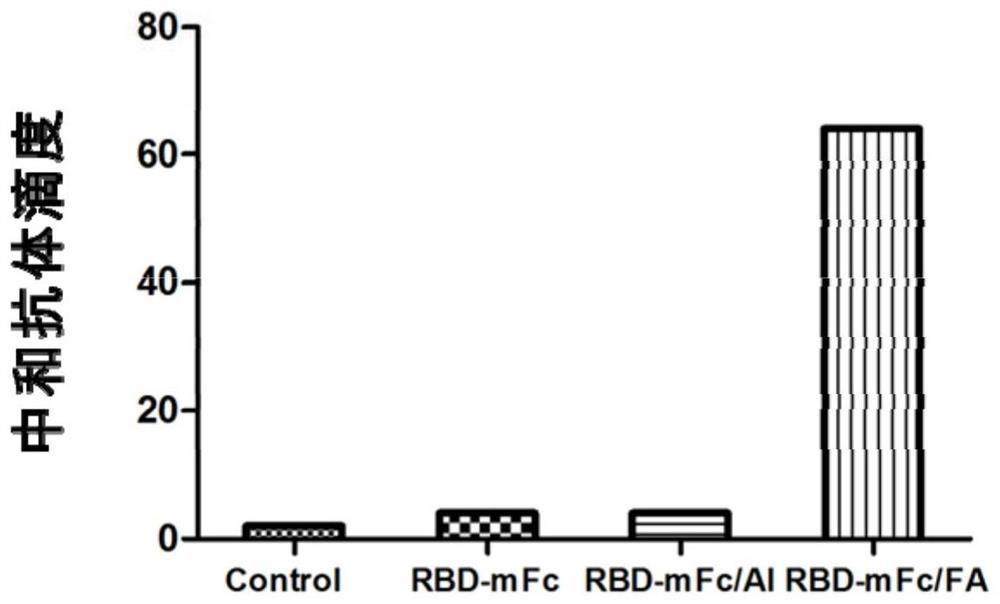
The invention discloses a fusion protein of a novel coronavirus envelope protein and an application of the fusion protein. The fusion protein (SARS2-RBD-Fc) is obtained by fusing an RBD structural domain of a novel coronavirus envelope protein S with an antibody Fc fragment; and as a subunit vaccine, the fusion protein can induce an organism to generate an efficient neutralizing antibody through nasal drip immunization and intramuscular injection. It indicates that the SARS2-RBD-Fc can be used as a candidate vaccine for preventing and treating new coronavirus infection.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Subunit vaccine against respiratory syncytial virus infection
The present invention relates generally to methods of treating or preventing RSV infections, and more specifically, to compositions, and the use thereof, comprising one or more RSV G protein immunogen or fragment thereof capable of eliciting protective immunity without eliciting an immunopathological response or eliciting a reduced immunopathological response.
Owner:ID BIOMEDICAL CORP LAVAL
Porcine circovirus type 2 subunit vaccine and preparation method thereof
InactiveCN101884787AImprove biological activityHighly species-specificViral antigen ingredientsVirus peptidesOpen reading frameAntigenicity
The invention mainly aims to provide a porcine circovirus type 2 (PCV2) subunit vaccine and a preparation method thereof. Particularly, a baculovirus vector expression system is utilized to express a large amount of recombinant open reading frame type 2 (ORF2) protein in insect cells, so that the subunit vaccine with good immunity effect is developed. A bac-to-bac baculovirus expression system is adopted to perform whole gene amplification on the porcine circovirus type 2 ORF2 gene, and a melittin signal peptide nucleotide sequence is introduced into a terminal 5', so that the recombinant baculovirus of an open reading frame containing the melittin signal peptide nucleotide sequence and a PVC2ORF2 gene sequence is established, wherein the infected insect cell expresses the recombinant ORF2 protein with efficient and high PCV2 antigenicity; and thus the subunit vaccine containing the PCV2 recombinant ORF2 protein is prepared. The inoculation experiments of piglets show that the subunit vaccine has a good immunity protection effect.
Owner:PU LIKE BIO ENG
Prophylactic and therapeutic influenza vaccines, antigens, compositions and methods
The present invention relates to the intersection of the fields of immunology and protein engineering, and particularly to antigens and vaccines useful in prevention of infection by influenza virus. Provided are recombinant protein antigens, compositions, and methods for the production and use of such antigens and subunit vaccine compositions. In some embodiments, influenza antigens include hemagglutinin polypeptides neuraminidase polypeptides, and / or combinations thereof.
Owner:FRAUNHOFER USA
P153 and P156 antigens for the immunodiagnosis of canine and human ehrlichioses and uses thereof
Sequences encoding two immunoreactive glycoproteins were cloned from Ehrlichia canis (p153 gene) and Ehrlichia chaffeensis (p156 gene). These two glycoproteins are species-specific immunoreactive orthologs that are useful as subunit vaccines and for serologic and molecular diagnostics for E. canis and E. chaffeensis.
Owner:RES DEVMENT FOUND
Porcine epidemic diarrhea recombinant baculovirus gene engineering subunit vaccine, preparation method and application thereof
InactiveCN103585625AImprove abilitiesTargetedMicroorganism based processesAntiviralsGenetic engineeringTGE VACCINE
The invention belongs to the technical field of biological vaccine preparation, and particularly relates to a porcine epidemic diarrhea (PED) recombinant baculovirus gene engineering subunit vaccine, a preparation method and an application thereof. According to the present invention, S1 gene and M gene of the current new PEDV epidemic strain are selected as reference sequences, a baculovirus expression system is adopted to express S1 protein or partial S1 protein and M protein, and the obtained recombinant protein is prepared into a subunit vaccine for effectively controlling PED occurrence; with the PED recombinant baculovirus gene engineering subunit vaccine produced by using the method, the defect of the current PEDV traditional vaccine is solved; and the PED recombinant baculovirus gene engineering subunit vaccine can be used for prevention and treatment of PEDV infections and related diseases caused by PEDV, and can further be used for preparation of coating antigen of PEDV detection antibody ELISA kits.
Owner:SOUTH CHINA AGRI UNIV
Influenza recombinant subunit vaccine
InactiveUS20080008725A1Avoids potential degradationAvoiding lysis of the host cellsSsRNA viruses negative-senseViral antigen ingredientsAdjuvantEnation
The invention provides influenza proteins, including subunit proteins and immunogenic compositions that can be utilized, with or without adjuvants, as vaccines to protect against influenza infection in animal models and humans. The recombinant proteins are expressed from transformed insect cells that contain integrated copies of the appropriate expression cassettes in their genome. The invention uses a Drosophila melanogaster expression system to provide high yields of recombinant subunit proteins with native-like conformation.
Owner:MERCK SHARP & DOHME CORP
Recombinant baculovirus strain of porcine circovirus type 2 Cap protein expression, construction method and application thereof
InactiveCN101358182AImprove immune activityViral antigen ingredientsAntiviralsMicroorganism preservationImmunocompetence
The present invention discloses a recombinant baculovirus strain rBac / PCV2Cap (microorganism preservation number: CGMCC NO.2083) efficiently expressing Porcine circovirus type 2 Cap protein and applications thereof. The recombinant baculovirus strain rBac / PCV2Cap constructed by the present invention can efficiently express recombinant PCV2-Cap protein in insect cells, and the expressed recombinant Cap protein, which has good immunocompetence and antigenicity, can serve as a subunit vaccine used to prevent the related plague caused by Porcine circovirus type 2 infection as well as a detecting and diagnostic antigen for Porcine circovirus type 2 serum antibody.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
PRRSV subunit vaccines
ActiveUS7722878B2SsRNA viruses positive-senseViral antigen ingredientsProtective immunityProtection sex
Vaccines effective against PRRSV include at least one portion of PRRSV ORF1. Such vaccines, upon administration, provoke an immune response in PRRSV-susceptible animals. Moreover, compositions in accordance with the present invention provide immune response up to and including protective immunity against PRRSV as well as reduce the severity of PRRSV and / or incidence of PRRSV. Selected portions of ORF1 can be used singularly, in combination with one another, in combination with other PRRSV ORFs, and in combination with other PRRSV vaccines.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Lipid nanoparticle vaccine adjuvants and antigen delivery systems
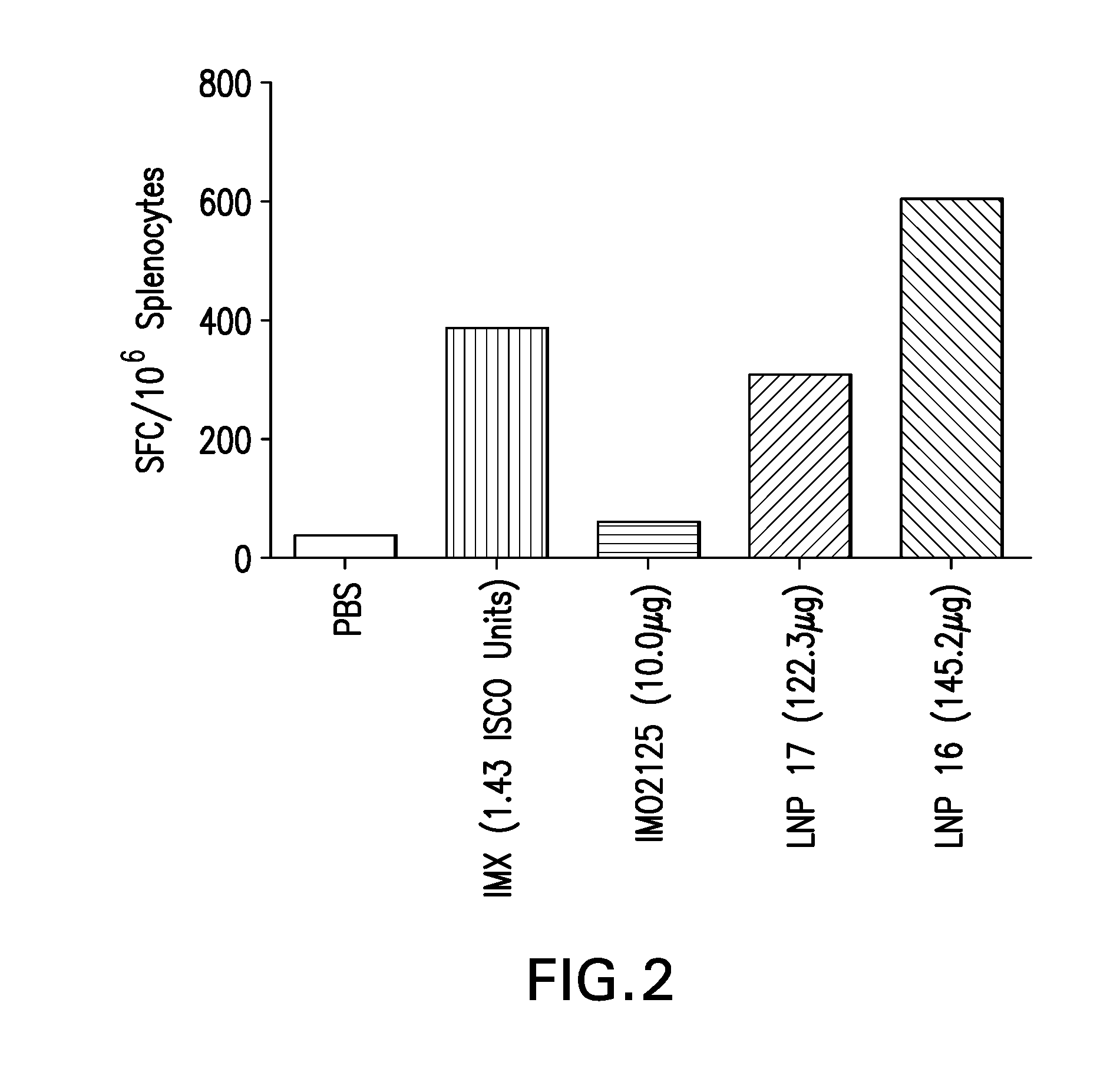
The instant invention provides for novel lipid nanoparticle (LNP) formulations, containing cationic lipids, for use as vaccine adjuvants and / or as antigen delivery systems. It is an object of the instant invention to provide LNP formulations that demonstrate enhancements in humoral and cellular immunogenicity of vaccine antigens, particularly subunit vaccine antigens, when utilized alone or in combination with immunostimulatory agents (e.g. small molecule or oligonucleotide TLR agonists). The instant invention further identifies physical and chemical properties of the LNP formulations that can be manipulated to enhance antigen efficiency and adjuvant tolerability in vivo.
Owner:MERCK SHARP & DOHME LLC
Method for assembling foot and mouth disease virus hollow capsid in insect with acidproof improvement
The present invention discloses a method for assembling foot-and-mouth disease virus empty capsids in insect cells via the alteration of acid-resistance. The method for assembling foot-and-mouth disease virus empty capsids in insect cells includes the following steps: (1) the altered P12A gene and the non-structural protein gene 3C of foot-and-mouth disease virus are introduced into bacteria via baculovirus vectors for recombination to produce recombinant rhabdovirus A; (2) the DNA of the recombinant rhabdovirus A is used to transfect the insect cells, so that the foot-and-mouth disease virus empty capsids are obtained. The method assembles the integral foot-and-mouth disease virus empty capsids in the insect cells for the first time, lays a foundation for the research and the development of gene-engineered subunit vaccines and novel diagnostic reagents.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
CHO (Chinese hamster ovary) cell strain with high-efficiency expression of CD2V protein of African swine fever (ASF)
ActiveCN110078801AHigh expressionEasy to purifyVirus peptidesMicroorganism based processesAfrican swine feverChinese hamster
The invention provides CD2V protein of African swine fever (ASF) capable of being expressed with high-efficiency in a CHO (Chinese hamster ovary) cell strain. The amino acid sequence of the CD2V protein is shown in SEQ ID NO:4; a recombinant plasmid constructed by the invention is used for expressing the CD2V protein of an African swine fever virus in CHO cells; the invention further provides a recombinant CHO cell strain prepared by transfecting the CHO cells through the recombinant plasmid, the recombinant CHO cell strain can be used to prepare the CD2V protein, and the prepared protein canbe used for differential diagnosis of the African swine fever. According to the cell strain with the expression of the CD2V protein of the African swine fever, the expression quantity is high, purification is easy, the cell strain can be used for the differential diagnosis, and a solid foundation is laid for the production of subunit vaccines and diagnostic reagents of the African swine fever.
Owner:YEBIO BIOENG OF QINGDAO
Influenza recombinant subunit vaccine
InactiveUS20070042001A1Avoids potential degradationAvoiding lysis of the host cellsSsRNA viruses negative-senseVirus peptidesAdjuvantEnation
The invention provides influenza proteins, including subunit proteins and immunogenic compositions that can be utilized, with or without adjuvants, as vaccines to protect against influenza infection in animal models and humans. The recombinant proteins are expressed from transformed insect cells that contain integrated copies of the appropriate expression cassettes in their genome. The invention uses a Drosophila melanogaster expression system to provide high yields of recombinant subunit proteins with native-like conformation.
Owner:HAWAII BIOTECH INC
A fowl adenovirus group I serum type 4 genetic engineering subunit vaccine, and a preparing method and applications thereof
ActiveCN106946995AHigh antigen contentImprove securitySsRNA viruses negative-senseViral antigen ingredientsDiseaseInclusion bodies
A fowl adenovirus group I serum type 4 genetic engineering subunit vaccine, and a preparing method and applications thereof are disclosed. The sequence of an antigen protein in the vaccine is shown as SEQ ID NO:1. The antigen protein has advantages of high safety, high immunity, no pathogenicity for chickens or other animals, and the like. The subunit vaccine can prevent chicken hydropericardium syndrome, inclusion body hepatitis and other diseases which are caused by infection of fowl adenovirus group I serum type 4.
Owner:苏州沃美生物有限公司
P153 and P156 antigens for the immunodiagnosis of canine and human ehrlichioses and uses thereof
Sequences encoding two immunoreactive glycoproteins were cloned from Ehrlichia canis (p153 gene) and Ehrlichia chaffeensis (p156 gene). These two glycoproteins are species-specific immunoreactive orthologs that are useful as subunit vaccines and for serologic and molecular diagnostics for E. canis and E. chaffeensis.
Owner:RES DEVMENT FOUND
Porcine O-type foot-and-mouth disease virus recombinant baculovirus as well as preparation method and application thereof
InactiveCN103122353AImprove expression levelHigh expressionGenetic material ingredientsAntiviralsEscherichia coliShuttle vector
The invention discloses porcine O-type foot-and-mouth disease virus recombinant baculovirus as well as a preparation method and application thereof. Sequences of VP0, VP1 and VP3 genes are artificially synthesized by referring to an FMDV (Foot And Mouth Disease Virus) O-type epidemic strain gene sequence; the VP0, VP1 and VP3 genes are connected to pFBDPHmHNM1P10eGFP plasmid by adopting the plasmid as a framework vector, so that a baculovirus transfer vector pFBDPHmVP013 is obtained. The baculovirus transfer vector pFBDPHmVP013 is mixed with DH10Bac escherichia coli competent cells, and the positive bacterial colony is selected to obtain a recombinant shuttle vector Bacmid; the shuttle vetcor Bacmid is transferred with a sf9 cell, and the recombinant baculovirus QP-Ac-FVLP is obtained by collecting the cell supernatant. The recombinant baculovirus can be used for efficiently expressing FMDVVP0, Vp1 and Vp3 proteins and forming virus-like particles. And the virus-like particles are used for preparing subunit vaccine, so that the organism is induced to generate specific immunity response after the mouse is immunized.
Owner:HUAZHONG AGRI UNIV
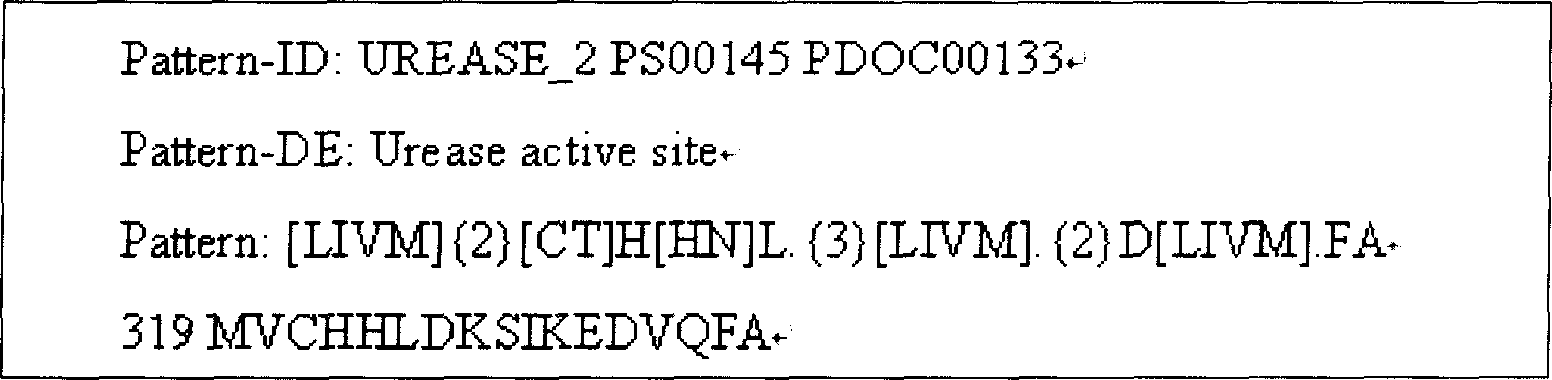
Helicobacter pylori vaccine based on urease B subunit active segment and its prepn process
InactiveCN1887349AGood immune protectionAntibacterial agentsBacterial antigen ingredientsProtective antigenAntigen
The present invention provides one kind of genetic engineering multivalent subunit vaccine for preventing and treating human helicobacter pylori infection and its preparation process. The vaccine consists of active helicobacter pylori UreB fragment UreB414 as the central antigen component, other combined protective antigens, and intramolecular or extramolecular adjuvant. Compared with univalent vaccine, the multivalent combined vaccine can stimulate the body to generate more powerful and more comprehensive helicobacter pylori resisting specific immune reaction.
Owner:ARMY MEDICAL UNIV
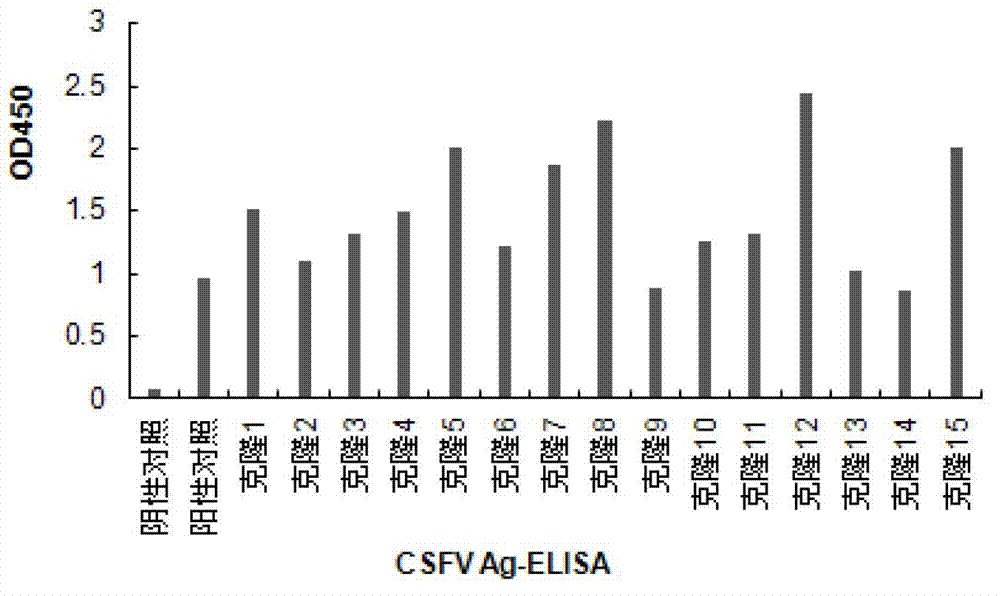
Recombinant cell line for stably expressing classical swine fever virus E2 protein, and applications of the same in preparation of subunit vaccines and diagnosis reagents of classical swine fever
ActiveCN103751774AStable in natureFight infectionMicroorganism based processesAntiviralsMaternal antibodyAntigen
The present invention discloses a strain of a recombinant cell line for stably expressing classical swine fever virus E2 protein, and applications of the recombinant cell line in preparation of subunit vaccines and diagnosis reagents of classical swine fever, wherein specifically the recombinant cell line is BCSFV-E2, is preserved in the China General Microbiological Culture Collection Center, and has the preservation number of CGMCC No.7719. The classical swine fever subunit vaccine prepared by using the recombinant cell line has characteristics of high safety, good immunization effect, easy mass production, less being susceptible to exogenous virus pollution or influence of antibodies, and no influence of the maternal antibody on immunization of swine, and can induce and produce high level classical swine fever virus neutralization antibodies after the swine is immunized. In addition, the present invention further discloses a method for constructing the recombinant mammalian cell line, a method for preparing the classical swine fever subunit vaccine, and applications of the antigen expressed by the recombinant cell line in preparation of classical swine fever prevention vaccines and diagnosis reagents.
Owner:HARBIN WEIKE BIOTECH DEV +1
Porcine circovirus type 2 recombinant cap protein and subunit vaccine
ActiveCN102174086AImprove securityNot pathogenicBacteriaViral antigen ingredientsAntigenEscherichia coli
The invention belongs to the field of molecular biology, and discloses a porcine circovirus type 2 recombinant cap protein and a subunit vaccine. The porcine circovirus type 2 cap protein expressed by recombinant Escherichia coli is obtained by steps of cloning a porcine circovirus type 2 cap protein in a nuclear localization signal area of which the N terminal is cut and which is rich in arginine into a prokaryotic expression vector to obtain a recombinant expression vector, transfecting the recombinant expression vector into Escherichia coli BL21(DE3), and expressing by using the recombinant Escherichia coli BL21(DE3). Tests prove that the constructed recombinant strain expresses a foreign protein stably. When the subunit vaccine is prepared from the expressed recombinant protein, an antigen has high purity and safety, does not have pathogenicity on animals such as pigs and the like, and passes safety evaluation easily.
Owner:NANJING AGRICULTURAL UNIVERSITY
Fusion protein of SARS-CoV-2, and vaccine composition of fusion protein
InactiveCN111662389AInhibition of replicationRefrain from rebroadcastingSsRNA viruses positive-senseAntibody mimetics/scaffoldsSpecific immunityVariant strain
The invention belongs to the technical field of biology, and particularly relates to a fusion protein of SARS-CoV-2, and a vaccine composition of the fusion protein. The fusion protein and the prepared vaccine composition containing the fusion protein overcome the defects of poor immunogenicity and the like of subunit vaccines, can induce the specific immune response aiming at SARS-CoV-2, and achieve the purposes of inhibiting the replication of SARS-CoV-2, inhibiting the spread of SARS-CoV-2 or preventing the settlement of SARS-CoV-2 common strains and variant strains in SARS-CoV-2 host bodies. The fusion protein and the vaccine composition containing the fusion protein can effectively prevent and / or treat novel coronavirus pneumonia (Corona Virus Disease 2019, COVID-19). The fusion protein can be recombined and expressed in large quantities by the aid of the genetic engineering technology, the consumed time is short, and the large-scale production can be facilitated.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Recombinant adenovirus of porcine reproductive and respirator syndrome virus and porcine Circovirus, and vaccine
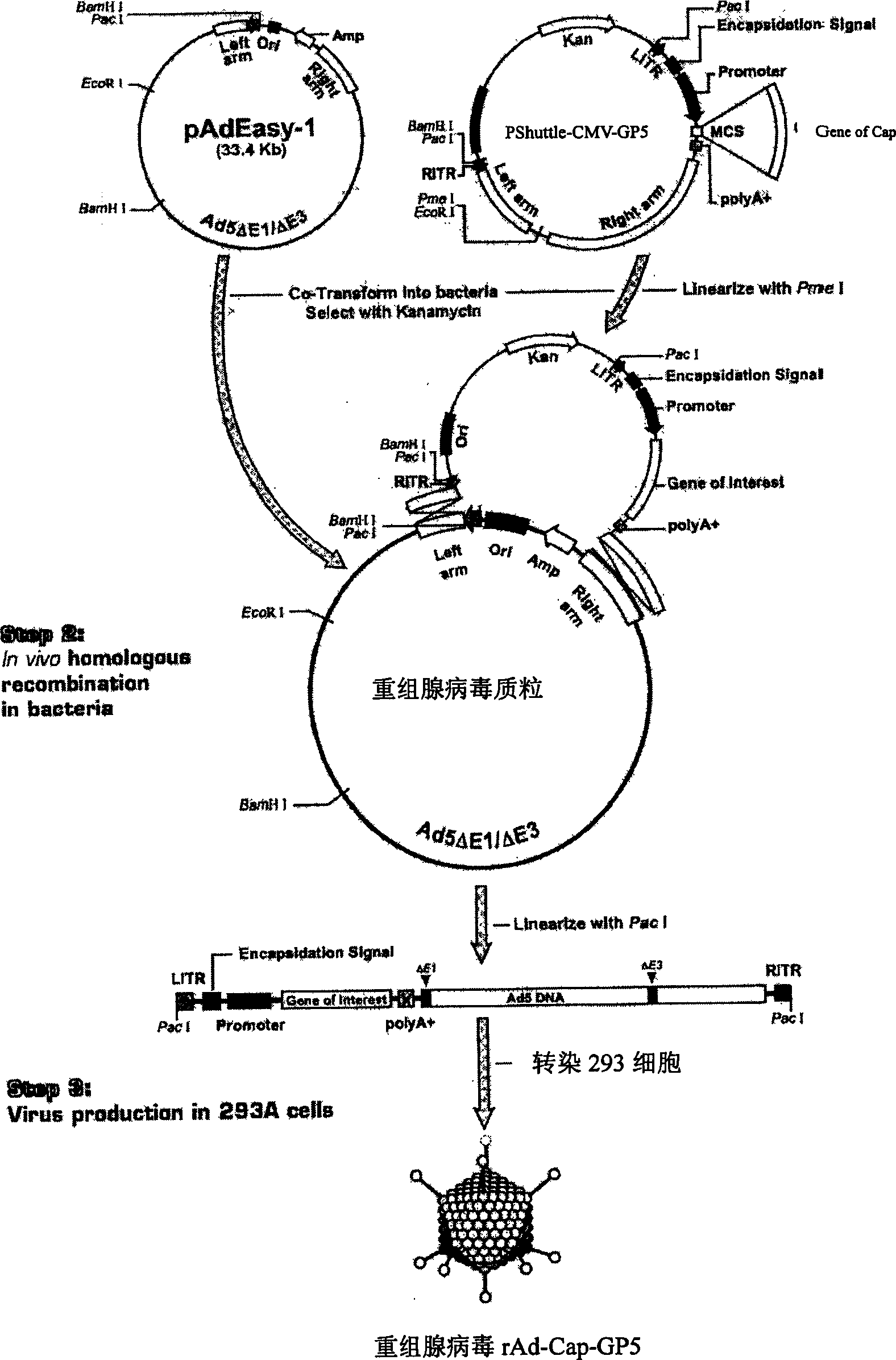
InactiveCN1800375AHas the copy featureStable potencyViruses/bacteriophagesAntibody medical ingredientsEscherichia coliCircovirus
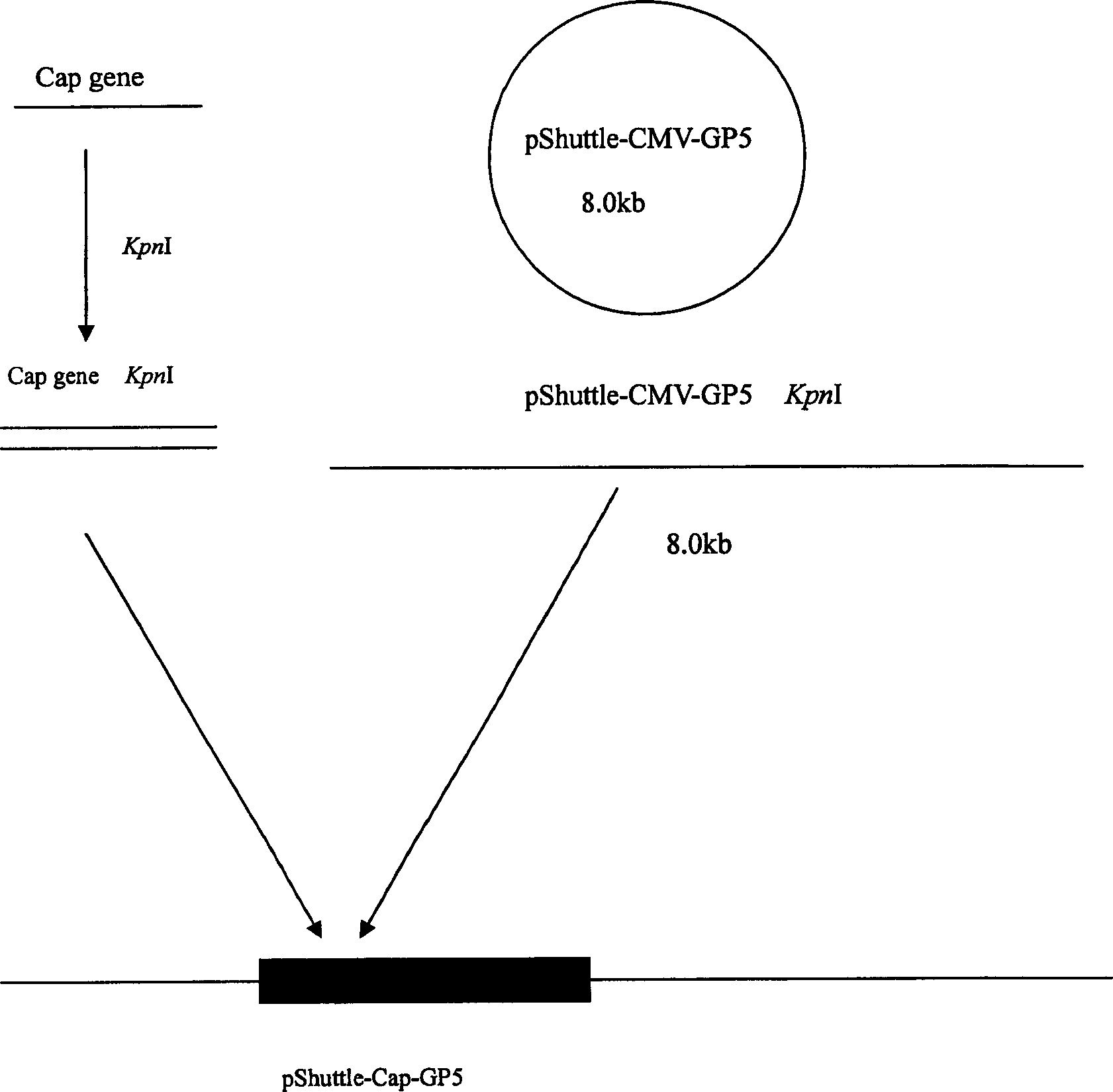
The invention relates to a pig breeding and breathing complex virus (PPRSV) and pig 2-type loop virus (PCV 2) series gene recombination adenovirus and vaccine in the field of high and new biotechnology. It uses PCR technology to clone the Cap protein gene into the plasmid carrier pShuttle-CMV-GP5 by open type reading rule and transforms tobacillus coli BJ5183strain with cage carrier pAdEasy-1 to capture the recombination plasmid; it uses recombination plasmid HEK293-A cell to capture recombination adenovirus and purifies it to express the recombination adenovirus rAd-Cap-GP5 of PRRSV GP5protein and PCV-2 Cap protein.
Owner:NANJING AGRICULTURAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com