Porcine circovirus type 2 recombinant cap protein and subunit vaccine
A technology of porcine circovirus and protein, which is applied in the field of molecular biology, can solve the problems of no prokaryotic expression of Cap protein immunoprotection, no Cap protein immunogenicity analysis, etc., and achieves low cost, simple preparation process, and high antigen purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
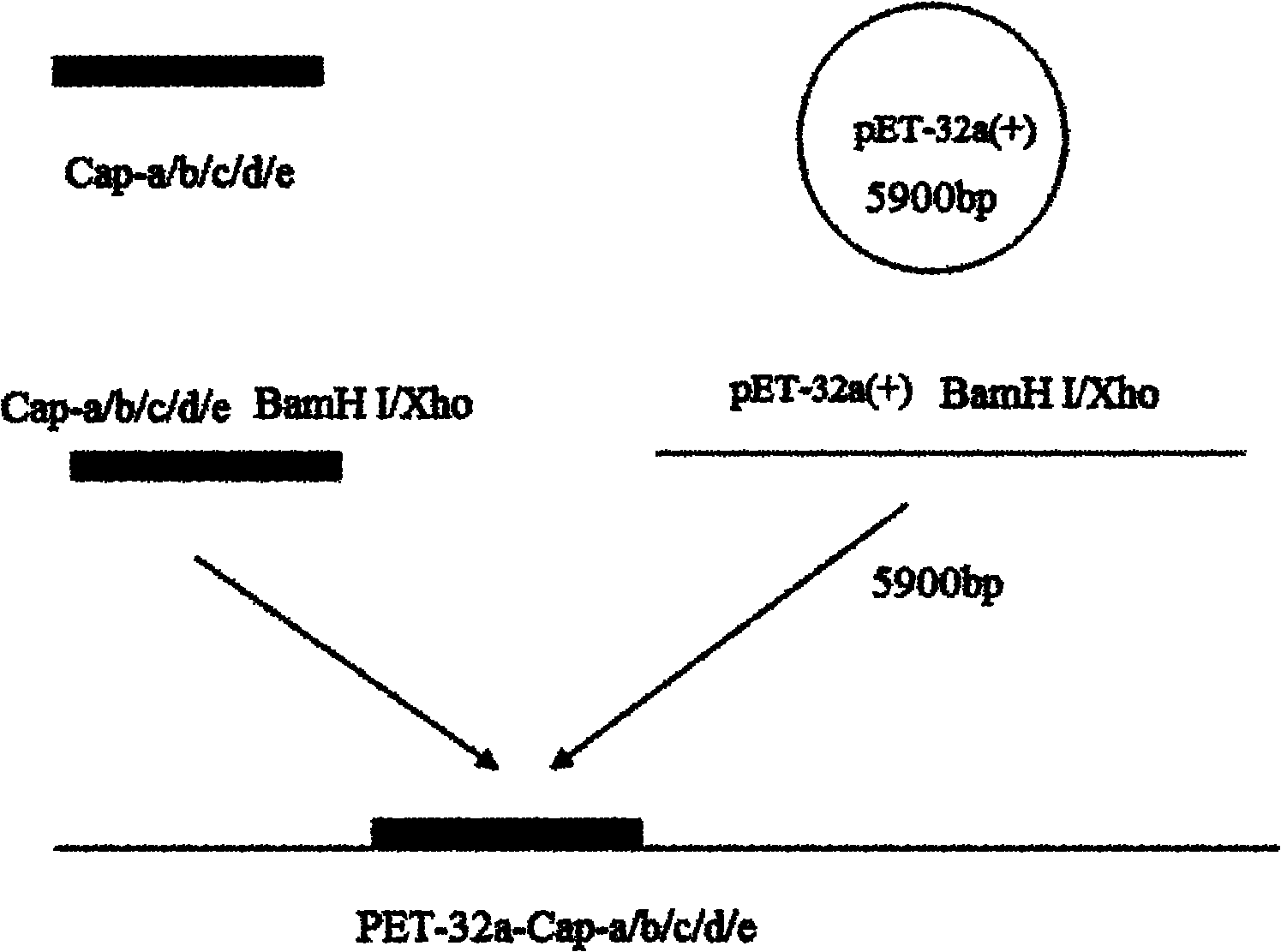
[0038] Example 1 PCR amplification of Cap protein a-e gene fragment and construction of prokaryotic expression plasmid
[0039] 1.1 Primer design
[0040] Primers were designed according to the PCV2 SH strain gene sequence (AY686763), and the upstream and downstream primers were respectively introduced into BamH I and Xho I sites to amplify ORF2 in 51-150, 51-200, 51-234, 101-200 and 101-233aa respectively. Gene fragments, and named Cap-a (SEQ ID NO.8), Cap-b (SEQ ID NO.9), Cap-c (SEQ ID NO.2), Cap-d (SEQ ID NO.10), Cap-e (SEQ ID NO. 11). The primer sequences are as follows:
[0041] Cap51F AA GGATCC CGCACCATCGGTTATAC (SEQ ID NO. 3)
[0042] Cap101F CC GGATCC GTTAAGGTTGAATTCTG (SEQ ID NO. 5)
[0043] Cap150R TAT CTCGAG TATGGTATGGCGGGAGG (SEQ ID NO. 6)
[0044] Cap200R TAA CTCGAG TGCCGAGGCCTACA (SEQ ID NO. 7)
[0045] Cap233R CTT CTCGAG TCACTTAGGGTTAAGTGGG (SEQ ID NO. 4)
[0046] The above primers were synthesized by Shanghai Yingjun Biotechnology Co., Ltd.
...
Embodiment 2
[0051] Example 2 Construction of recombinant expression strain BL21-Cap-a / b / c / d / e
[0052] 2.1 Transformation of competent Escherichia coli BL-21
[0053] In Example 1, the correct recombinant plasmid was sequenced to transform Escherichia coli BL-21 (NEB), and the recombinant expression strains BL21-Cap-a, BL21-Cap-b, BL21-Cap-c, BL21-Cap-d, BL21-Cap-e, meanwhile, the empty plasmid pET-32a was transformed into competent Escherichia coli BL-21 in the same way.
[0054] 2.2 Optimization of recombinant protein expression conditions
[0055] Pick the recombinant expression strain BL21-Cap-a / b / c / d / e and Escherichia coli BL-21 containing the empty plasmid pET-32a and inoculate them in LB liquid medium containing ampicillin, overnight at 37°C Shake culture.
[0056] (1) Inoculate 10 μL of the overnight cultured bacterial solution into 3 ml of LB liquid medium containing ampicillin (50 μg / ml), shake and culture at 200 rpm at 37°C for about 3 hours, and make the OD 600 When it rea...
Embodiment 3
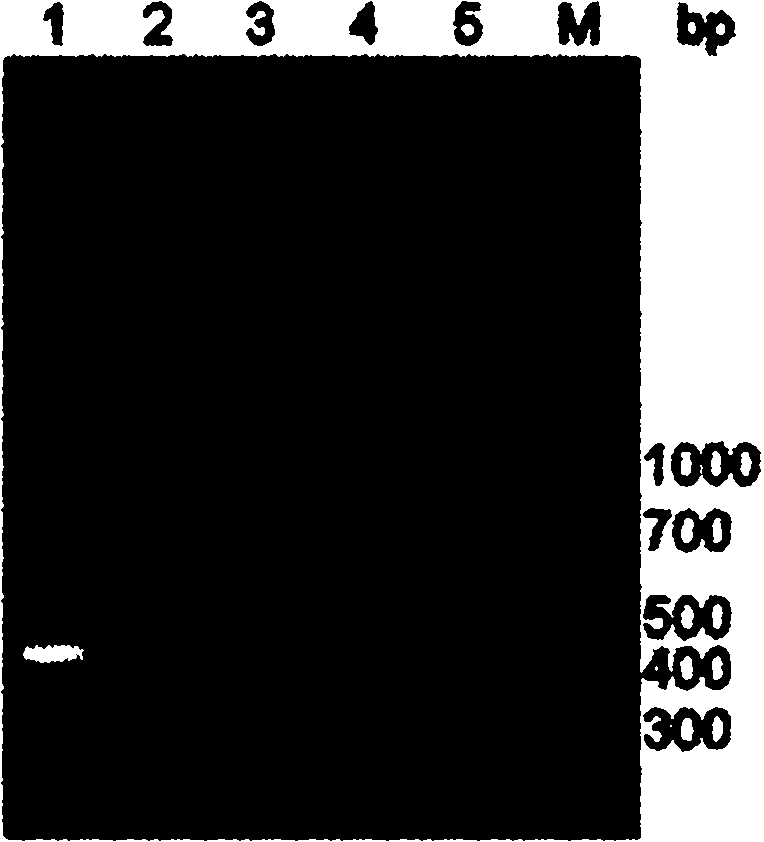
[0066] Example 3 Mass expression, purification and antigenic identification of fusion protein
[0067] 3.1 Mass expression and purification of fusion protein
[0068] According to the best induction expression condition explored in Example 2, induce the expression of 500ml of genetically engineered recombinant bacteria, collect the thalline by centrifugation, add 5ml of PBS to resuspend the thallus for every 100ml of bacterium liquid, centrifuge after ultrasonic lysis, separate the supernatant and inclusion bodies, and include The body was resuspended with an equal volume of PBS, and the inclusion body was washed and purified with the inclusion body washing solution. The steps are as follows:
[0069] (1) Centrifuge at 8000rpm for 15min, resuspend the pellet in inclusion body washing solution I (50mM Tris-HCl; 100mM NaCl; 10mM EDTA; 1% Triton X-100), 4°C for 1-2h;
[0070] (2) Centrifuge at 8000rpm for 15min, resuspend the pellet in inclusion body washing solution II (50mM Tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com