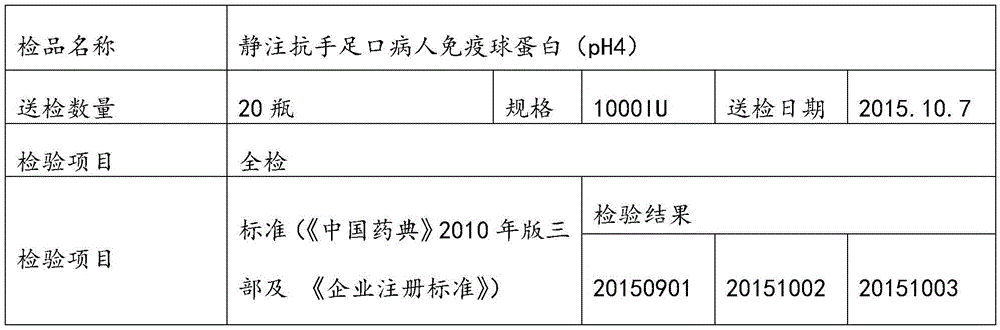
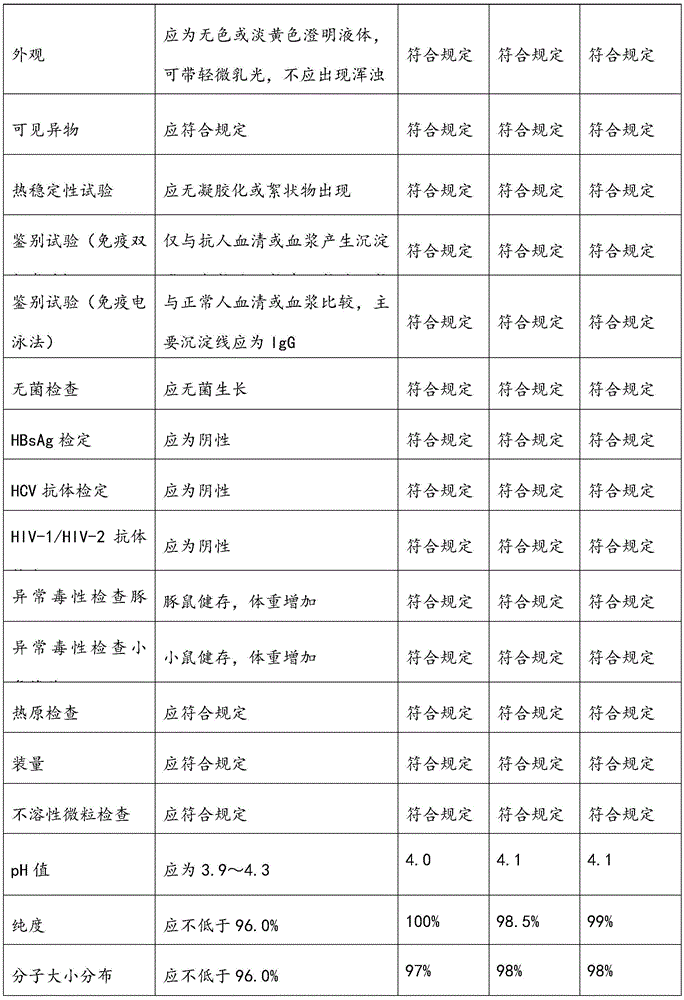
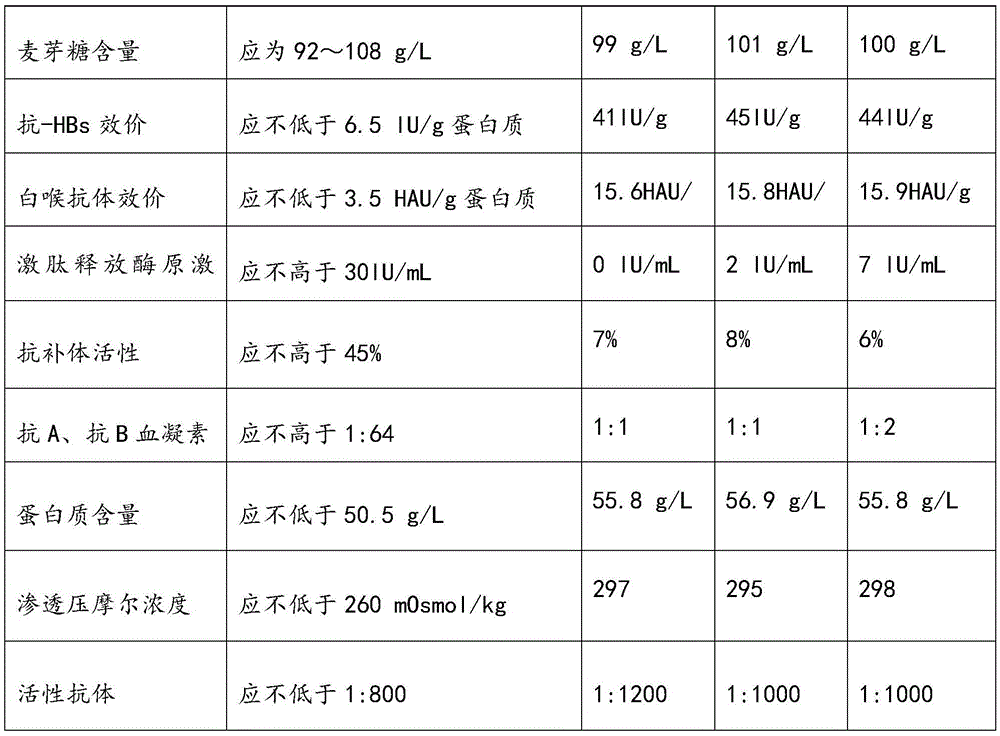
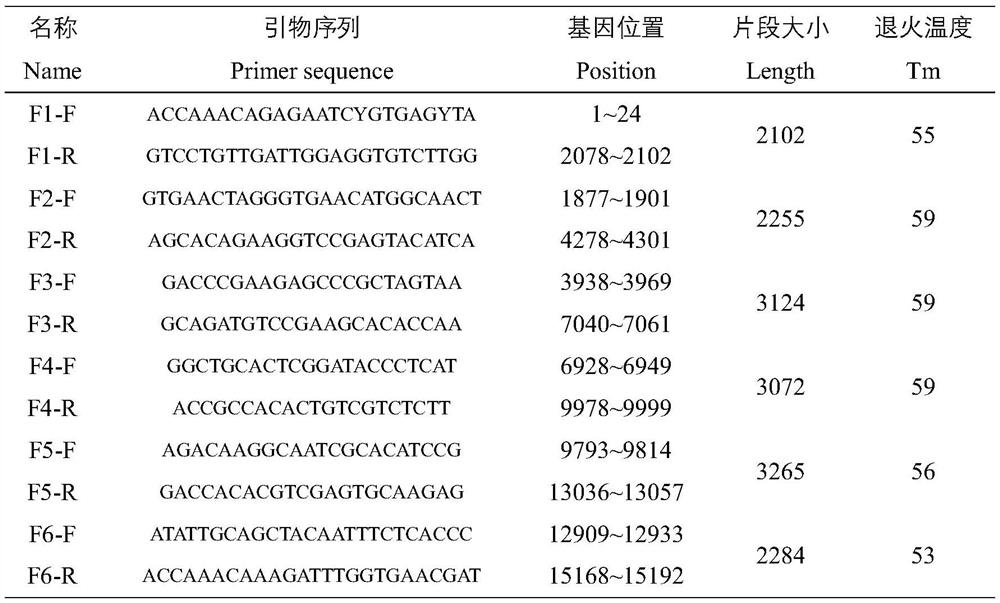
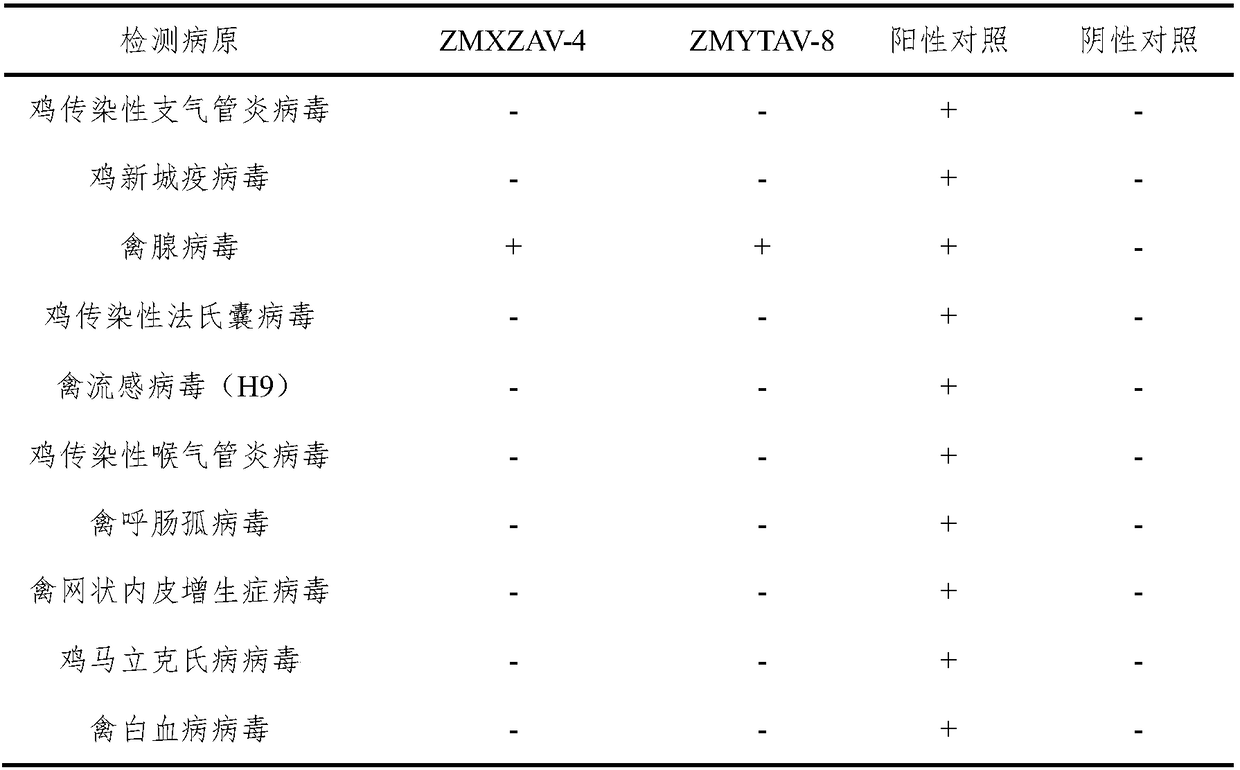
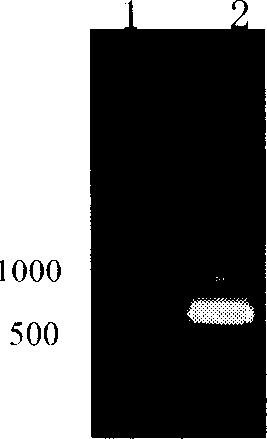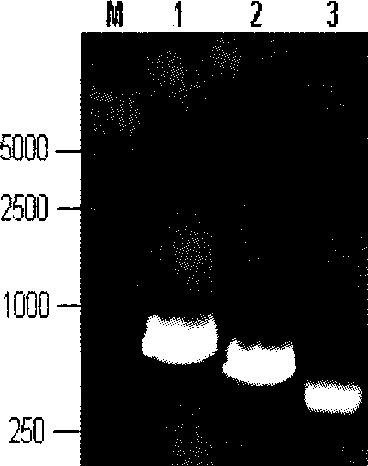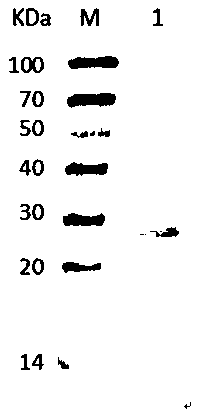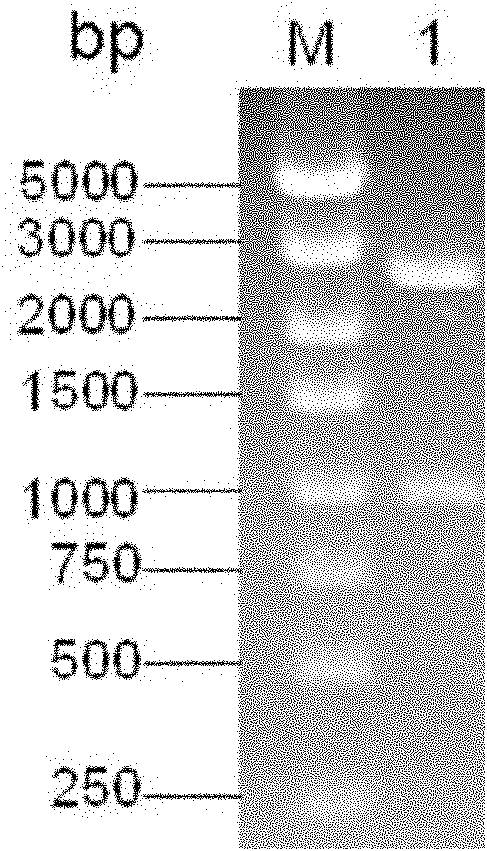Patents
Literature
75results about How to "Stable potency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tanshinon IIA sulfonic acid sodium glucose injection liquid and its preparing method
InactiveCN1640391ASolve discolorationFix instabilityOrganic active ingredientsPharmaceutical delivery mechanismAntioxidantTherapeutic effect
The persent invention relates to a sodium tanshinon IIA sulfonate glucose injection and its preparation method. Said injection contains (by wt%) 0.002-0.016% of sodium tanshinon IIA sulfonate, 5-25% of glucose, 0.05-0.2% of antioxidant and the rest is injection water. Said injection can obtain good therapeutic effect, and its preparation method is simple.
Owner:昆明希捷医药研发有限公司
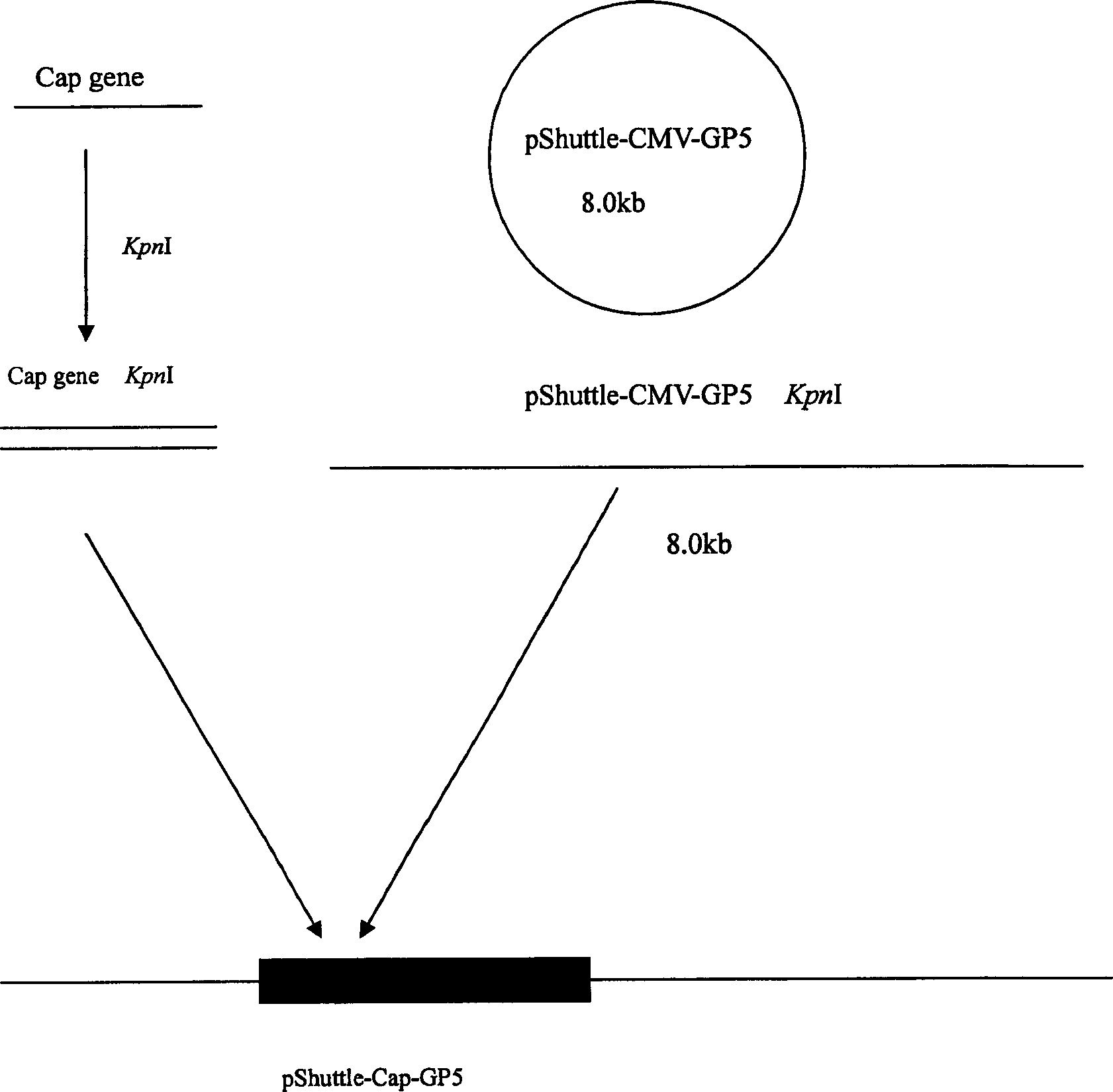
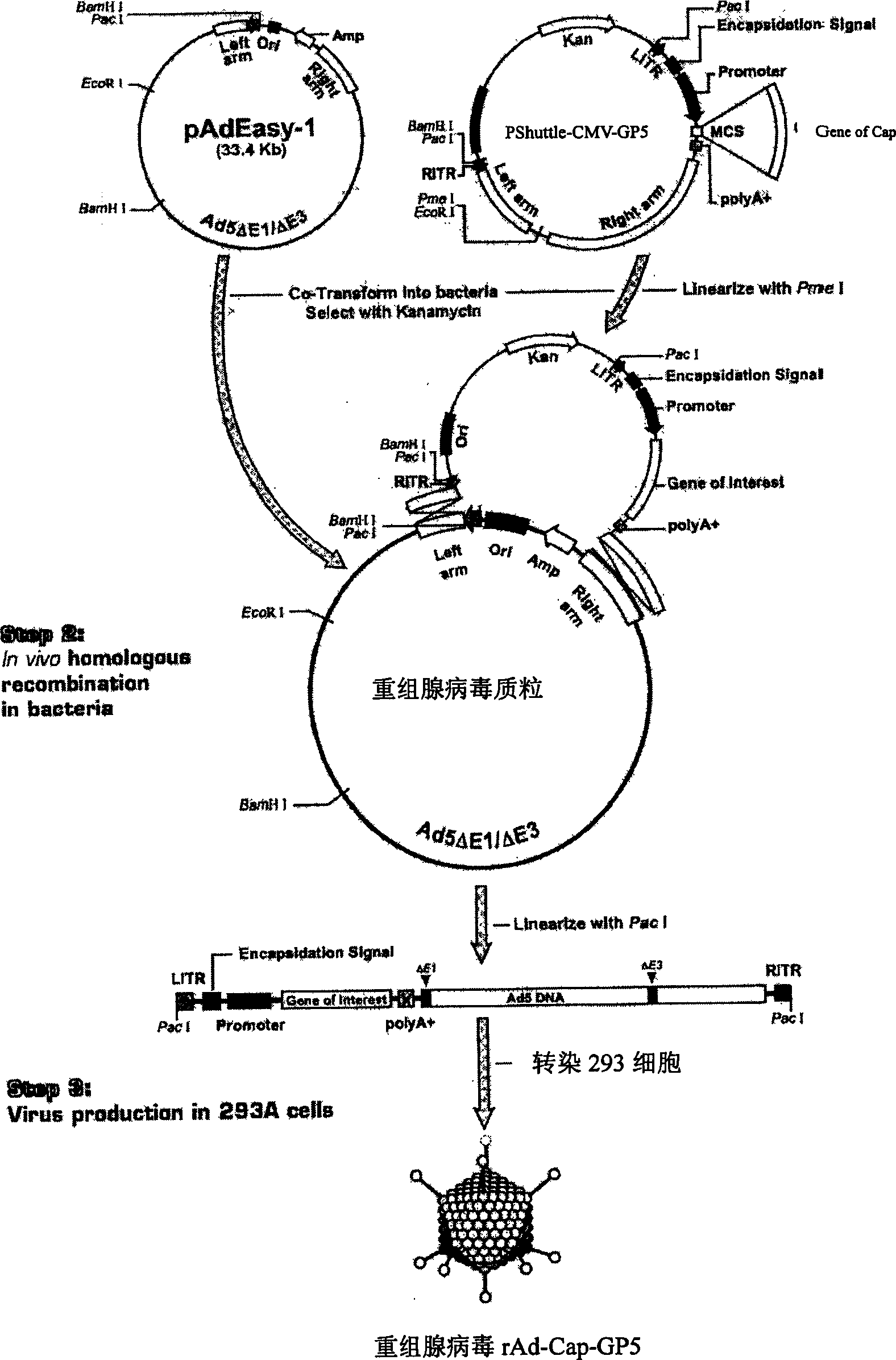
Recombinant adenovirus of porcine reproductive and respirator syndrome virus and porcine Circovirus, and vaccine
InactiveCN1800375AHas the copy featureStable potencyViruses/bacteriophagesAntibody medical ingredientsEscherichia coliCircovirus
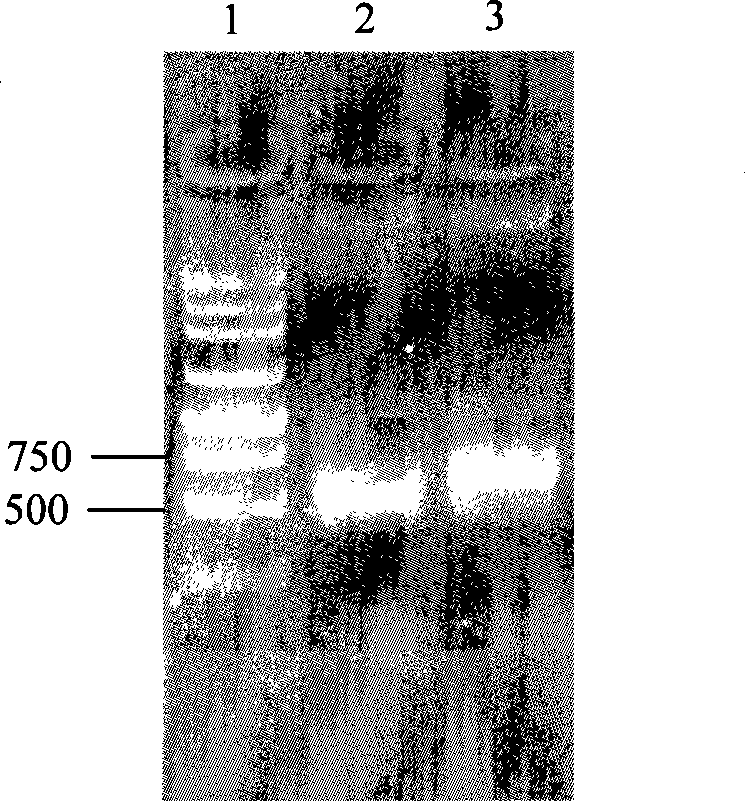
The invention relates to a pig breeding and breathing complex virus (PPRSV) and pig 2-type loop virus (PCV 2) series gene recombination adenovirus and vaccine in the field of high and new biotechnology. It uses PCR technology to clone the Cap protein gene into the plasmid carrier pShuttle-CMV-GP5 by open type reading rule and transforms tobacillus coli BJ5183strain with cage carrier pAdEasy-1 to capture the recombination plasmid; it uses recombination plasmid HEK293-A cell to capture recombination adenovirus and purifies it to express the recombination adenovirus rAd-Cap-GP5 of PRRSV GP5protein and PCV-2 Cap protein.
Owner:NANJING AGRICULTURAL UNIVERSITY
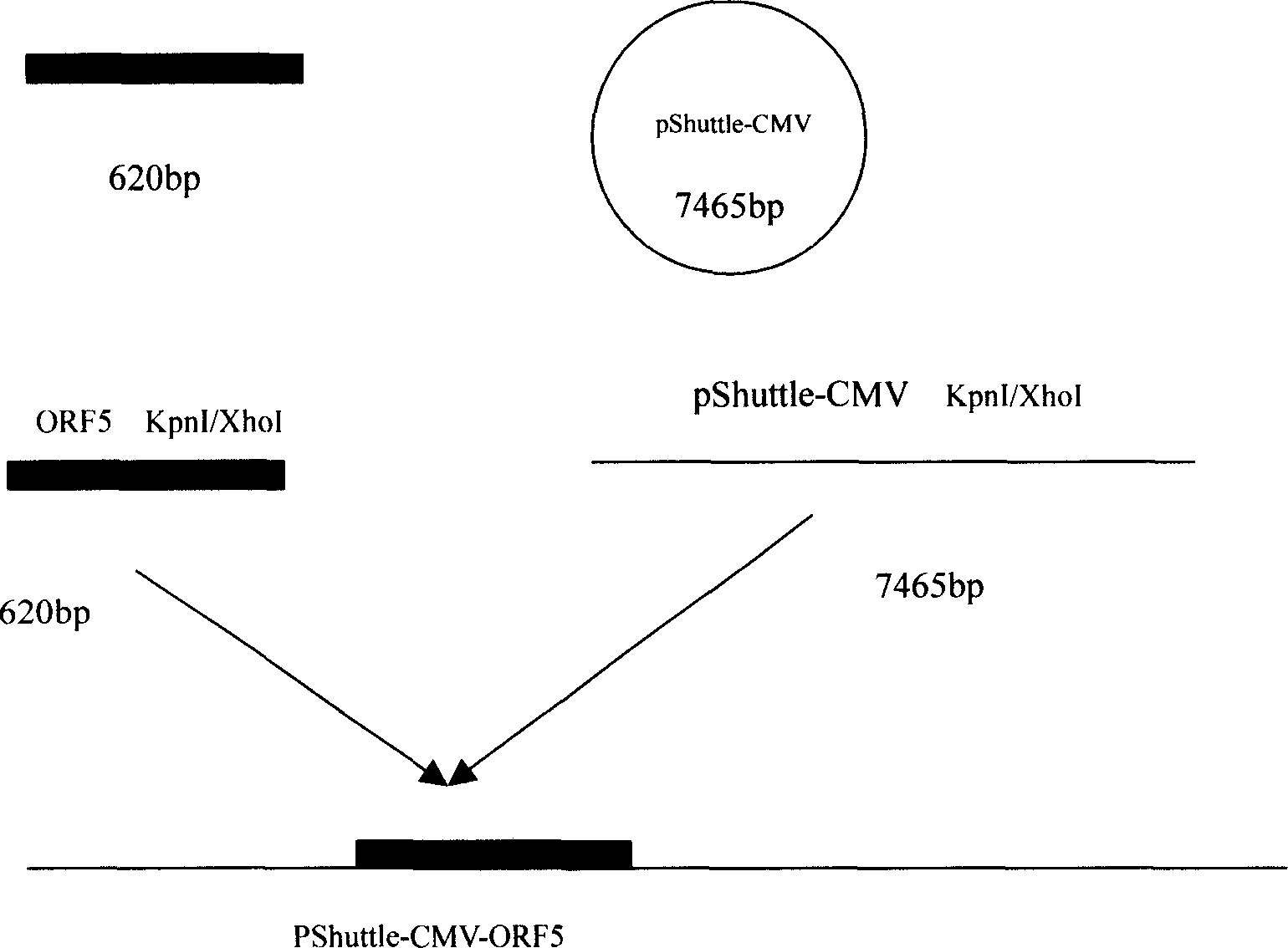
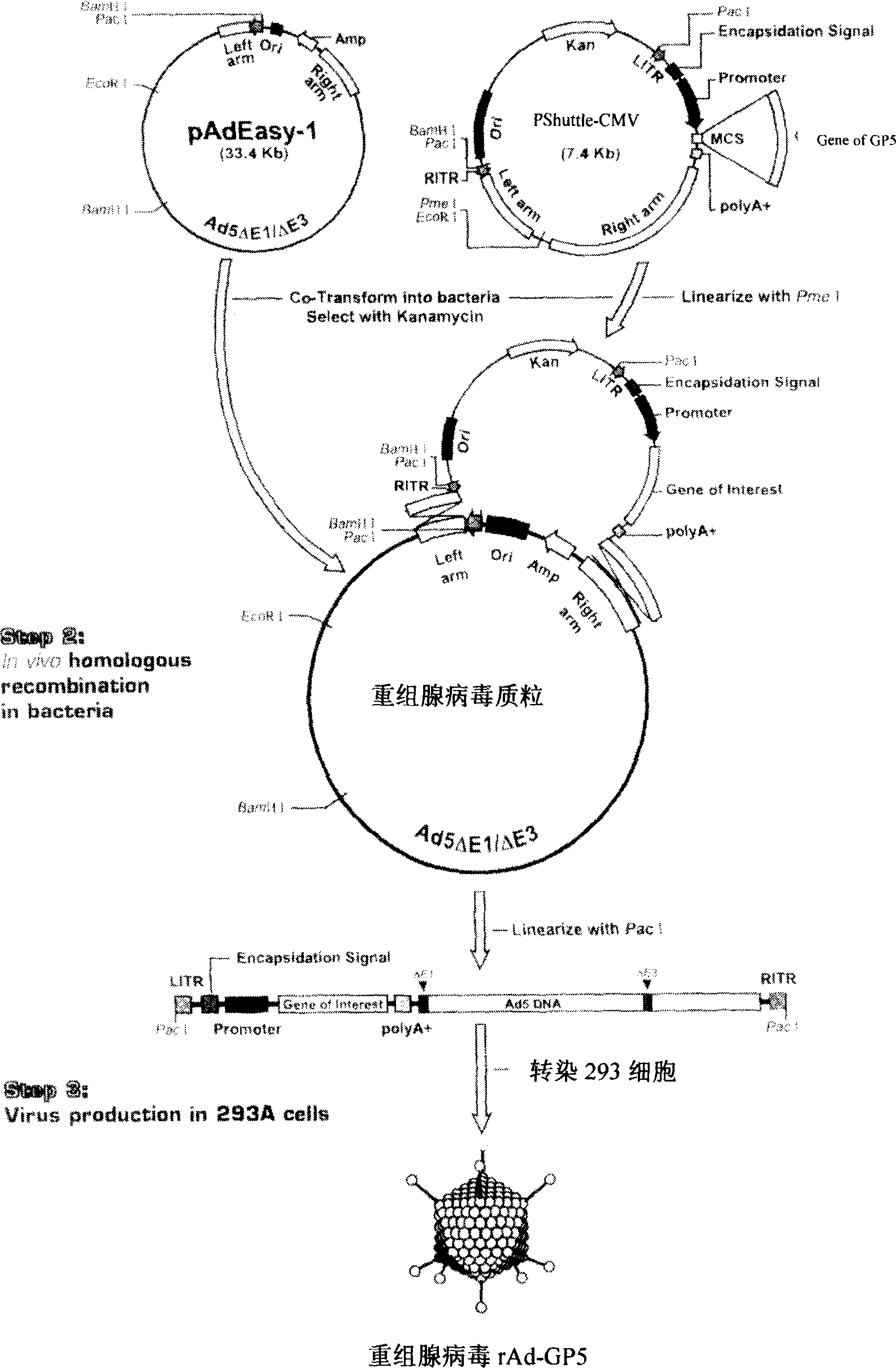
Pig breeding and respiratory syndrome recombined adenovirus and vaccine
InactiveCN1554766ALittle changeChange propertiesGenetic material ingredientsInactivation/attenuationEscherichia coliBiotechnology
The present invention relates to pig reproduction and respiratory syndrome virus recombined adenovirus and vaccine, and belongs to the field of biological high-tech. Through RT-PCR process to proliferate whole PRRSV GP5 sequence, cloning the gene sequence to the shuttle vector pShuttle-CMV of adenovirus carrier system, cotransforming colibacillus BJ5183 strain together with the skeleton vector of adenovirus carrier system to obtain recombinant plasmid, transfecting HEK293-A cell to obtain recombinant adenovirus and plaque purification, and RT-PCR and indirect immunofluorescence technique inspection, the recombinant adenovirus rAd-GP5 expressing PRRSV GP5 protein is constituted. The recombinant adenovirus can set ahead the expression of PRRSV GP5 protein and raise the expression amount to simulate the immune protecting reaction of body effectively.
Owner:NANJING AGRICULTURAL UNIVERSITY
Porcine reproductive and respiratory syndrome bivalence recombinant adenovirus vaccine and preparation method thereof
InactiveCN101380468AImmediately exert cellular immune functionNot pathogenicViral antigen ingredientsAntiviralsEukaryotic plasmidsAttenuated vaccine
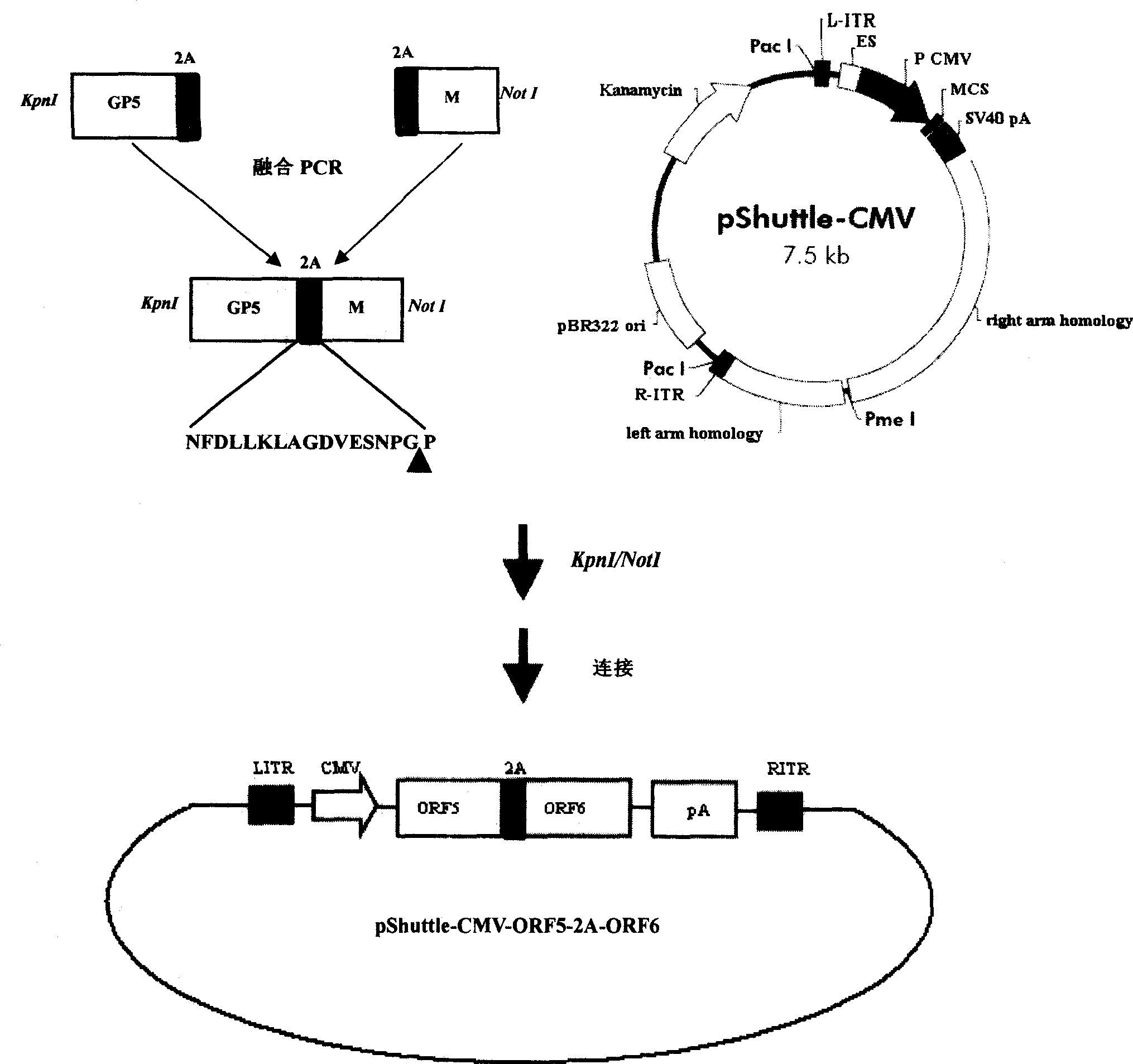
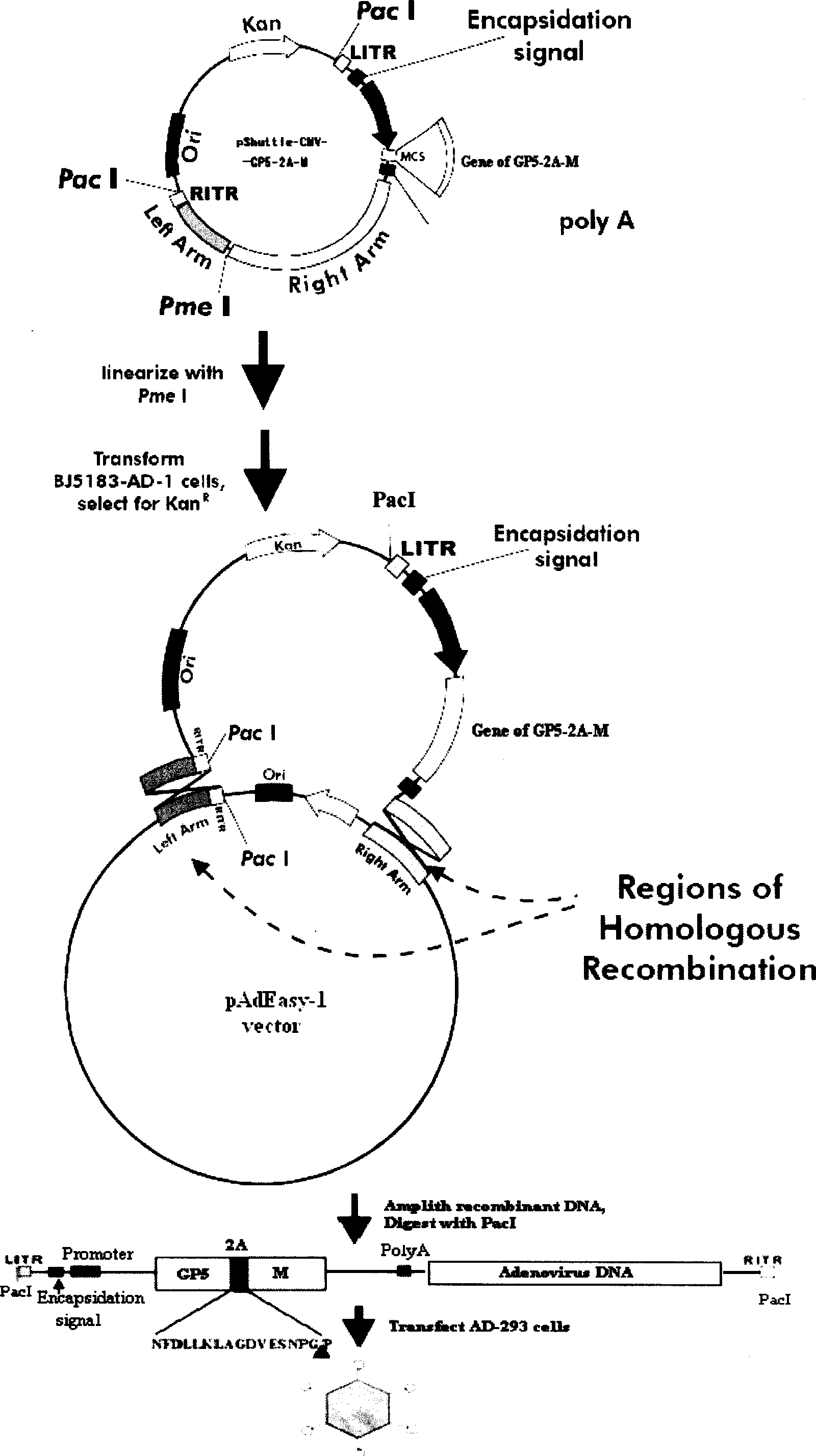
The invention discloses a porcine reproductive and respiratory syndrome divalent recombination adenovirus vaccine and the preparation method thereof. The invention belongs to the technical field of biological vaccine preparation. The vaccine can be prepared by the following steps: a GP5-2A-M fusion protein gene can be constructed by inserting an FMDV2A gene with self craking between PRRSV GP5 and M protein; homologous recombination is carried out on the GP5-2A-M fusion protein gene and adenovirus backbone plasmid pAdEasy-1; recombination adenovirus rAd-GP5-2A-M is prepared by restriction enzyme and HEK-293A cells transfection, and the divalent recombination adenovirus vaccine is prepared by the technology and the steps such as purification, amplification, and the like. After expression, the aggregate protein GP5-2A-M constructed by the invention is self cracked into GP5 and M protein, as well as exerts the viral neutralization of GP5 and the immune function of the M protein; the vaccine has stable titer with the virulent valence being 10<10.43>TCID<50> / 1.0ml, as well as has both the duplication characteristic of a routine attenuated vaccine and the safety of an inactivated vaccine; the divalent recombination adenovirus vaccine can be popularized in and applied to the control work of porcine reproductive and respiratory syndrome.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Method for preparing infectious chicken Fabricius bursa refined yolk cryodesiccation antibody
InactiveCN101113176AEmergency prevention is goodGood treatment effectEgg immunoglobulinsAntiviralsAntigenYolk
A preparation method of a purified vitelline freezing antibody for chickens infectious bursal disease is characterized in that: high purity antigens are adopted to vaccinate IBDV B87 to SPF chickens, then velvet urine, idiosome, velvet urine membrence are acquired, grinded, filtered and inactivated to antigens, and the steps are: producing immune eggs, collecting high immune egg, separating vitelline, diluting with distilled water, inactivating, extraction, decentering, inactivating, freezing and checking; the eggs is radiated by Co60 to kill viruses and bacterium, and finally froze by novel immuno-enhancer - Astragali Polysaccharoses and preserved under a temperature of 2-8 DEG C. Composite drugs for prevention and therapy are prepared by adopting the vitelline antibody for chickens infectious bursal disease as major components and cooperating with a novel immuno-enhancer, to decrease the jeopardy of the chicken breeding industry, and improve the living level of people and benefit mankind.
Owner:PU LIKE BIO ENG
Immunodetection kit and measurement method and preparation method thereof
The invention provides an immunodetection kit. The immunodetection kit comprises 1) a conjugate carrier and 2) an immunochromatography test strip, wherein the conjugate carrier comprises a ligand, a marker and a solid-phase carrier, the ligand is used for generating specificity reaction with a to-be-measured analysis substance, the marker is used for signal detection, the ligand is connected withthe marker, and the solid-phase carrier is used for bearing the ligand and the marker, the conjugate carrier and the immunochromatography test strip are separately arranged. The kit can be stored andtransported under a room temperature and also has the characteristics of favorable accuracy, and the operation method is simple and rapid and is suitable for immediate detection. During measurement ofthe kit, a sample solution and the conjugate carrier are uniformly mixed and then are added into the immunochromatography test strip for detection, and a conjugate is more fully released. The kit isconvenient and rapid to fabricate, is low in manufacturing cost and can be compatible with existing assembly line equipment very well, and complicated process and high-cost equipment are not needed.
Owner:DONGGUAN HEC MEDICAL INTELLIGENT DEVICE R&D CO LTD
Cryoprotectant and lyophilization method for latex coupled antibody
ActiveCN105709235AGood freeze-drying protection effectActivity is not destroyedPowder deliveryAntibody ingredientsPolyethylene glycolBovine serum albumin
The invention relates to the technical field of medicine, in particular to a cryoprotectant and a lyophilization method for a latex coupled antibody. The cryoprotectant comprises BSA (bovine serum albumin), PEG (polyethylene glycol) and PVP (polyvinylpyrrolidone). The cryoprotectant is designed for the latex coupled antibody and can protect the activity of the latex coupled antibody from being damaged in a lyophilization process in combination with the provided lyophilization method, non-specific aggregation in a redissolution process after lyophilization cannot happen, and the antibody titer keeps stable after redissolution. Repeated experiments prove that the deviation is not obvious, the relative deviation is plus or minus 10% or smaller, and the cryoprotectant has good lyophilization protection effect.
Owner:SHENZHEN GOLDSITE DIAGNOSTICS
Recombinant anine interferon alpha standard substance, preparation method and potency determination method thereof
InactiveCN106282279AHigh potencyStable potencyCompound screeningApoptosis detectionFreeze-dryingTotal protein
The invention discloses a recombinant anine interferon alpha standard substance, a preparation method and potency determination method thereof. BL21 / pET-28 alpha-rCaIFN alpha recombinant bacteria of the expression recombinant anine interferon alpha is fermented and induced, and supernatant after bacterial cell disruption is collected directly to extract the total protein; thereafter, a pure product of the preparation is obtained by three-step purification, a freeze-drying protective additive is added in the pure product for vacuum freeze drying, then a standard substance is obtained, wherein the valence is 1.0*1.04 IU / mL, the purity is 97.34% by a SDS-PAGE detection, the purity is 99.79% by a HPLC detection, and the relative molecular weight is 33 KD. According to the preparation methd, the prepared pecombinant anine interferon alpha as a standard substance can be used for comparison of characteristic identification and potency determination of the recombinant anine interferon alpha, when the standard substance is taken as a standard, the determining results of the potency determination of different batches of the recombinant anine interferon alpha are more reliable and more dependable.
Owner:ANHUI JIUCHUAN BIOTECH
Improved body fluid color-developing fungi 1,3-beta-D-glucan detection kit and application method thereof
ActiveCN106290890ASimple production processImprove stabilityMaterial analysis by observing effect on chemical indicatorC factorBeta d glucan
The invention relates to an improved body fluid color-developing fungi 1,3-beta-D-glucan detection kit, which comprises a reaction main-agent, a main agent combination solution, a sample treatment fluid, sterile water, a standard substance and a quality control material. With Tachypleus tridentatus Leach or Limulus polyphemus Linnaeus hemocyte lysate as a main raw material, the reaction main-agent contains G factor, B factor, C factor, clottable protein and a polypeptide chromogenic substrate. By a new preparation process, the reaction main-agent has characteristics of high stability and small intra-assay and inter-assay difference. The main agent combination solution adopts a new formula. After the main agent combination solution is mixed with the reaction main-agent, Tachypleus Amebocyte Lysate endotoxin reaction branch can be effectively shielded. According to the kit, rate-method enzyme kinetics detection is carried out by ELIASA. The detection speed is fast, and anti-interference performance is strong. In addition, the preparation technology is simple, and the product has stronger stability and higher specificity and sensitivity.
Owner:KOCH BIOTECHNOLOGY(BEIJING) CO LTD
Lytic vibrio parahaemolyticus bacteriophage RDP-VP-19003 and application thereof
The invention discloses a lytic vibrio parahaemolyticus bacteriophage RDP-VP-19003 and an application thereof to pathogenic vibrio parahaemolyticus infection of aquatic animals. According to the invention, the bacteriophage is separated from wastewater of Qingdao Jimo aquaculture farmers by using a double-layer plate method, is named as vibrio parahaemolyticus bacteriophage RDP-VP-19003, and has apreservation number of CGMCC No. 19385. The vibrio parahaemolyticus bacteriophage RDP-VP-19003 provided by the invention has relatively high temperature tolerance and a relatively wide acid-base tolerance range, and is beneficial to industrial production and preparation. The bacteriophage has a strong cracking effect on the vibrio parahaemolyticus BV-19004, can effectively prevent and control generation and propagation of the vibrio parahaemolyticus, and can be used for efficiently and reliably treating aquatic animal diseases caused by the vibrio parahaemolyticus and improving the water environment.
Owner:RECOM QINGDAO BIOTECH CO LTD
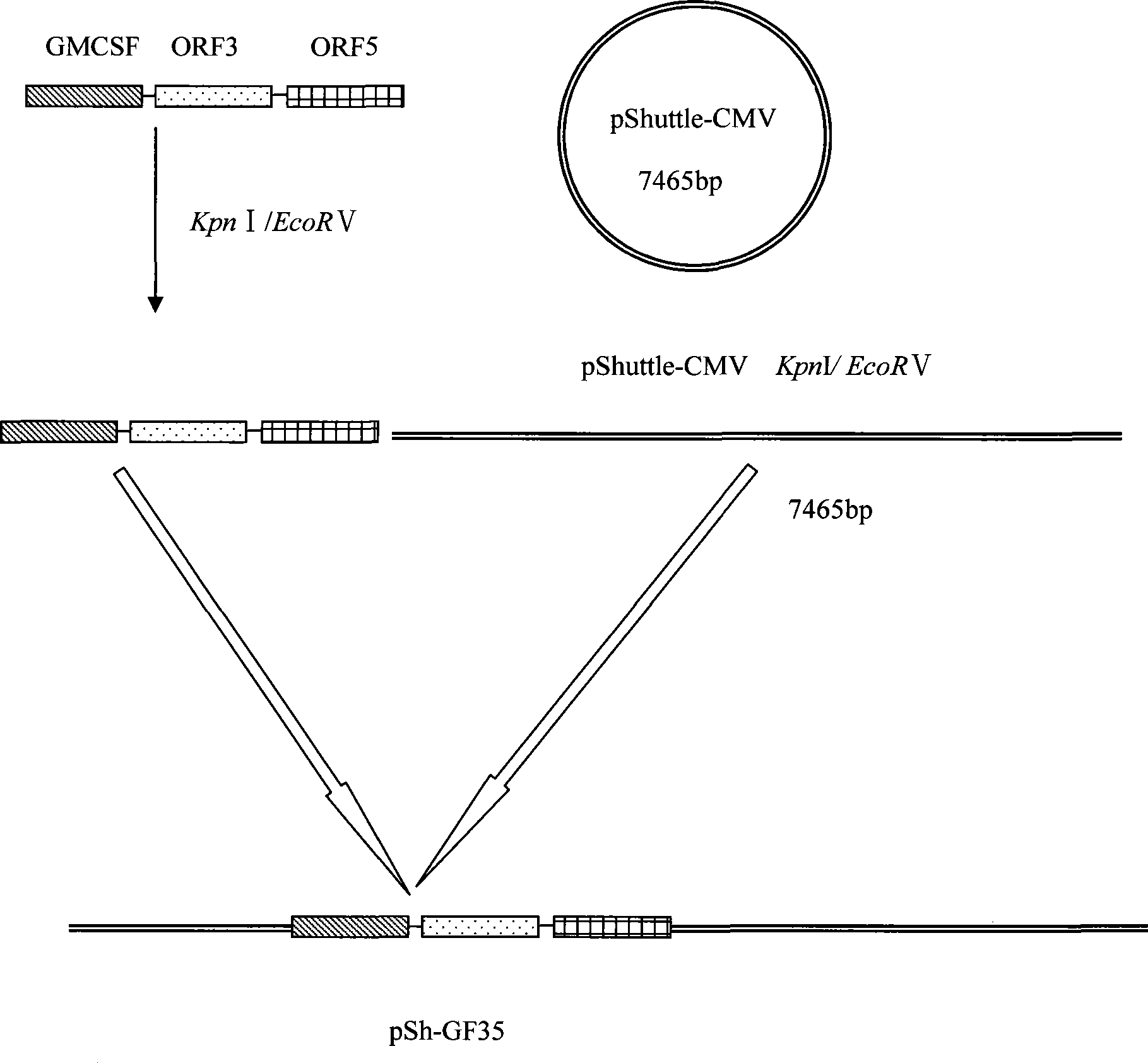
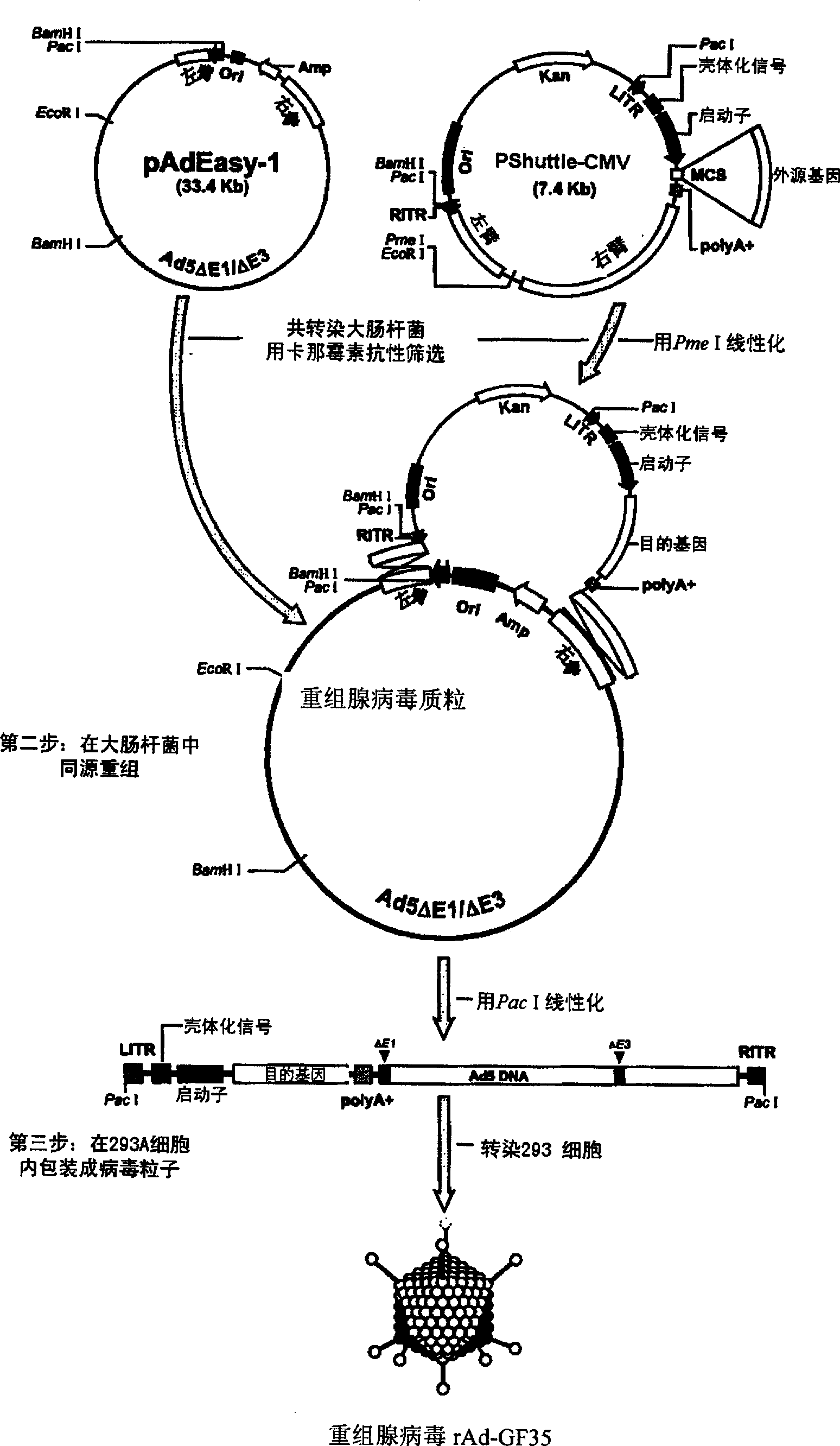
Porcine reproductive and respiratory syndrome recombinant adenovirus rAd-GF35
InactiveCN101423823AHas the copy featureIncreased effect of bioactive adjuvantsViral antigen ingredientsAntiviralsEscherichia coliAntigen
The invention discloses a virus (PRRSV) recombinant adenovirus rAd-GF35 of porcine reproduction and respiratory syndrome, which belongs to the technical field of high-tech biotechnology. Highly pathogenic PRRSV SY0608 separation strains of GP3 and GP5 protein genes and porcine colony sell stimulating factor (GMCSF) whole gene sequences are amplified, connected in series and cloned into pShuttle-CMV to be transformed into escherichia coli BJ5183 together with pAdEasy-1 so as to obtain recombinant plasmids; and HEK293A cells are transfected to obtain the recombinant adenovirus so as to successfully express PRRSV SY0608 strains GP3 and GP5 and a GMCSF protein. The recombinant virus can express the GMCSF correctly, and play a role of an adjuvant so as to improve the immune activity of the PRRSV GP3 and GP5 proteins and stimulate the immune protective reaction of an organism more effectively. The recombinant adenovirus vaccine has the safety of a subunit vaccine and the antigen proliferating ability of an attenuated vaccine, thereby having wide development and application prospect.
Owner:NANJING AGRICULTURAL UNIVERSITY
Medicinal composition containing fiber eliminating enzyme
InactiveCN1456352AStable potencyFree from adsorptionPeptide/protein ingredientsRespiratory disorderFiberMedicine
Owner:江苏浦金药业有限公司
Shuttling intracellular antibody TAT-4F for H3N2-type canine influenza virus
ActiveCN108728461AInterfere with copyingPlay an antiviral rolePolypeptide with localisation/targeting motifAntiviralsHemagglutininVirus influenza
The invention discloses a shuttling intracellular antibody TAT-4F for H3N2-type canine influenza virus. The H3N2 virus is A-type influenza virus, the main neutralizing antibody is from hemagglutinin (HA), therefore, the HA becomes an original main research target; then the influenza virus has high variability, the immune cross reaction among different variable branches is weaker, M1 antibody can be combined with M1 protein to inhibit the activity and disturb multiplication, transcription and release of the influenza virus so as to play a role in resisting the virus; therefore, the M1 protein is selected for preparing the corresponding antibody to obtain stable titer, coupling the antibody with TAT protein PTD (Protein Transduction Domain) capable of transducing biomacromolecules into cellsand expressing fusion protein TAT PTD-M1 ScFv, and then a shuttling antibody for resisting the canine influenza virus is prepared to provide a new path for treating the canine influenza virus.
Owner:ACAD OF MILITARY SCI PLA CHINA ACAD OF MILITARY MEDICAL SCI INST OF MILITARY VETERINARY MEDICINE
Porcine parvovirus BQ-C strain and application of porcine parvovirus BQ-C strain to preparation of inactivated porcine parvovirus vaccine
ActiveCN102719407AImproving immunogenicityStable potencyMicroorganism based processesAntiviralsDiseaseAdjuvant
The invention discloses a porcine parvovirus BQ-C strain and application of the porcine parvovirus BQ-C strain to the preparation of an inactivated porcine parvovirus vaccine, and belongs to the field of biological vaccines. The porcine parvovirus BQ-C strain has the advantages of high immunogenicity, good cultural character and the like; and porcine parvovirus (BQ-C strain) are inoculated to swine testis (ST) cells, a culture is harvested and inactivated by using binary ethylenimine (BEI), and the culture and a commercial MontanideTM ISA15AVG adjuvant of SEPPIC are mixed and emulsified to form the inactivated porcine parvovirus vaccine. The experiment proves that: diseases caused by porcine parvoviruses can be prevented by immunizing primiparous healthy female sows by using the prepared inactivated porcine parvovirus vaccine; and moreover, the invention has the advantages that the vaccine is safe, an antibody can be quickly generated, the immune period is long-lasting, and the like.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Human immunoglobulin resisting hand-foot-and-mouth disease and preparation method of human immunoglobulin
ActiveCN105669860AImprove survival rateStrong specificityImmunoglobulins against virusesPeptide preparation methodsEnterovirusUltrafiltration
The invention relates to human immunoglobulin resisting the hand-foot-and-mouth disease and a preparation method of the human immunoglobulin. The hand-foot-and-mouth virus-neutralizing antibody titer is larger than or equal to 1:800. The preparation method comprises steps as follows: (1) efficient positive plasma with the hand-foot-and-mouth virus-neutralizing antibody titer larger than or equal to 1:80 is screened; (2) the screened efficient positive plasma is mixed; (3) the mixed plasma is separated with a low-temperature ethanol filter pressing method, immunoglobulin components II are purified and separated with an ion-exchange column chromatography method, and the immunoglobulin with the purity ranging from 98.5% to 100% is obtained through filtration, chromatography, ultrafiltration, preparation, incubation of inactivated viruses at low pH, virus removal through nanofilm filtration and subpackaging. By means of the preparation and production method, the hand-foot-and-mouth virus-neutralizing antibody titer, the purity and the recovery rate are high, enteroviruses can be treated in a targeted manner, and the human immunoglobulin is an effective medicine for treating an enterovirus infectious disease, is safe and reliable and has higher social benefits and economic benefits.
Owner:哈尔滨派斯菲科生物制药有限公司
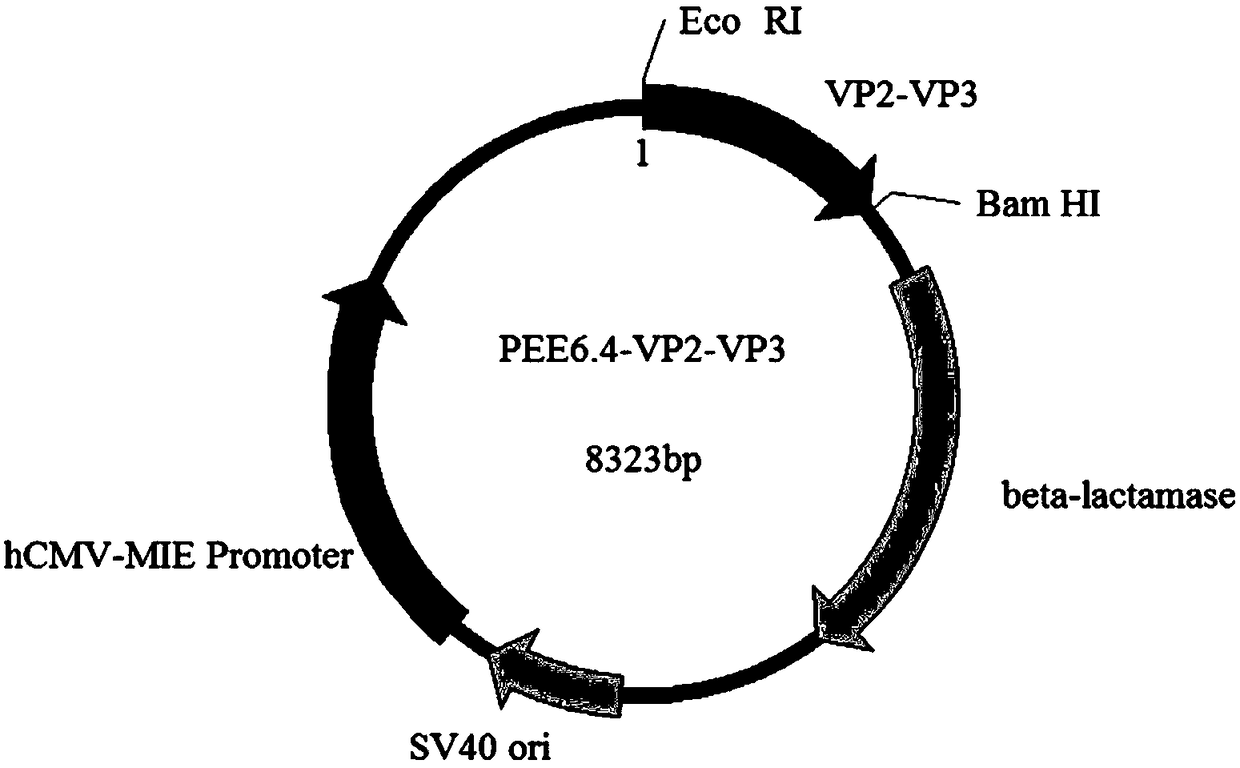
Fusion protein of infectious bursal disease virus, preparation method, application, expression system of fusion protein and vaccine comprising fusion protein
InactiveCN109134668AImprove securityWill not causeAntibody mimetics/scaffoldsVirus peptidesSide effectInfectious bursitis
The invention relates to the technical field of biology, and particularly provides a fusion protein of an infectious bursal disease virus, a preparation method, application, an expression system of the fusion protein and a vaccine comprising the fusion protein. The fusion protein comprises a VP2 section and a VP3 section, wherein the VP2 section is expressed by a nucleotide sequence shown in SEQ ID NO.1, the VP3 is expressed by a nucleotide sequence shown in SEQ ID NO.2. The fusion protein is obtained by tandem expression of the gene sequences of the VP2 and the VP3 of the infectious bursal disease virus. By analyzing the gene sequences of the VP2 and the VP3 and selecting zones high in antigenicity and easy for high expression to link tandemly, the obtained fusion protein has the advantages of high antigenicity and high expression amount. By adopting the fusion protein as an antigen to prepare the vaccine, the prepared vaccine has the advantages of good safety, stable potency and no toxic and side effects. The invention further provides the preparation method and application of the fusion protein, and the vaccine prepared from the fusion protein.
Owner:TECON BIOLOGY CO LTD
Shuttling intracellular antibody TAT-2C for H3N2-type canine influenza virus
ActiveCN108728462AInterfere with copyingPlay an antiviral rolePolypeptide with localisation/targeting motifAntiviralsBiological macromoleculeNeutralizing antibody
The invention discloses a shuttling intracellular antibody for H3N2-type canine influenza virus. The H3N2 virus is A-type influenza virus, the main neutralizing antibody is from hemagglutinin (HA), therefore, the HA becomes an original main research target; however the influenza virus has high variability, the immune cross reaction among different variable branches is weaker, M1 antibody can be combined with M1 protein to inhibit the activity and disturb multiplication, transcription and release of the influenza virus so as to play a role in resisting the virus; therefore, the M1 protein is selected for preparing the corresponding antibody to obtain stable titer, coupling the antibody with TAT protein PTD (Protein Transduction Domain) capable of transducing biomacromolecules into cells andexpressing fusion protein TAT PTD-M1 ScFv, and then a shuttling antibody for resisting the canine influenza virus is prepared to provide a new path for treating the canine influenza virus.
Owner:ACAD OF MILITARY SCI PLA CHINA ACAD OF MILITARY MEDICAL SCI INST OF MILITARY VETERINARY MEDICINE
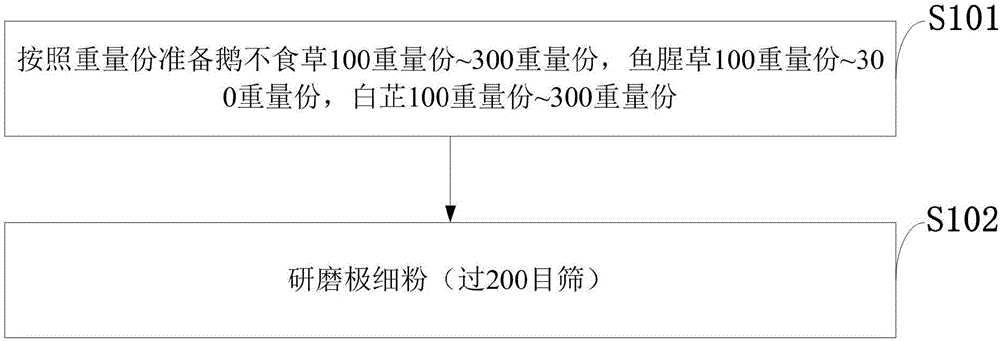
Traditional Chinese medicine for treating rhinitis and sinusitis and preparation method of traditional Chinese medicine
InactiveCN106798778AStable potencyEasy to usePharmaceutical delivery mechanismRespiratory disorderNasal cavitySinusitis
The invention belongs to the technical field of traditional Chinese medicines and discloses a traditional Chinese medicine for treating rhinitis and sinusitis and a preparation method of the traditional Chinese medicine. The traditional Chinese medicine for treating rhinitis and sinusitis comprises 100-300 parts by weight of centipeda minima, 100-300 parts by weight of cordate houttuynia, and 100-300 parts by weight of radix angelicae; a cotton swab is used to dip powder of the traditional Chinese medicine for treating rhinitis and sinusitis, the powder is fed into a nasal cavity, a patient takes a deep breath, the powder reaches a diseased region, the medicine is taken twice each day, and a course of treatment includes 14 days. The powder of the traditional Chinese medicine is used to treat rhinitis and sinusitis through an external inhalation method, is convenient to use, takes effects quickly, has good treatment effects, is non-toxic, and does not cause untoward effects; a patient with a mild symptom can also be treated by smelling the odor. Particularly, the traditional Chinese medicine has better treatment effects on purulent nasal mucus, nasal obstruction, airway obstruction and forehead ache caused by rhinitis and sinusitis, and takes effects quickly when being used to improve the symptoms of ventilation, purulent nasal mucus, forehead ache and the like.
Owner:荆门市中医医院
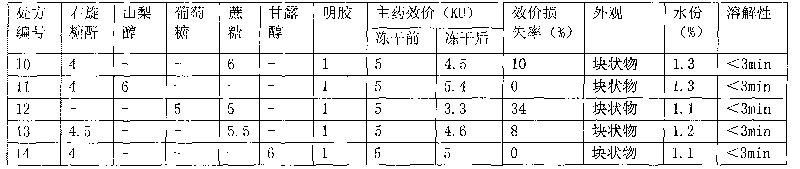
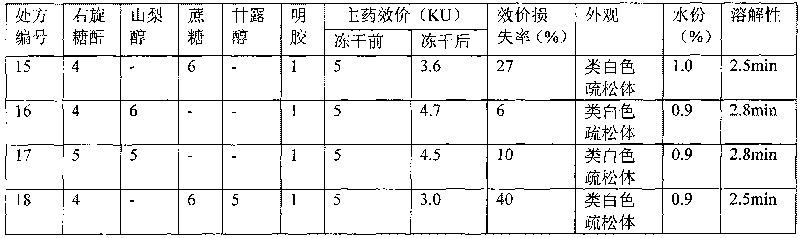
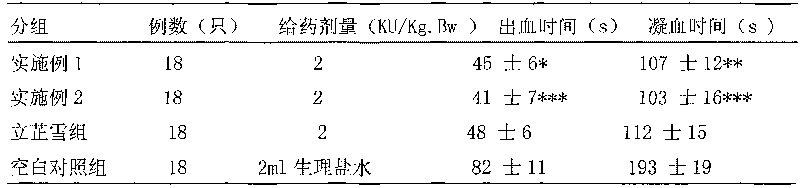
Hemocoagulase freeze-dried powder injection and preparation method thereof
InactiveCN101756912ARetain activityStable potencyPowder deliveryPeptide/protein ingredientsFreeze-dryingBULK ACTIVE INGREDIENT
The invention provides a hemocoagulase freeze-dried powder injection and a preparation method thereof, and belongs to the field of biological products and preparations. The hemocoagulase freeze-dried powder injection takes hemocoagulase as an active ingredient, and a freeze-dried protective agent consists of gelatin, dextran-20, and sorbierite. The hemocoagulase freeze-dried powder injection can be used as a haemostatic and the like.
Owner:PEKING UNIV FOUNDER GRP CO LTD +2
Recombination low-dosage human alpha-2b(IFN-alpha-2b) interferon lozenge and preparing method thereof
InactiveCN1471970ASignificant effectExcellent clinical effectPeptide/protein ingredientsAntiviralsMagnesium stearateInterferon alpha
A buccal lozenge for treating SARS, hepatitis B, hepatitis C and tumor is prepared from low-dosage recombinant human alpha-2b(IFN-alpha-2b) interferon and medical auxiliaries including mycose, maltose, sugar powder, staroh, gelatin and magnesium stearate. Its advantages are high curative effect, low poison, and low cost.
Owner:山东泉港药业有限公司
Lysozyme hydrochloride toothpaste and preparation method thereof
InactiveCN105902407AGood curative effectImprove mucopolysaccharide metabolismCosmetic preparationsToilet preparationsFoaming agentDental plaque induced gingivitis
The invention discloses a lysozyme hydrochloride toothpaste and a preparation method thereof. The lysozyme hydrochloride toothpaste comprises an active ingredient and auxiliary materials, wherein the active ingredient is lysozyme hydrochloride, and the weight percentage of lysozyme hydrochloride in toothpaste is 0.3-4.6 percent; the auxiliary materials are any acceptable auxiliary materials for preparing the toothpaste, and comprise a humectant, a thickener, a foaming agent, a protective agent, an abrasive, a flavoring agent and other additives. The lysozyme hydrochloride toothpaste has the advantages that the cleaning capability is strong, the mouth feel of tooth brushing is excellent, the effect of treating periodontal diseases, such as inhibiting of dental plaque and / or relieving of gingivitis and periodontitis, are realized, and more safety in use is realized.
Owner:SHANDONG SBOND PHARMA
Salmonella abortus equi horse derived strain and application thereof in preparation of salmonella equina inactivated vaccine
ActiveCN111100817AImproving immunogenicityStable potencyAntibacterial agentsBacteriaLaboratory cultureImmunogenicity
The invention discloses a salmonella abortus equi horse derived strain and an application thereof in preparation of a salmonella equina inactivated vaccine, and belongs to the technical field of biological vaccines. The salmonella abortus equi horse derived strain is named as 20180316.H.AES.G and is preserved in the common microorganism center of the horse China General Microbiological Culture Collection Center, wherein the culture collection number is CGMCC No.18341. The salmonella abortus equi horse derived strain 20180316.H.AES.G provided by the invention has the advantages of good immunogenicity, good culture characteristics and the like. An inactivated vaccine is prepared by the steps of inoculating the salmonella abortus equi horse derived strain 20180316.H.AES.G to a salmonella equienrichment broth, performing culturing, and then performing inactivating with 0.2% v / v formaldehyde. Experiments prove that when a mouse is immunized by the prepared inactivated vaccine of the salmonella abortus equi, the mouse can be free from death after challenge, and safety is good after a horse body is immunized. Therefore, the vaccine has the advantages of safety, low-dose immunization andthe like. The invention provides the inactivated vaccine of salmonella abortus equi for the first time, and the inactivated vaccine can be used for preventing abortion caused by salmonella abortus equi.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Avian adenovirus 4, 8 and 11 type trivalent vaccine as well as preparation method and application thereof
PendingCN114395536AImprove performanceHigh titerViral antigen ingredientsMicroorganism based processesFowl adenovirusTGE VACCINE
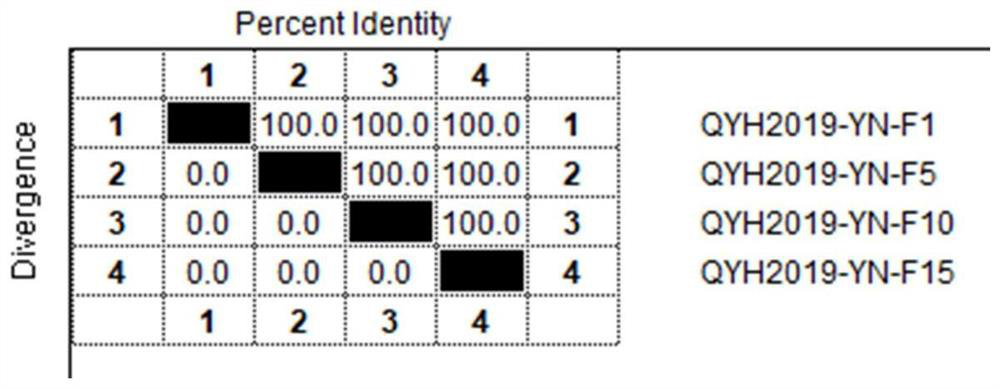
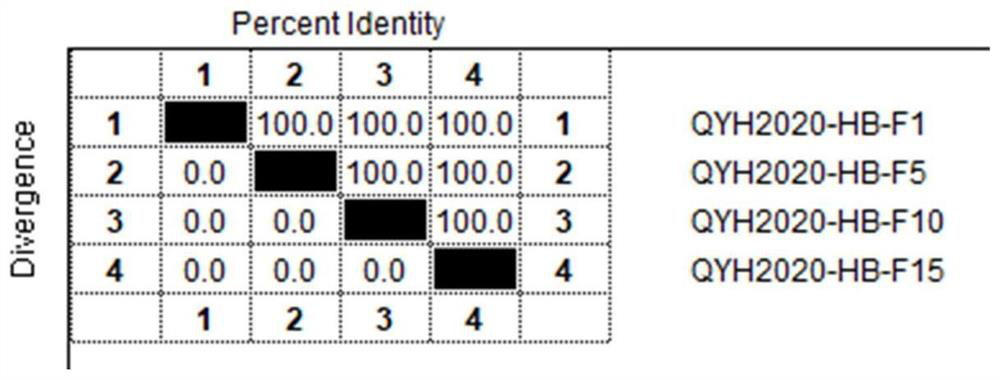
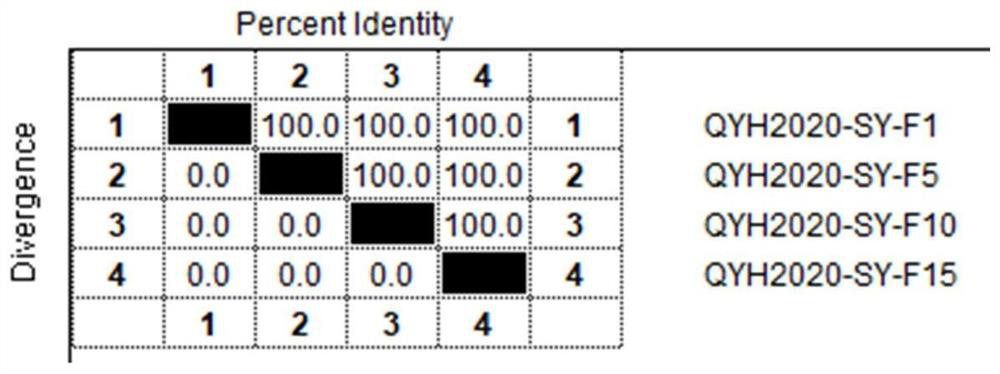
The invention relates to the technical field of veterinary biological products, in particular to an avian adenovirus 4, 8 and 11 type trivalent vaccine as well as a preparation method and application thereof. The fowl adenovirus trivalent vaccine comprises a fowl adenovirus type 4 vaccine strain QYH2019-YN, a fowl adenovirus type 8 vaccine strain QYH2020-HB and a fowl adenovirus type 11 vaccine strain QYH2020-SY. The invention provides a fowl adenovirus trivalent vaccine which is prepared from a fowl adenovirus type 4 vaccine strain, a fowl adenovirus type 8 vaccine strain and a fowl adenovirus type 11 vaccine strain which are obtained by newest separation, and has an effective prevention effect on fowl adenovirus type 4, fowl adenovirus type 8 and fowl adenovirus type 11 as well as chicken inclusion body hepatitis and hydropericardium-hepatitis syndrome caused by the fowl adenovirus type 4, fowl adenovirus type 8 and fowl adenovirus type 11.
Owner:乾元浩生物股份有限公司
Pigeon paramyxo virus type 1 PPMV-1/BJ-C strain and application thereof
InactiveCN112063596AImproving immunogenicityGood cultivation characteristicsSsRNA viruses negative-senseViral antigen ingredientsAdjuvantVirus type
The invention provides a pigeon paramyxo virus type 1 PPMV-1 / BJ-C strain and application thereof. The attenuated strain PPMV-1 / BJ-C is constructed by mutating an F protein cleavage site of the attenuated strain PPMV-1 / BJ-C into a La Sota strain corresponding site on the basis of a pigeon plague virus virulent strain PPMV-1 / BJ. PPMV-1 / BJ-C has the advantages of good immunogenicity, good culture characteristics, stable titer and the like, the pigeon plague virus PPMV-1 / BJ-C strain is inoculated to 9-11-day-old SPF chick embryos through an allantoic cavity, allantoic fluid of infected chick embryos is harvested and inactivated with diethyleneimine, and then an aluminum hydroxide adjuvant is added to prepare a pigeon plague virus inactivated vaccine. The inactivated vaccine prepared from the PPMV-1 / BJ-C strain can generate a relatively high antibody, can prevent ND caused by pigeon plague virus, and the vaccine has the advantages of safety, quick response, lasting immune period and the like, and has wide application prospects.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Group I avian adenovirus type 8 strain and application thereof
ActiveCN109207437AImprove securityImprove protectionViral antigen ingredientsDigestive systemDiseaseInclusion bodies
The invention relates to the technical field of veterinary biological products, in particular to a group I avian adenovirus type 8 strain and application thereof. The group I avian adenovirus type 8 strain ZMYTAV-8 strain, with an accession number of CGMCC No. 14297 has the characteristics of high toxin production and good immunogenicity. The strain can effectively prevent chicken inclusion body hepatitis caused by serum avian adenovirus type 8. Therefore, the invention also provides application of the avian adenovirus type 8 strain ZMYTAV-8 in the preparation of drugs for the prevention of inclusion body hepatitis. Further, the present invention provides a bivalent inactivated vaccine of avian adenovirus serotype 4, serotype 8 for the prevention of pericardial effusion-hepatitis syndromeand inclusion body hepatitis. The vaccine has good immunogenicity and can prevent avian diseases caused by adenovirus, such as inclusion body hepatitis and pericardial effusion-hepatitis syndrome, which are popular in recent years. The vaccine has 100% protection rate against local isolates, and has high safety, rapid antibody production and stable efficacy.
Owner:乾元浩生物股份有限公司
Bovine parainfluenza virus PBIV3-B strain and application thereof
ActiveCN107338227AImproving immunogenicityStable potencySsRNA viruses negative-senseViral antigen ingredientsBovine parainfluenza virusEmulsion
The present invention discloses bovine parainfluenza virus PBIV3-B strain and application thereof, the bovine parainfluenza virus PBIV3-B strain has the advantages of good immunogenicity and culture characteristics and the like, the bovine parainfluenza virus PBIV3-B strain is inoculated into MDBK cell to obtain a culture matter, after the culture matter is inactivated with (beta-Propiolactone), the culture matter is mixed with commercial MontanideTMISA15AVG adjuvant of SEPPIC of France for emulsion to obtain a bovine parainfluenza virus inactivated vaccine. Results showed that when the bovine parainfluenza virus inactivated vaccine prepared by the method is used for immunization of a weaned healthy negative cow, diseases caused by the bovine parainfluenza virus type 3 can be prevented, and the bovine parainfluenza virus inactivated vaccine has the advantages of safety, fast antibody production and lasting immunity period and the like.
Owner:INST OF ANIMAL SCI CAAS
Nucleic acid and recombinant protein co-immune vaccine based on classical swine fever virus gene as well as preparation method and application of co-immune vaccine
PendingCN111973738AImproving immunogenicityImmunization blank period is shortSsRNA viruses positive-senseViral antigen ingredientsClassical swine fever virus CSFVAdjuvant
The invention discloses a nucleic acid and recombinant protein co-immune vaccine based on a classical swine fever virus gene as well as a preparation method and application of the co-immune vaccine. The preparation method comprises the steps that firstly, optimized recombinant plasmids and recombinant classical swine fever vaccine antigen protein are obtained by combining gene engineering and cellengineering; then the nucleic acid sequence and the recombinant protein are compounded into different liquid phase layers of a biphasic adjuvant, so that the obtained co-immune vaccine is good in immunogenicity and short in immune blank period, can rapidly induce an organism to generate high-level, high-affinity and durable antibody response and immune memory, effectively protects a target animalfrom being attacked by classical swine fever virulent viruses, and the method can be applied to preparation of products for preventing / treating classical swine fever.
Owner:天康生物制药有限公司
Rumen compound preparation, preparation method thereof and application of preparation
PendingCN110250326AIncrease profitImprove conversion rateAnimal feeding stuffAccessory food factorsBetaineNiacin
The invention belongs to the technical field of ruminant nutrient substance rumen protection, and particularly relates to a rumen compound preparation, a preparation method thereof and an application of the preparation, wherein the rumen compound preparation is used for ruminant feed, can improve the use performance of the feed and is high in nutrient substance content and good in thermal-sensitive properties. The rumen compound preparation comprises components A, B, C, D and E, wherein the component A is prepared from, by weight, 11 parts of methionine, 16 parts of lysine, 3 parts of niacin and 10 parts of glycine betaine, the component B is a ruminant enzyme preparation, the component C is a ruminant probiotics, the component D is a vitamin for a ruminant, and the component E is an rumen auxiliary material. The component A, the component B, the component C, the component D and the component E are mixed according to the weight ratio of A:B:C:D:E=2:1:1:1:5 to prepare the rumen compound preparation. The rumen compound preparation has the advantages that various nutrient substances can be simultaneously provided, absorption of the nutrient substances in the feed can be promoted, feed use ratio is increased, and the added nutrient substances are high in use ratio.
Owner:河南大华生物技术有限公司
Construction method of gene engineering strain of Lactobacillus paracasei with high bacteriocin yield
InactiveCN105755031AGood genetic stabilityGood antibacterial effectBacteriaTransferasesBacteriocinMutant strain
The invention provides a construction method of a gene engineering strain of Lactobacillus paracasei with high bacteriocin yield, relates to a construction method of the gene engineering strain of the Lactobacillus paracasei and aims to solve the problem of low bacteriocin yield of the Lactobacillus paracasei at present. The method comprises steps as follows: step one, a prcK gene is amplified from Lactobacillus paracasei HD1.7; step two, a plasmid pMD18-T-prcK is constructed; step three, a recombinant overexpression vector pRSFDuet-1-prcK is constructed; step four, a recombinant overexpression vector pRR-tet is constructed; step five, the overexpression vector pRR-tet is used for performing electrotransformation on Lactobacillus paracasei HD1.7 competent cells; step six, overexpression mutant strains of the prcK gene are screened and identified. The bactericidal capacity of the gene engineering strain is increased by 17.86% compared with that of original strains; bacteriocin titer of fermentation broth after passage is 2372.46 plus or minus 46.27 AU / mL, and the titer after the passage is more stable; the method is applied to bacteriocin production.
Owner:HEILONGJIANG UNIV
Swine influenza virus H3N2 subtype hemagglutinin (HA)-1 protein recombinant suipoxvirus and preparation method thereof
InactiveCN102154366AObvious blue spotEasy to filterMicroorganism based processesAntiviralsHemagglutininTransfer vector
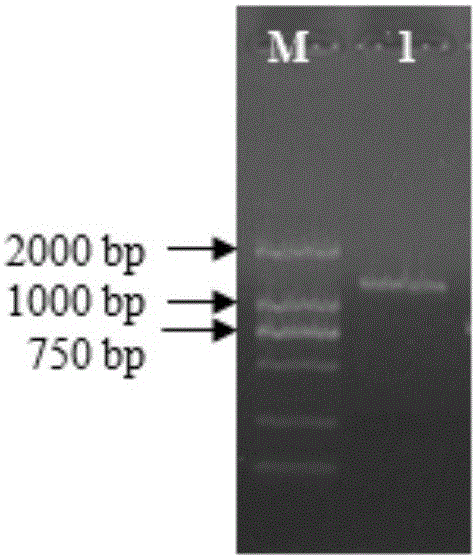
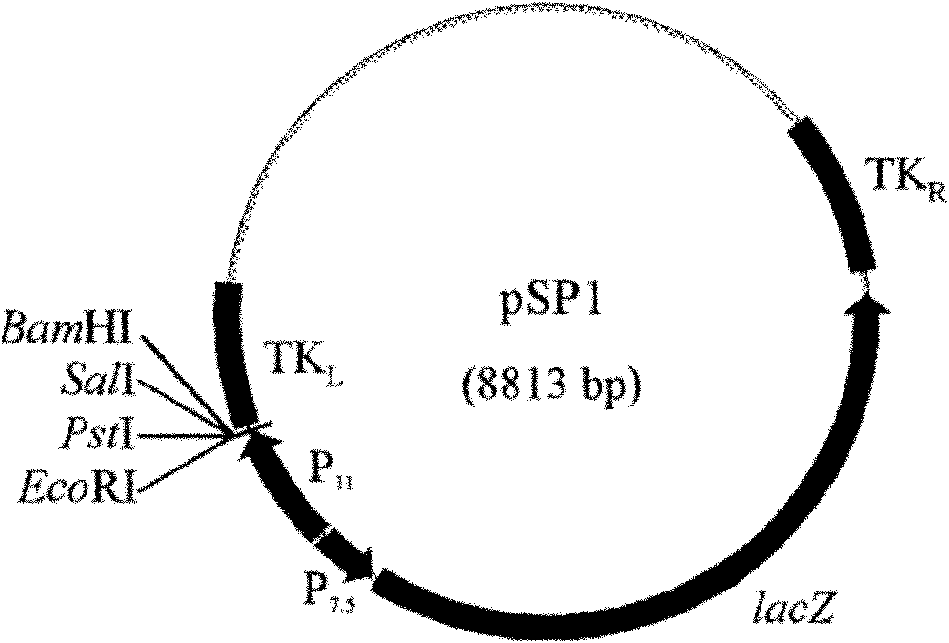
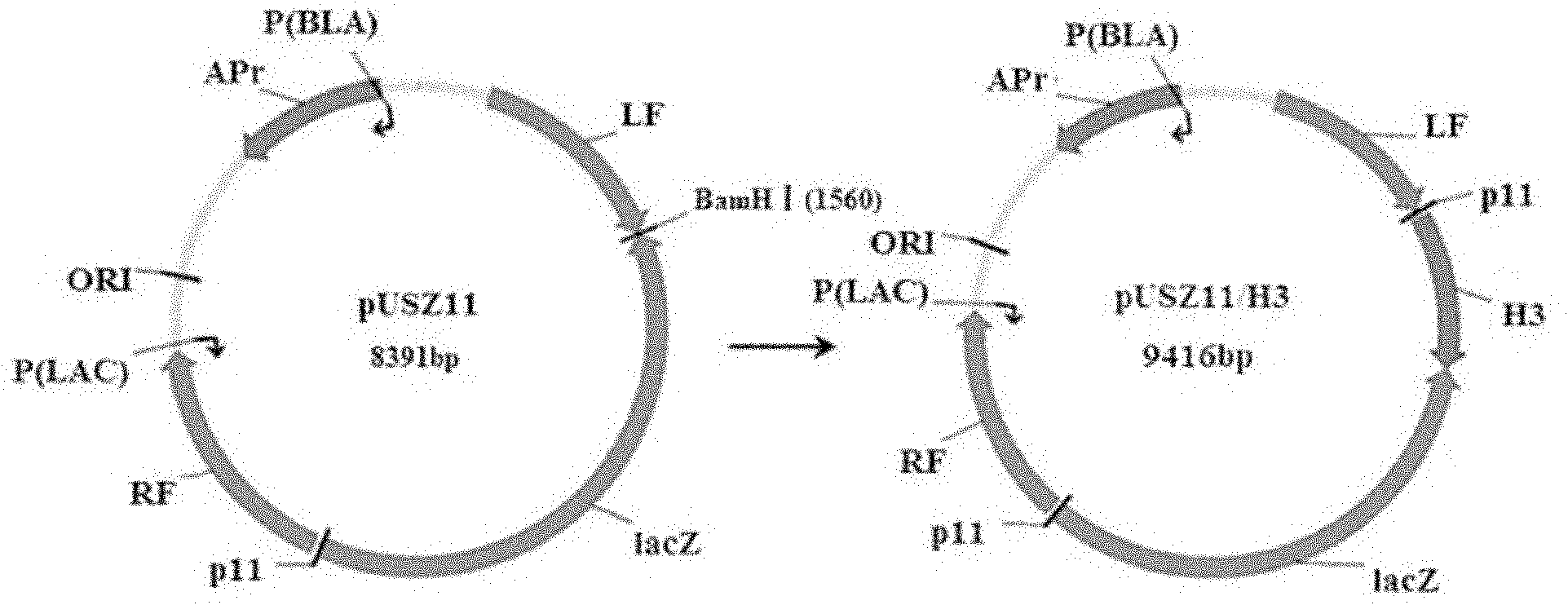
The invention belongs to the technical field of biology and discloses swine influenza virus H3N2 subtype HA-1 protein recombinant suipoxvirus and a preparation method thereof. An HA1 gene is designed and synthesized according to the gene sequence of HA1 of a A / swine / Guangxi / 1 / 2004(H3N2) strain of the swine influenza virus, and the gene is cloned in a suipoxvirus vector pUSZ11 to obtain a transfer vector pUSZ11 / H3. The recombinant virus is a positive clone obtained by infecting and transfecting PK15 cells with the suipoxvirus and the transfer vector pUSZ11 / H3 and performing homologous recombination. The recombinant suipoxvirus can express HA1 protein stably, and after infection with the virus, the cytopathy becomes regular, the toxic effect of the breeding virus is stabilized at 107TCID50 / mL, the breeding virus can effectively stimulate the immune protect reaction of organisms and can be used in the preparation of medicines for preventing and / or treating infection with swine influenza virus H3N2 subtype.
Owner:NANJING AGRICULTURAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com