Human immunoglobulin resisting hand-foot-and-mouth disease and preparation method of human immunoglobulin
An immunoglobulin, hand, foot and mouth technology, applied in the preparation method of antiviral immunoglobulin, immunoglobulin, peptide, etc., can solve the problem of low specificity, achieve good specificity, strong operability, improve filtration effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] 1. Plasma screening and collection of plasma donors:
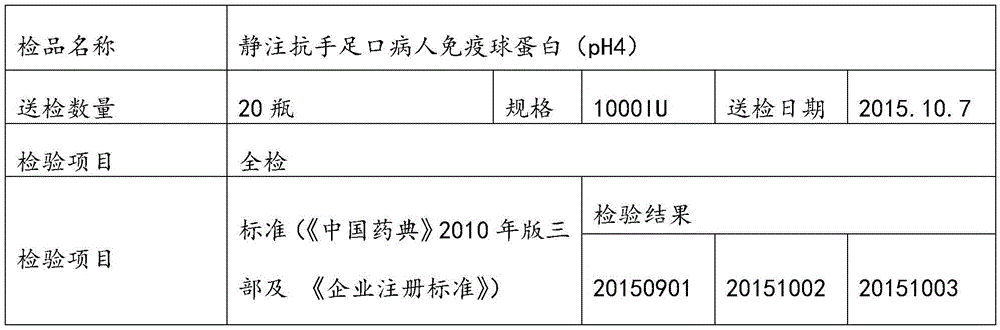
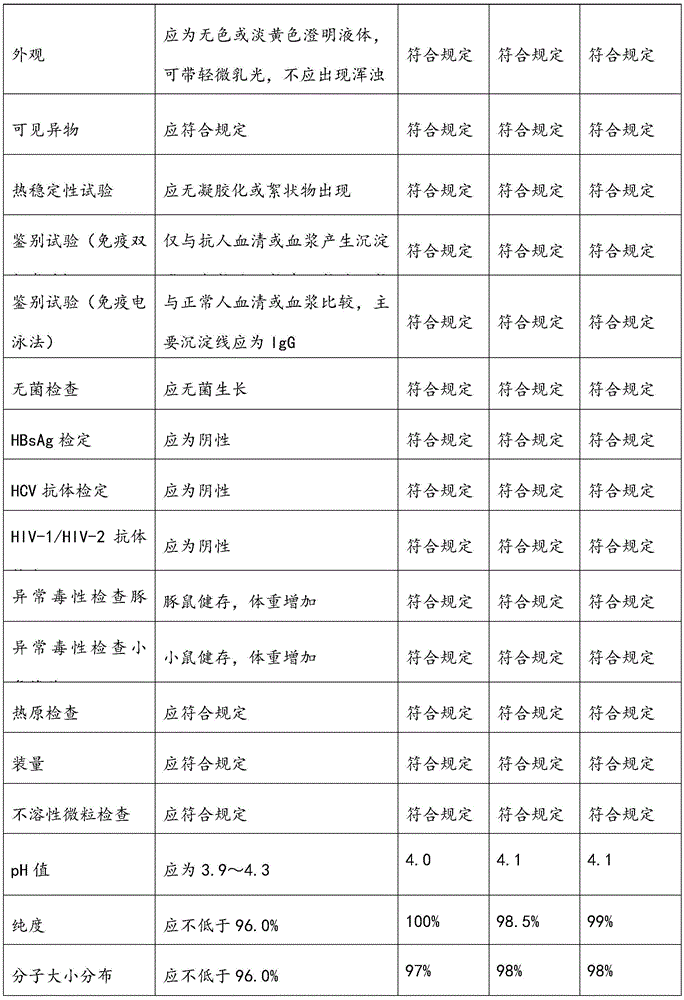
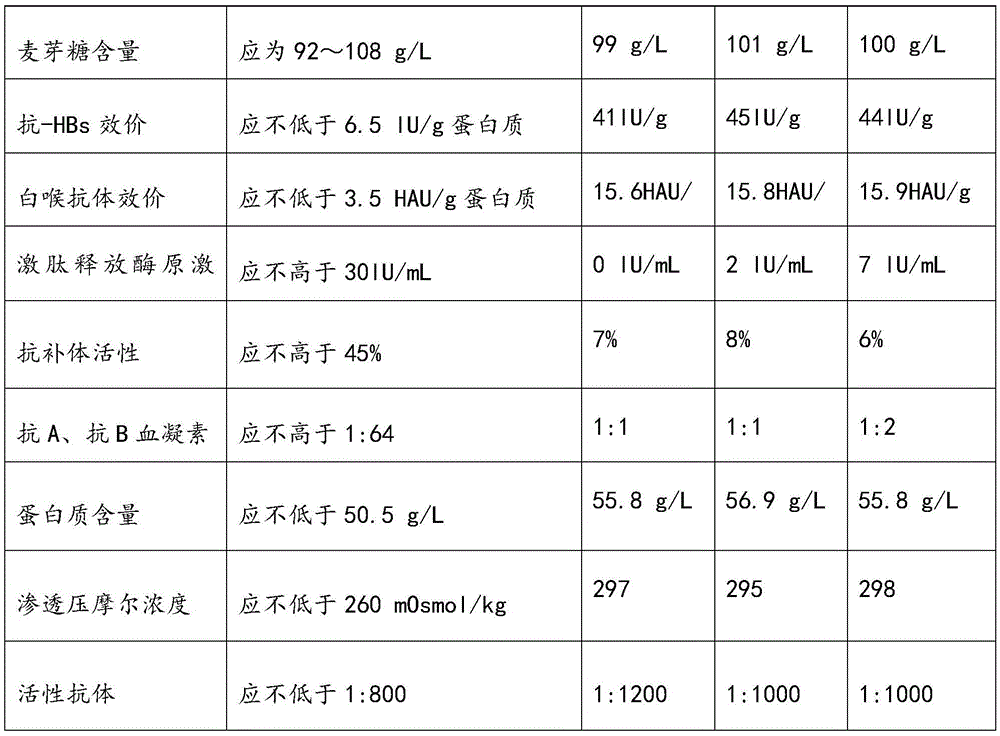
[0058] Select plasma, and use the neutralization test method to detect the neutralization titer of anti-hand-foot-mouth disease antibodies in the raw plasma should meet the following conditions: the neutralization titer is not less than 1:80; the protein content (detected by the biuret method) is greater than or equal to 55g / L; Alanine aminotransferase (monitored by Lai's method) is not higher than 35 units; ELISA method is negative for syphilis, hepatitis B surface antigen, HIV-1 / HIV-2 antibodies, and HCV antibodies. Frozen storage at low temperature below -20℃, and the shelf life is not more than 2 years.
[0059] 2. Neutralization experiment method to determine antibody titer in raw plasma:
[0060] (A) Pre-dilute the plasma sample with the cell maintenance solution (DMEM cell culture medium containing 3% fetal bovine serum), inactivate it at 56°C for 30 minutes, and then continue to dilute it by 10 times, namely The ...
Embodiment 2
[0089] 1. Plasma screening and collection of plasma donors:
[0090] Select plasma, and use the neutralization test method to detect the neutralization titer of anti-hand-foot-mouth disease antibodies in the raw plasma should meet the following conditions: the neutralization titer is not less than 1:80; the protein content (detected by the biuret method) is greater than or equal to 55g / L; Alanine aminotransferase (monitored by Lai's method) is not higher than 35 units; ELISA method is negative for syphilis, hepatitis B surface antigen, HIV-1 / HIV-2 antibodies, and HCV antibodies. Frozen storage at low temperature below -20℃, and the shelf life is not more than 2 years.
[0091] 2. Neutralization experiment method to determine antibody titer in raw plasma:
[0092] (A) Pre-dilute the plasma sample with the cell maintenance solution (DMEM cell culture medium containing 3% fetal bovine serum), inactivate it at 56°C for 30 minutes, and then continue to dilute it by 10 times, namely The ...
Embodiment 3
[0113] 1. Plasma screening and collection of plasma donors:
[0114] Select plasma, and use the neutralization test method to detect the neutralization titer of anti-hand-foot-mouth disease antibodies in the raw plasma should meet the following conditions: the neutralization titer is not less than 1:80; the protein content (detected by the biuret method) is greater than or equal to 55g / L; Alanine aminotransferase (monitored by Lai's method) is not higher than 35 units; ELISA method is negative for syphilis, hepatitis B surface antigen, HIV-1 / HIV-2 antibodies, and HCV antibodies. Frozen storage at low temperature below -20℃, and the shelf life is not more than 2 years.
[0115] 2. Neutralization experiment method to determine antibody titer in raw plasma:
[0116] (A) Pre-dilute the plasma sample with the cell maintenance solution (DMEM cell culture medium containing 3% fetal bovine serum), inactivate it at 56°C for 30 minutes, and then continue to dilute it by 10 times, namely The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com