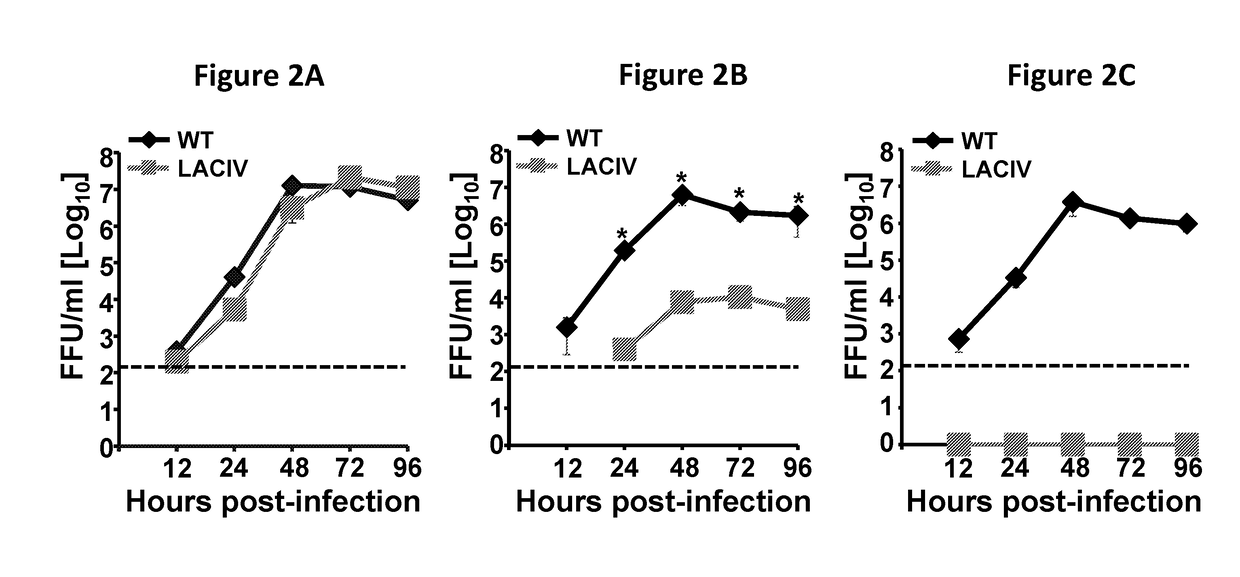
Patents
Literature
62 results about "Canine influenza" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Canine influenza (dog flu) is influenza occurring in canine animals. Canine influenza is caused by varieties of influenzavirus A, such as equine influenza virus H3N8, which was discovered to cause disease in canines in 2004. Because of the lack of previous exposure to this virus, dogs have no natural immunity to it. Therefore, the disease is rapidly transmitted between individual dogs. Canine influenza may be endemic in some regional dog populations of the United States. It is a disease with a high morbidity (incidence of symptoms) but a low incidence of death.
Vaccines and methods to treat canine influenza
The present invention relates to providing new vaccines and treatments for the diseases related to canine influenza virus. It discloses influenza viral antigens, and methods of presenting these antigens to canines, especially dogs. It relates to attenuated and killed vaccines. The present invention relates to experimentally generated canine and equine influenza viruses. The invention also includes influenza A, including H3, N8, H3N8, H7N7 and viruses which contain at least one genome segment from an canine or equine influenza virus. The present invention also relates to the use of these viruses in therapeutic compositions to protect canines, dogs in particular, from diseases caused by influenza viruses.
Owner:ZOETIS SERVICE LLC
Canine influenza virus
ActiveUS7682619B2Avoid seizuresAvoid it happening againSsRNA viruses negative-senseVirus peptidesHemagglutininCanine influenza
The present invention relates to an isolate canine influenza virus. The present invention relates to an isolated nucleic acid molecule encoding a hemagglutinin from a canine influenza virus. The present invention also relates to the protein or polypeptide encoded by the isolated nucleic acid molecule. Vaccines and detection and treatment methods relating to canine influenza viruses are also disclosed.
Owner:CORNELL RES FOUNDATION INC
Canine influenza virus
ActiveUS20070253981A1Avoid seizuresAvoid it happening againSsRNA viruses negative-senseVirus peptidesHemagglutininCanine influenza
The present invention relates to an isolate canine influenza virus. The present invention relates to an isolated nucleic acid molecule encoding a hemagglutinin from a canine influenza virus. The present invention also relates to the protein or polypeptide encoded by the isolated nucleic acid molecule. Vaccines and detection and treatment methods relating to canine influenza viruses are also disclosed.
Owner:CORNELL RES FOUNDATION INC
Compositions and methods for the treatment of canine influenza virus disease
InactiveUS20070092537A1SsRNA viruses negative-senseViral antigen ingredientsEquine influenza virusVirus diseases
Compositions, including vaccine compositions, and methods for treating, preventing or ameliorating canine influenza virus (CIV) disease by utilizing one or more canine influenza virus (CIV) or equine influenza virus (EIV) strain or immunogens thereof are set forth herein. Also set forth are challenge models useful in assessing the efficacy of a composition against canine influenza virus, comprising an equine influenza virus (EIV) or canine influenza virus (CIV) strain or immunogens thereof.
Owner:WYETH LLC
Canine influenza virus monoclonal antibody hybridoma cell strain F112 and application thereof
ActiveCN107034197AStrong antiviral activityRapid test diagnosisImmunoglobulins against virusesAntiviralsLymphocyteTiter
The invention relates to canine influenza virus monoclonal antibody hybridoma cell strain F112 and belongs to the technical field of biology. Immune antigens are prepared by using canine influenza virus epidemic strain A / Canine / Nanjing / 11 / 2012 (H3N2) through lymphocyte hybridoma technique, the efficient hybridoma cell strain F112 is screened out from the antigens, and the canine influenza virus monoclonal antibody hybridoma cell strain F112 has hemagglutination inhibition titer of 212 and neutralizing titer of 105 for canine influenza virus. A canine influenza preventive therapeutic agent prepared by using F112 monoclonal antibody is used for preventing and treating canine influenza, under the effective rate of 100%. The efficient F112 monoclonal antibody is applicable to the detection and diagnosis of canine influenza and the preventive therapy, and has a promising application prospect.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Canine influenza virus and related compositions and methods of use
The present invention provides an isolated canine influenza virus of subtype H3N8 comprising an HA having SEQ ID NO: 4 or an amino acid sequence that is greater than 99% identical to SEQ ID NO: 4 , with the proviso that the amino acids at positions 94 and 233 are identical to SEQ ID NO: 4; a composition comprising attenuated or inactivated virus; isolated or purified HA, NM, NP, M1 , NS1 , PA, PB1 , and PB2 proteins and fragments thereof and compositions comprising same or nucleic acids, optionally as part of a vector, encoding same; and a method of inducing an immune response to canine influenza virus in an animal comprising administering to the animal an aforementioned composition.
Owner:IOWA STATE UNIV RES FOUND
Canine influenza virus and related compositions and methods of use
ActiveUS20070098742A1SsRNA viruses negative-sensePeptide/protein ingredientsImmunity responseHypotype
The present invention provides an isolated canine influenza virus of subtype H3N8 comprising an HA having SEQ ID NO: 4 or an amino acid sequence that is greater than 99% identical to SEQ ID NO: 4, with the proviso that the amino acids at positions 94 and 233 are identical to SEQ ID NO: 4; a composition comprising attenuated or inactivated virus; isolated or purified HA, NM, NP, M1, NS1, PA, PB1, and PB2 proteins and fragments thereof and compositions comprising same or nucleic acids, optionally as part of a vector, encoding same; and a method of inducing an immune response to canine influenza virus in an animal comprising administering to the animal an aforementioned composition.
Owner:IOWA STATE UNIV RES FOUND
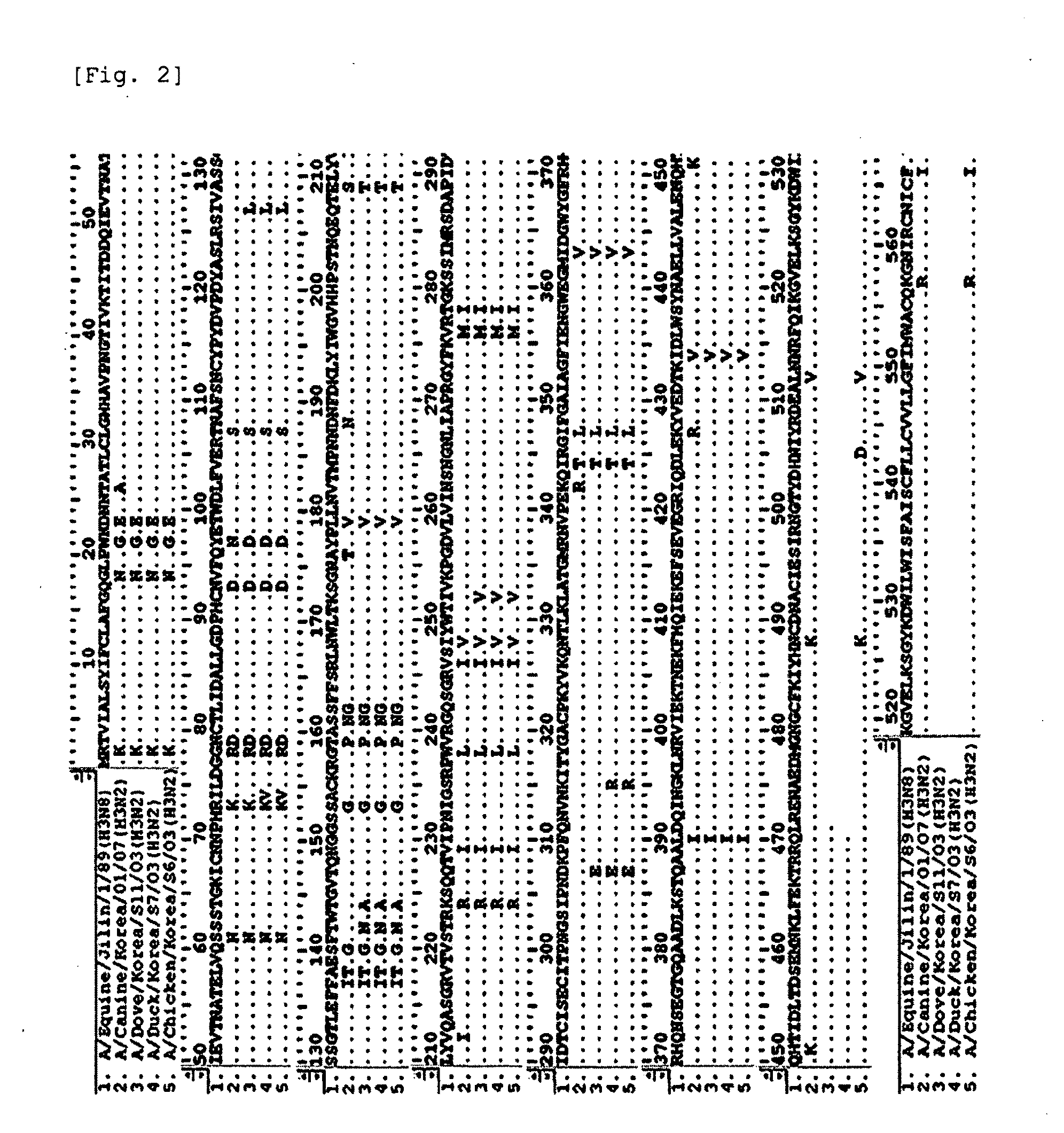
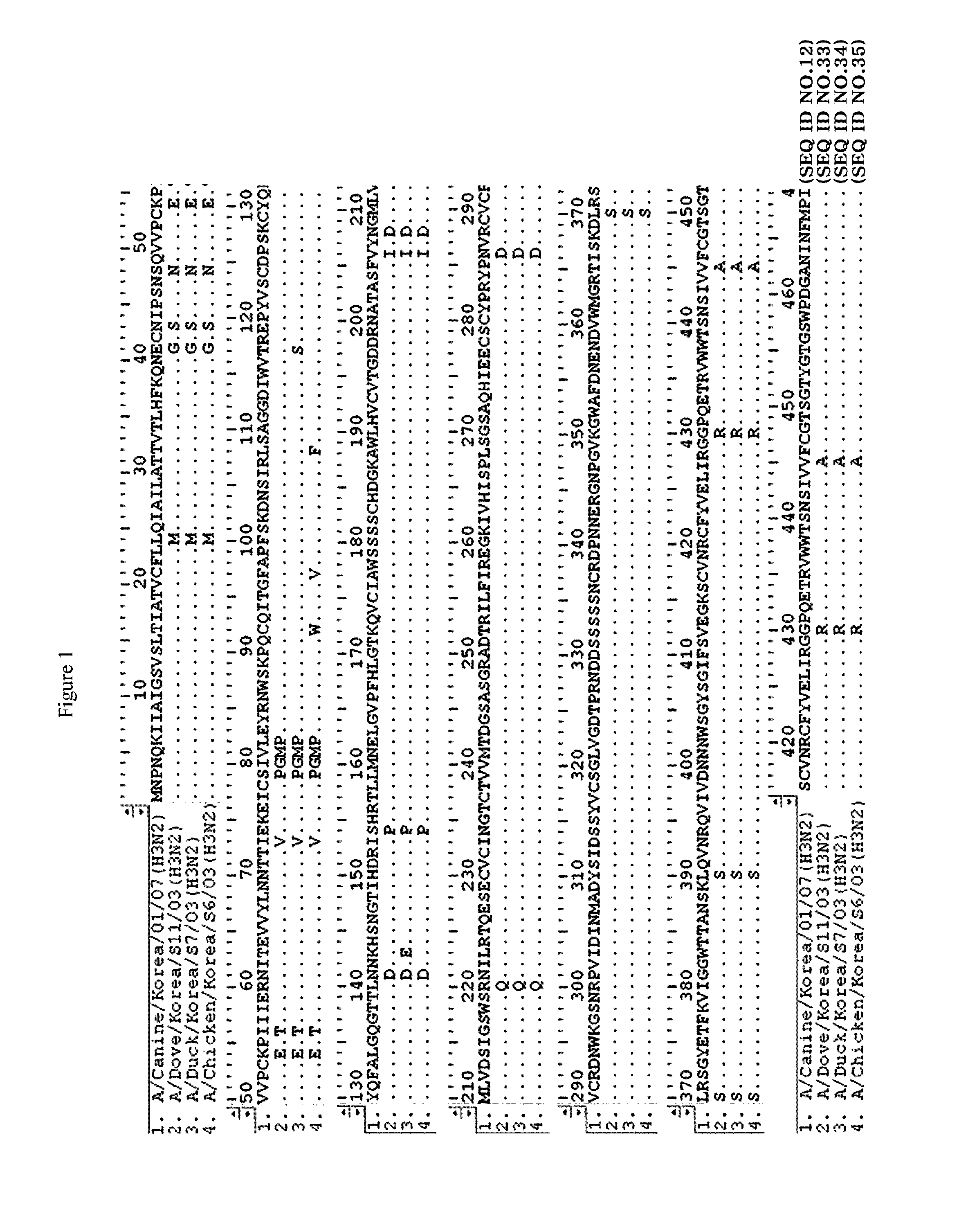
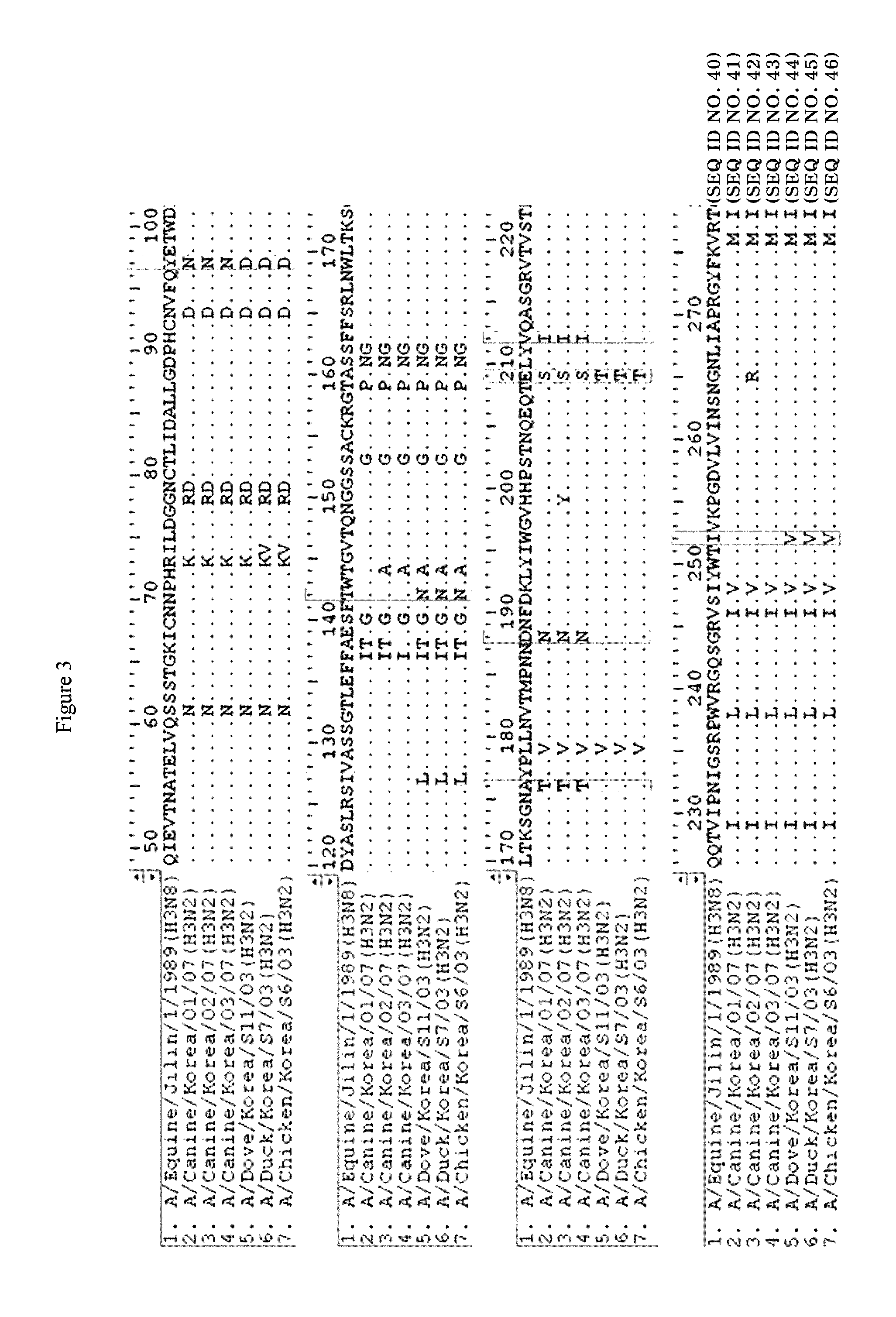
Novel canine influenza virus and vaccine therefore
Novel influenza viruses A / Canine / Korea / 01 / 07 (H3N2), A / Canine / Korea / 02 / 07 (H3N2) and A / Canine / Korea / 03 / 07 (H3N2) are disclosed. A vaccine composition comprising at least one of the viruses, a method for preventing or treating diseases resulting from influenza virus infection by administering the vaccine composition, and an assay kit for detecting the viruses are also disclosed.
Owner:BIONOTE INC
Method for producing influenza virus vaccine
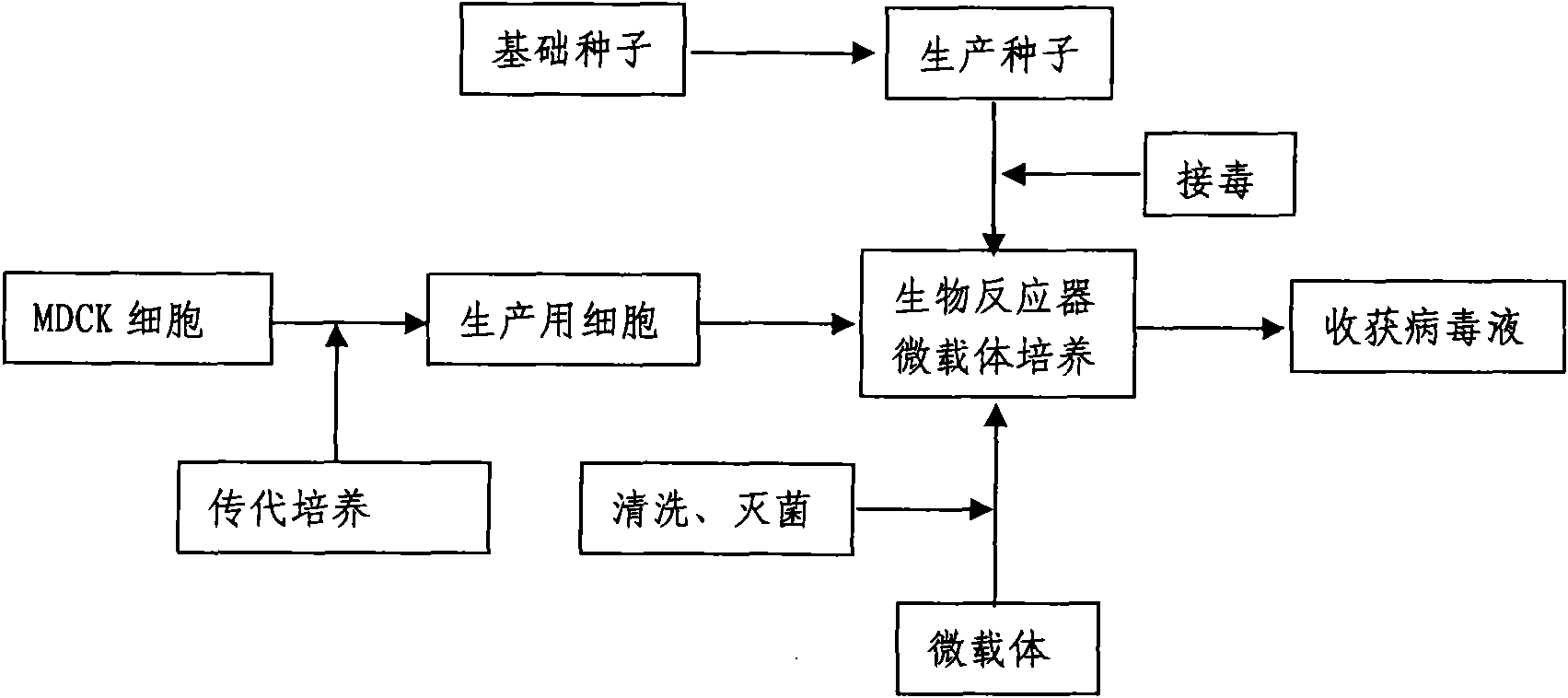
ActiveCN101955915ASolve pollutionGuaranteed to be pureAntiviralsViruses/bacteriophagesCanine kidneyEquine influenza vaccine
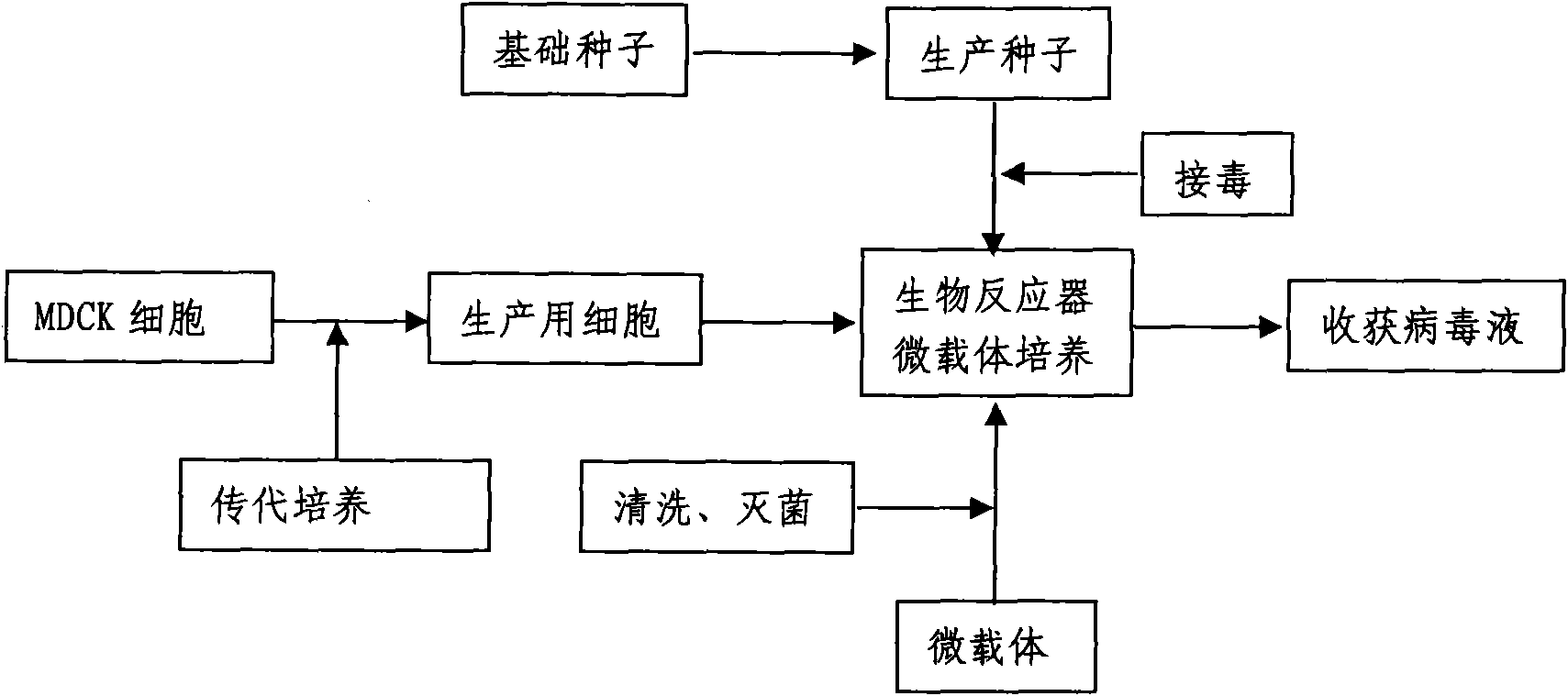
The invention discloses a method for producing a vaccine for avian influenza viruses and other influenza viruses such as a swine influenza virus, a canine influenza virus and an equine influenza virus. The method comprises the following steps of: subculturing cells for preparing the vaccine; (2) reproducing cytotoxic varieties; (3) performing microcarrier suspension culture on darby canine kidney (MDCK) cells in a bioreactor; (4) reproducing venom for preparing the vaccine; and (5) harvesting virus liquid. The method has the advantages of great reduction in production cost, short production period, no restriction to raw material supply, small occupied area, easy and quick expansion in production scale, low and readily treated environmental pollution, high degree of automation, few labors, easy equilibrium and stabilization in quality and obvious improvement on the yield and the quality of the vaccines.
Owner:成都史纪生物制药有限公司
Application of H3N2 canine influenza virus CGD1
ActiveCN103223162AReduce severityShorten the detox periodAntiviralsAntibody medical ingredientsTGE VACCINEMicrobiological culture
The invention belongs to the technical field of preparation of virus vaccine, and concretely discloses an application of an H3N2 canine influenza virus CGD1. According to the invention, three self-isolated, identified and preserved strains of H3N2 canine influenza virus CGD1, CGD2 and CGD3 are used to inoculate chick embryo for subcultring, and the CGD1 is found to be good in genetic stability. Therefore, the CGD1 is selected for plaque purification to breed an H3N2 canine influenza virus vaccine strain CIVGDYM1, and the vaccine strain has been preserved in China general microbiological culture collection center, with an accession number being CGMCCNO: 7218. By using the H3N2 canine influenza virus vaccine strain CIVGDYM1 to inoculate the chick embryo for virus breeding, and through gaining allantoic fluid of the chick embryo, inactivating, preparing vaccine and other steps, the safe and effective H3N2 canine influenza virus inactivated vaccine can be prepared.
Owner:SOUTH CHINA AGRI UNIV
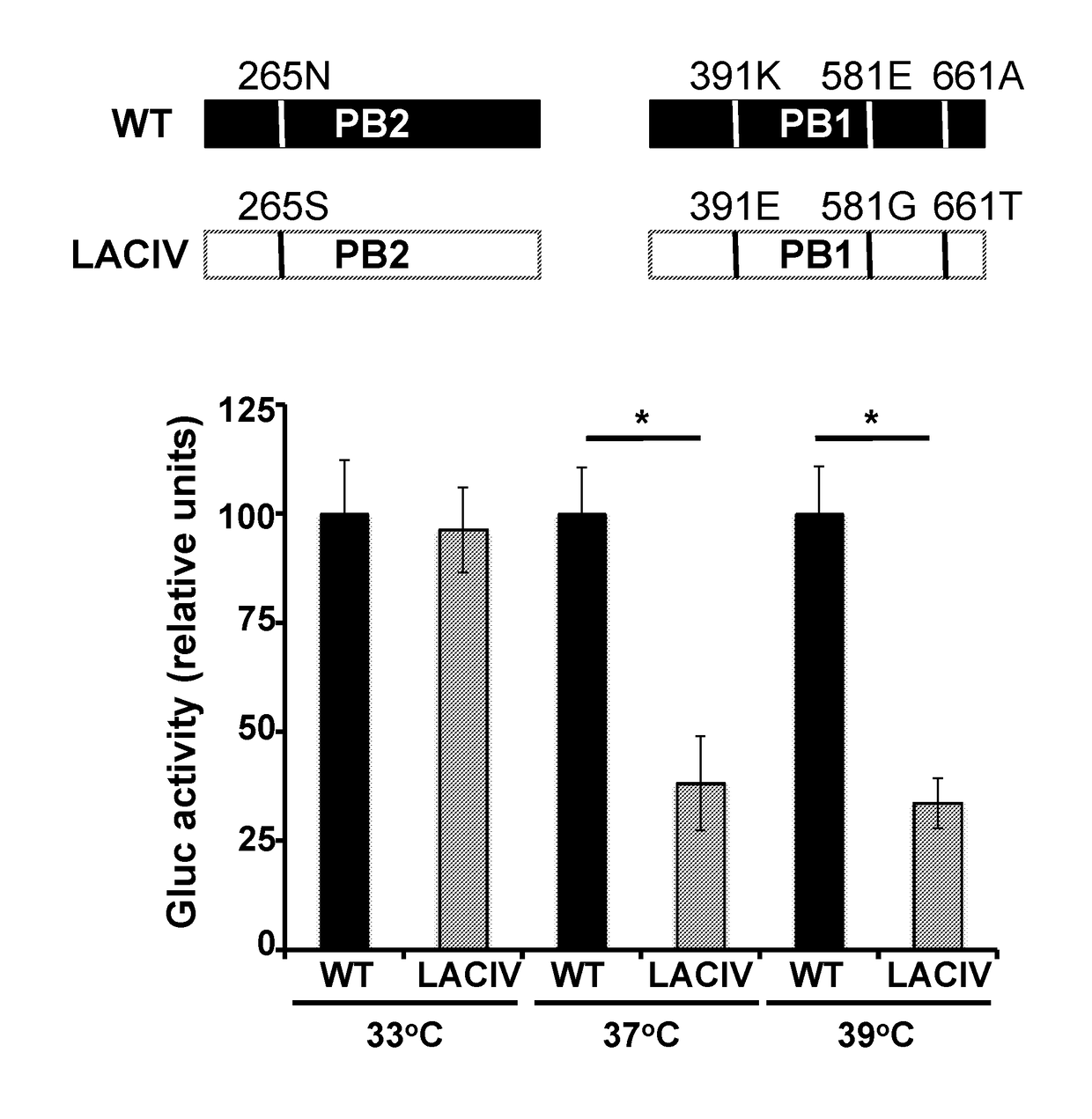
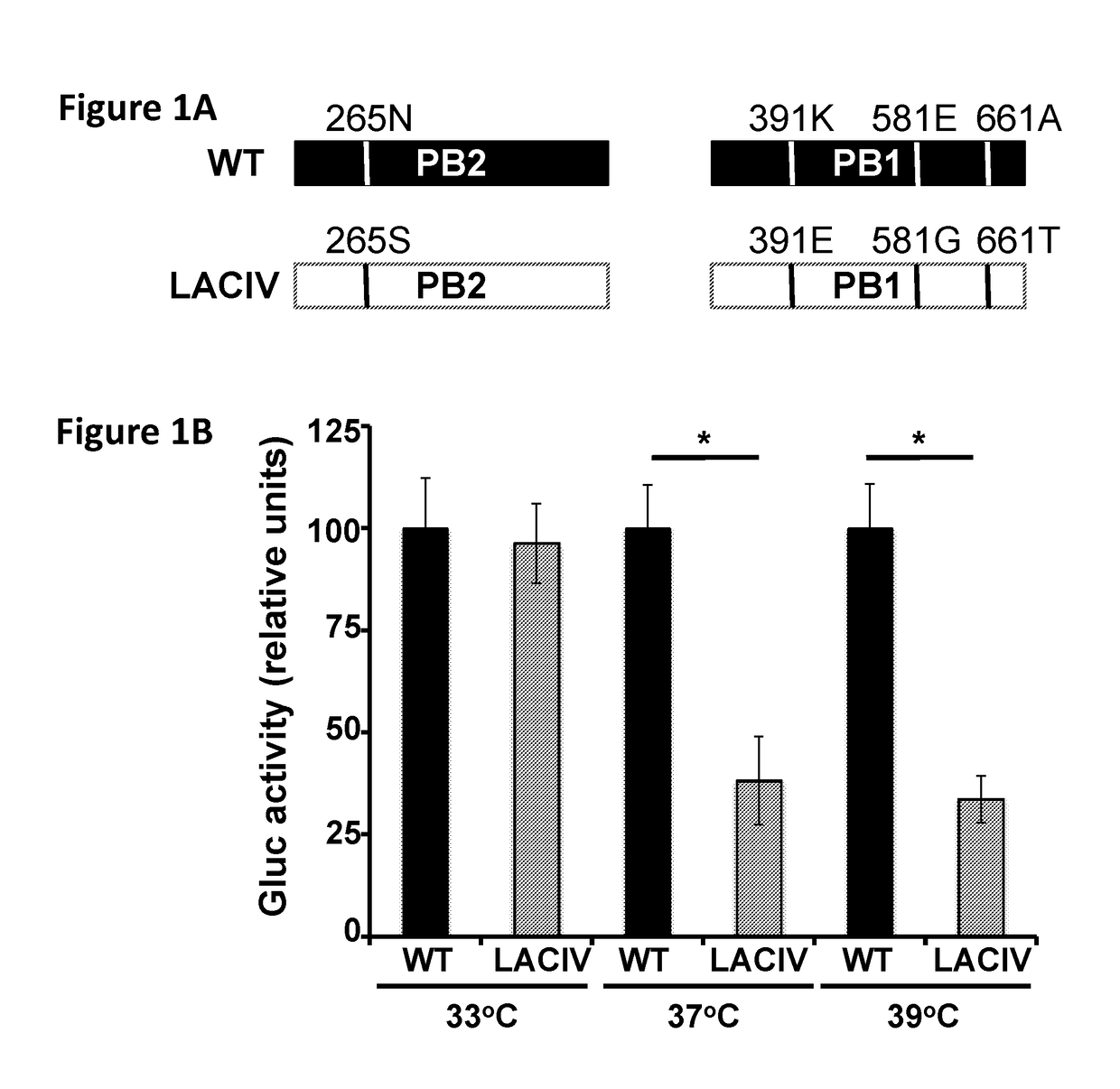
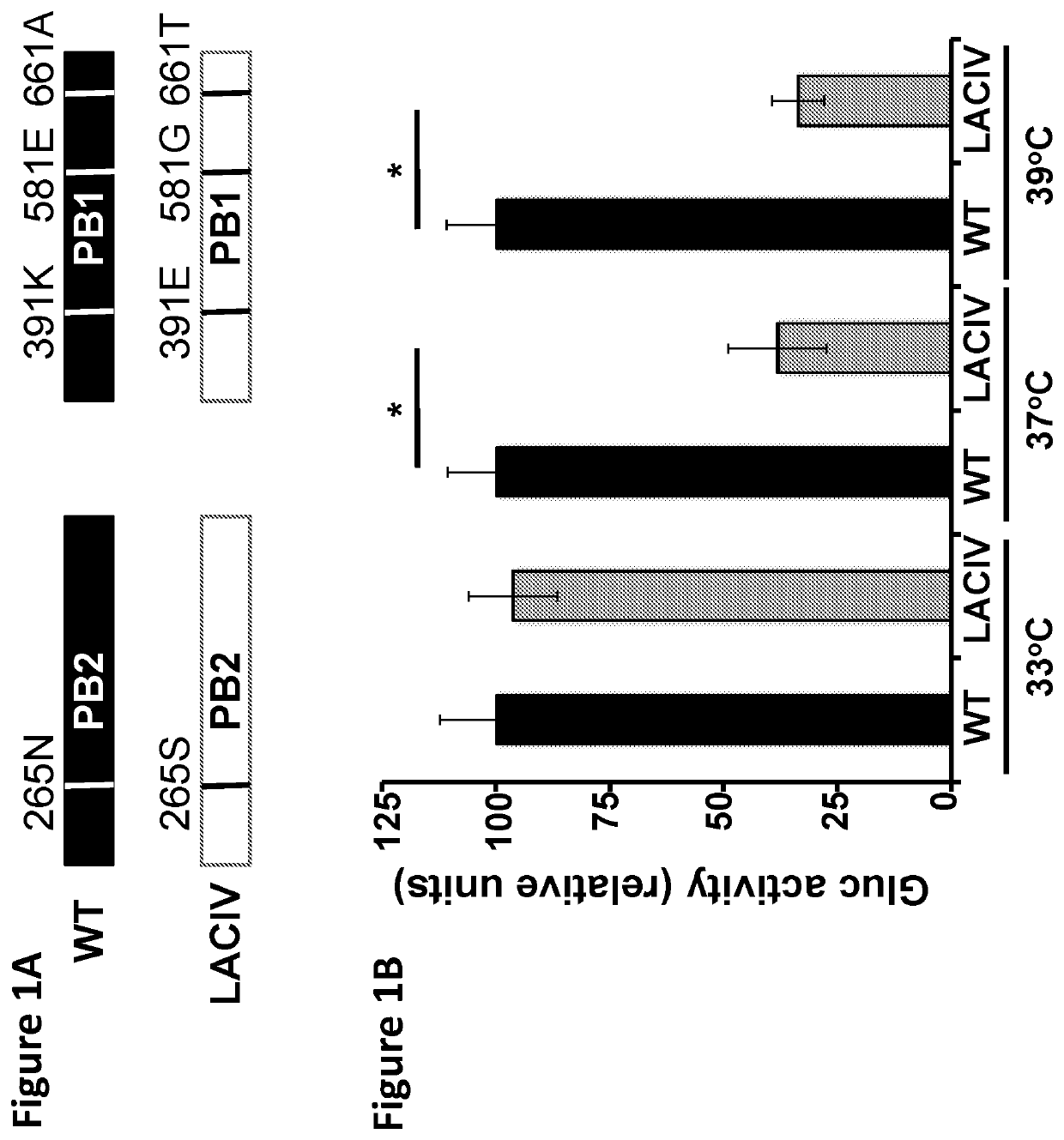
Live-Attenuated Vaccine Having Mutations in Viral Polymerase for the Treatment and Prevention of Canine Influenza Virus
ActiveUS20180243401A1SsRNA viruses negative-senseViral antigen ingredientsVirus influenzaPolymerase L
The present invention relates to compositions and methods for the treatment and prevention of canine influenza virus (CIV) and CIV-related pathology. The present invention is based in part upon the discovery that various mutations in segment 1 and segment 2 of the CIV genome, thereby encoding mutant PB2 and PB1 protein, render the virus to be temperature-sensitive.
Owner:CORNELL UNIVERSITY +1
Canine influenza virus and vaccine therefore
Novel influenza viruses A / Canine / Korea / 01 / 07 (H3N2), A / Canine / Korea / 02 / 07 (H3N2) and A / Canine / Korea / 03 / 07 (H3N2) are disclosed. A vaccine composition comprising at least one of the viruses, a method for preventing or treating diseases resulting from influenza virus infection by administering the vaccine composition, and an assay kit for detecting the viruses are also disclosed.
Owner:BIONOTE INC
Shuttling intracellular antibody TAT-4F for H3N2-type canine influenza virus
ActiveCN108728461AInterfere with copyingPlay an antiviral rolePolypeptide with localisation/targeting motifAntiviralsHemagglutininVirus influenza
The invention discloses a shuttling intracellular antibody TAT-4F for H3N2-type canine influenza virus. The H3N2 virus is A-type influenza virus, the main neutralizing antibody is from hemagglutinin (HA), therefore, the HA becomes an original main research target; then the influenza virus has high variability, the immune cross reaction among different variable branches is weaker, M1 antibody can be combined with M1 protein to inhibit the activity and disturb multiplication, transcription and release of the influenza virus so as to play a role in resisting the virus; therefore, the M1 protein is selected for preparing the corresponding antibody to obtain stable titer, coupling the antibody with TAT protein PTD (Protein Transduction Domain) capable of transducing biomacromolecules into cellsand expressing fusion protein TAT PTD-M1 ScFv, and then a shuttling antibody for resisting the canine influenza virus is prepared to provide a new path for treating the canine influenza virus.
Owner:ACAD OF MILITARY SCI PLA CHINA ACAD OF MILITARY MEDICAL SCI INST OF MILITARY VETERINARY MEDICINE
Establishment method of monoclonal antibody hybridoma cell strain for H3N2 canine influenza virus and preparation method and application of monoclonal antibody of monoclonal antibody hybridoma cell strain
ActiveCN104911150AHigh affinityImprove securityImmunoglobulins against virusesMicroorganism based processesHemagglutininVaccination
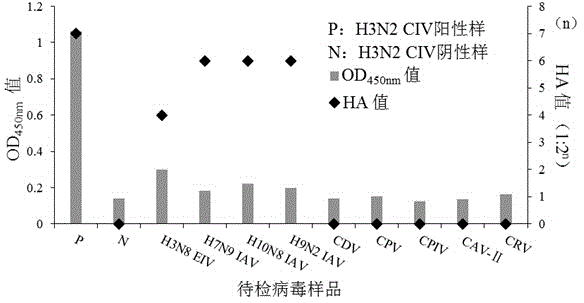
The invention relates to the technical field of cell engineering and discloses an establishment method of a monoclonal antibody hybridoma cell strain for an H3N2 canine influenza virus and a preparation method and application of a monoclonal antibody of the monoclonal antibody hybridoma cell strain. The monoclonal antibody hybridoma cell strain is preserved in China Center for Type Culture Collection on May 13, 2015; the preservation number is CCTCC C201555. The monoclonal antibody secreted by the monoclonal antibody hybridoma cell strain is combined with specificity of hemagglutinin of the H3N2 canine influenza virus and is high in appetency; the titer after purification is 1: 320,000, and the monoclonal antibody does not react with CDV, CPV, CPIV, CAV-II, CRV, H7N9, H10N8 or H9N2 subtype influenza viruses. By injecting the monoclonal antibody used for preventing and controlling the infection of the influenza viruses, compared with a traditional vaccination method, the method is higher in specificity, lower in risk and higher in safety, and has the actual practical value.
Owner:SOUTH CHINA AGRI UNIV
Single-Cycle Virus for the Development of Canine Influenza Vaccines
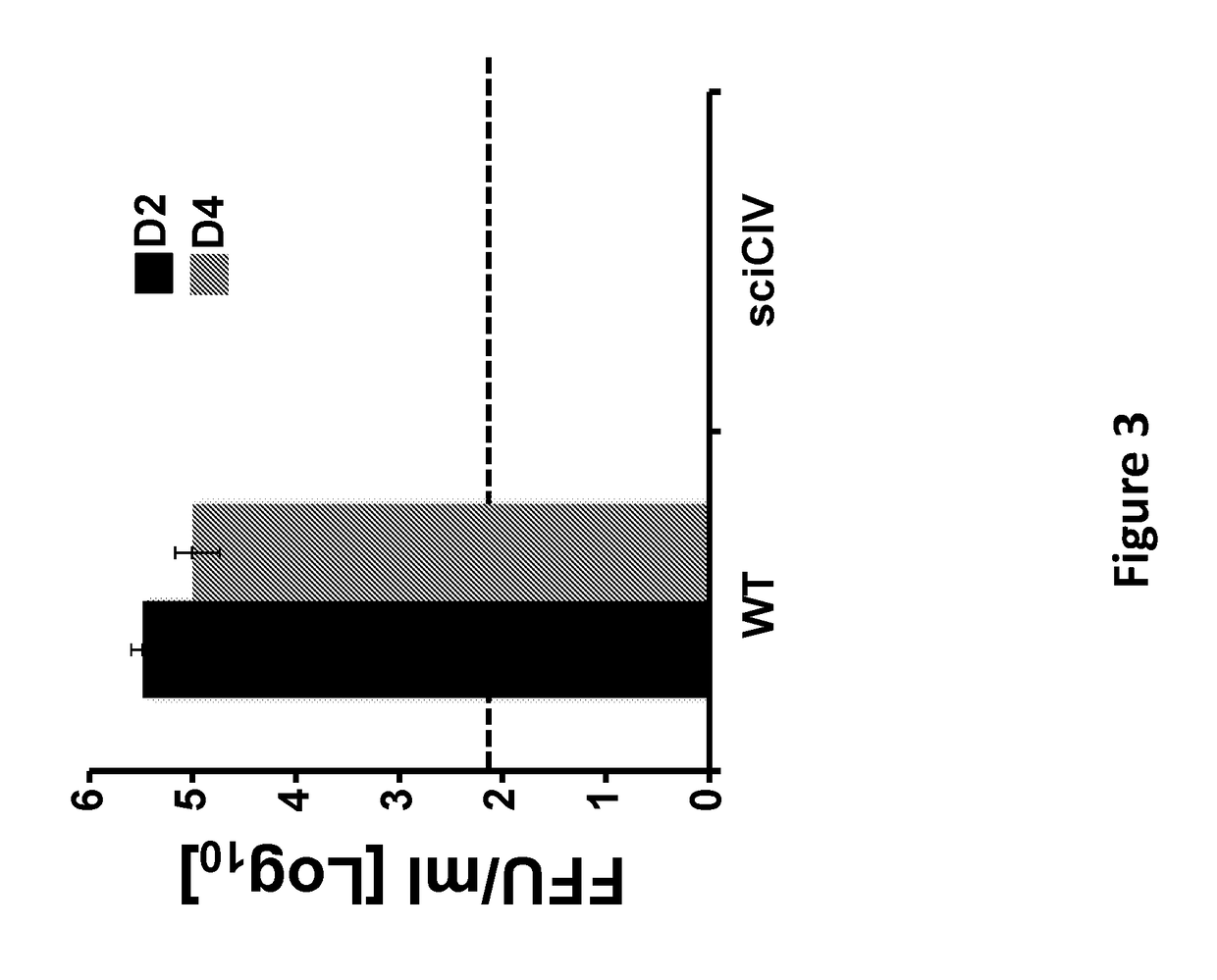
The present invention relates to compositions and methods for the treatment and prevention of canine influenza virus (CIV) and CIV-related pathology. The present invention is based in part upon the discovery that one or more mutations in segment 4 of the viral genome produces a single cycle infectious CIV (sciCIV). The sciCIV does not allow for the production of infectious progeny, but is able to induce a CIV-specific immune response.
Owner:CORNELL UNIVERSITY +1
Primer composition for detecting different influenza viruses and application thereof
ActiveCN104131009AEasy to operateWide coverageMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceA-DNA
The invention discloses a primer composition for detecting different influenza viruses and an application thereof. The primer composition disclosed by the invention comprises any one of primer pairs represented by the following (1)-(4): (1) a DNA molecule shown in SEQ ID No.1 and a DNA molecule shown in SEQ ID No.2; (2) a DNA molecule shown in SEQ ID No.3 and a DNA molecule shown in SEQ ID No.4; (3) a DNA molecule shown in SEQ ID No.5 and a DNA molecule shown in SEQ ID No.6; and (4) a DNA molecule shown in SEQ ID No.7 and a DNA molecule shown in SEQ ID No.8. The primer composition has positive meanings on timely diagnosing and timely treating pathogenetic canine, timely forecasting epidemic trends, preventing influenza large pandemic, and implementing large-scale canine influenza virus epidemiology monitoring.
Owner:CHINA AGRI UNIV
RT-LAMP primer combination and kit for detecting canine influenza virus as well as application of RT-LAMP primer combination
PendingCN105821158AHigh sensitivityAvoid pollutionMicrobiological testing/measurementMicroorganism based processesAgricultural scienceVirus
The invention discloses an RT-LAMP primer combination and kit for detecting canine influenza virus as well as an application of the RT-LAMP primer combination. The LAMP primer combination consists of a forward outer primer F3 as shown in SEQ ID NO.1, a reverse outer primer B3 as shown in SEQ ID NO.2, a forward inner primer FIP as shown in SEQ ID NO.3, a reverse inner primer BIP as shown in SEQ ID NO.4 and a loop primer LB as shown in SEQ ID NO.5. The LAMP primer combination is applied to the detection of canine influenza virus. With the adoption of the screened LAMP primer combination for detecting canine influenza virus provided by the invention, a method for detecting canine influenza virus through LAMP is established, and the method has the advantages of being high in specificity, high in sensitivity, quick, simple, convenient and free of special instruments.
Owner:NANJING POLICE DOG RES INST OF MINIST OF PUBLIC SECURITY
Method for detecting canine influenza
InactiveCN101838708AEnough detection analysisIncreased sensitivityMicrobiological testing/measurementBiotechnologyElectrophoresis
The invention belongs to the field of biotechnology and particularly relates to a method for detecting canine influenza. In the invention, a PCR detection method is adopted, and a PCR reaction system comprises the following materials: primers having sequences SEQ ID No.1 and SEQ ID No.2 respectively, as well as dNTPs, Ex Taq, 10*Ex Taq buffer and cDNA templates. At the end of a PCR reaction, whether a target strip exists is detected by electrophoresis and ultra-violet analysis, so as to determine if a dog catches canine influenza. The detection method provided by the invention has high sensitivity and specificity, is simple, convenient and quick in operation, has low requirements on the quality of initial materials and is convenient to use in clinic.
Owner:SOUTH CHINA AGRI UNIV
RT-PCR kit for detection of poultry source pedigree H3N2 subtype canine influenza virus and application thereof
ActiveCN103409559AFast identification testIncrease coverageMicrobiological testing/measurementMicroorganism based processesNucleotideNucleotide sequencing
The invention discloses an RT-PCR kit for detection of poultry source pedigree H3N2 subtype canine influenza virus and application thereof. The invention provides a primer pair for assisting detection of poultry source pedigree H3N2 subtype canine influenza virus. The nucleotide sequence of one primer is shown as a SEQ ID No.1, and the nucleotide sequence of the other primer is shown as a SEQ ID No. 2. Detection of poultry source pedigree H3N2 subtype canine influenza virus by the kit provided by the present invention is faster, and has higher singularity, sensibility and sensitivity than a traditional method. The kit can detect virus allantoic fluid as well as clinically acquired samples, and is easy for popularization as a useful tool for diagnosis and epidemiological investigation of poultry source pedigree H3N2 subtype canine influenza virus.
Owner:CHINA AGRI UNIV
H3N2 and H3N8 subgenetype dog influenza divalent inactivated vaccine and preparation method and application thereof
ActiveCN110680912ASsRNA viruses negative-senseViral antigen ingredientsVirus influenzaVaccine antigen
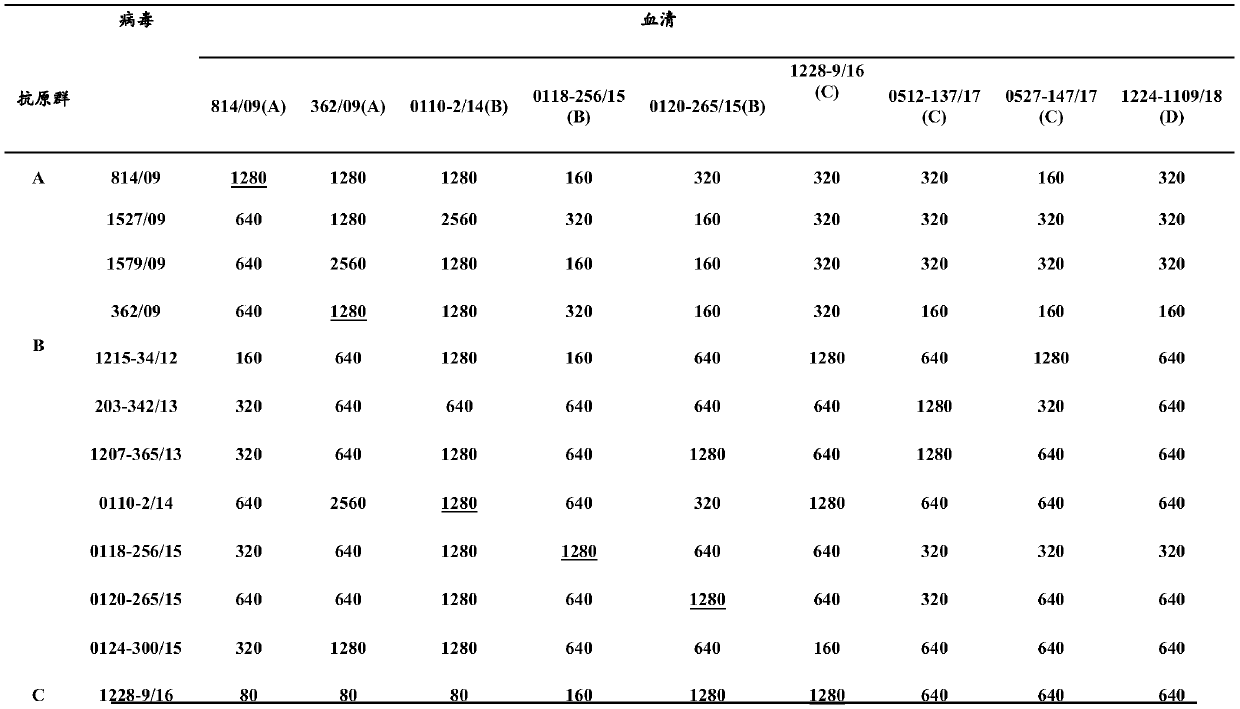
The invention discloses an H3N2 and H3N8 subgenetype dog influenza divalent inactivated vaccine and a preparation method and application thereof, and provides a dog influenza virus vaccine. The vaccine comprises a vaccine antigen which is totivirus bivalent inactivated vaccine antigen consisting of (1) or (2): (1) A / canine / Quanzhou / 1224-1109 / 2018(H3N2)CGMCCNO.18180 and an H3N8 subgenetype dog influenza virus influenza virus strain; and (2)a recombinant virus bivalent inactivated vaccine antigen consisting of a recombinant H3N2 influenza virus obtained by A / canine / Quanzhou / 1224-1109 / 2018(H3N2)CGMCC and a recombinant H3N8 subgenetype influenza virus strain obtained by the H3N8 subgenetype influenza virus strain. The H3N2 and H3N8 subgenetype dog influenza totivirus bivalent inactivated vaccine and the recombinant virus(H3N2 / PR8+H3N8 / PR8) bivalent inactivated vaccine both have favorable protective effects on different branch H3N2 subgenetype dog influenza virus and H3N8 subgenetype dog influenza virus.
Owner:CHINA AGRI UNIV
Compound Bupleurum injection for curing common cold of dog and preparation thereof
InactiveCN101297826AControl secondary infectionFast recoveryAntibacterial agentsOrganic active ingredientsDiseaseSulfite salt
The invention discloses a compound bupleurum injection for the treatment for canine influenza and a preparation method thereof, which aims at providing the compound bupleurum injection that can control the canine secondary infection at the same time of treating the canine influenza, have rapid onset of action, reduce the canine suffering and accelerate the canine healing speed, and the preparation method that has simple technology and easy realization. Each 100l of the injection contains: bupleurum extract liquid which is prepared by 50 to 200kg of bupleurum, 1 to 10kg of lincomycin hydrochloride, 0.05 to 1kg of chlorpheniramine, 0.2kg of sodium sulfite, 0.01kg of EDTA-2Na, 0.5kg of Tween-80 and the rest of water for injection. The injection of the invention adopts a compound preparation which combines the traditional Chinese medicine and the western medicine, the bupleurum and the chlorpheniramine can control the clinical symptoms caused by canine influenza and reduce the suffering caused by the canine influenza, the lincomycin hydrochloride can control the bacterial infection, the three are combined together for the usage, thus having rapid onset of action for the canine influenza and controlling the canine secondary infection, significantly ease the clinical symptoms caused by the canine influenza, treat both manifestation and root cause of disease and accelerate the canine recovery.
Owner:TIANJIN SHENGJI GRP CO LTD
Canine primary bronchial epithelial cell and application thereof in preparation of immortalized cells
ActiveCN104531607AHigh purityOvercome the disadvantage of very limited proliferative abilityArtificial cell constructsVertebrate cellsTelomeraseBeagle
The invention provides a canine primary bronchial epithelial cell and an application thereof in preparation of immortalized cells, and belongs to the technical field of biology. The canine primary bronchial epithelial cell is prepared by taking 45-day-old clean male beagles; separating canine tracheal tissues, cutting into pieces, adding collagenase and trypsin to digest, so as to obtain intact bronchiole tissues; cultivating the obtained cells, and further carrying out indirect immunofluorescence assay to detect keratin 18 expression, wherein an experiment proves that the canine primary bronchial epithelial cell is successfully obtained. A eukaryotic expression vector pEGFP-hTERT containing telomerase genes (hTERT) is built; after the canine primary bronchial epithelial cell is transfected, the hTERT gene is highly expressed; the bronchial epithelial cell can be stably subcultured for at least 20 generations. According to the canine primary bronchial epithelial cell provided by the invention, important biological materials are provided for research of infection mechanism of canine pathogens such as canine influenza viruses.
Owner:NANJING AGRICULTURAL UNIVERSITY
Live-Attenuated Vaccine Having Mutations in Viral Polymerase for the Treatment and Prevention of Canine Influenza Virus
The present invention relates to compositions and methods for the treatment and prevention of canine influenza virus (CIV) and CIV-related pathology. The present invention is based in part upon the discovery that various mutations in segment 1 and segment 2 of the CIV genome, thereby encoding mutant PB2 and PB1 protein, render the virus to be temperature-sensitive.
Owner:UNIV OF ROCHESTER +1
Kit for detecting H3N2 subtype canine influenza virus
ActiveCN104965083AHigh sensitivityImprove the detection rateBiological material analysisRepeatabilityImmunologic Technique
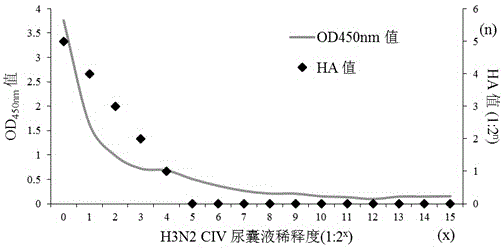
The invention relates to the technical field of immunology, and concretely discloses a kit for detecting an H3N2 subtype canine influenza virus. The kit includes a monoclonal antibody generated by a hybridoma cell strain H3N2-CIV-HA-2C5, and the hybridoma cell strain is preserved in China Center for Type Culture Collection on May 13, 2015 with the preservation number of CCTCC NO:C201555. The detection kit has the advantages of high sensitivity, strong specificity, very good repeatability and stability, clinic sample detection, realization of a detection rate being greatly higher than that of routine detection, and practical clinic application significance.
Owner:SOUTH CHINA AGRI UNIV
Live-attenuated vaccine having mutations in viral polymerase for the treatment and prevention of canine influenza virus
ActiveUS10478489B2SsRNA viruses negative-senseViral antigen ingredientsAvian influenza virusPolymerase L
The present invention relates to compositions and methods for the treatment and prevention of canine influenza virus (CIV) and CIV-related pathology. The present invention is based in part upon the discovery that various mutations in segment 1 and segment 2 of the CIV genome, thereby encoding mutant PB2 and PB1 protein, render the virus to be temperature-sensitive.
Owner:CORNELL UNIVERSITY +1
H3N2 subtype canine influenza virus inactivated vaccine, and preparation method and application thereof
InactiveCN103937752AImprove securityGood immune protectionAntiviralsViruses/bacteriophagesMicroorganismImmunogenicity
The invention discloses an H3N2 subtype canine influenza virus inactivated vaccine, and preparation method and application thereof, and also discloses an H3N2 subtype canine influenza virus strain used for preparing the inactivated vaccine, and the preparation method of the inactivated vaccine. The H3N2 subtype canine influenza virus strain is named as a CIV-HLJ strain, is preserved in China General Microbiological Culture Collection Center, and has a preservation number of CGMCC NO.8760. Results of safety and immunogenicity evaluation tests of the canine influenza inactivated vaccine prepared in the invention show that the vaccine is safe to canines, and can induce the canine to to generate an ideal immune protection effect. The vaccine provides an effective means for controlling H3N2 subtype canine influenza diseases.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Primer set for detecting American canine influenza virus subtype H3N8 and application thereof
InactiveCN106636475AStrong specificityIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesAgricultural scienceEpidemiologic survey
The invention discloses a primer set for detecting American canine influenza virus subtype H3N8 and application thereof. The primer set provided by the invention comprises a primer pair A and a primer pair B; the primer pair A is composed of a primer F1 and a primer R1; the primer pair B is composed of a primer F2 and a primer R2; the primer F1 is as shown in a sequence 1; the primer R1 is as shown in a sequence 2; the primer F2 is as shown in a sequence 3; and the primer R2 is as shown in a sequence 4. The application of the primer set comprises (c1) assistant identification of the American canine influenza virus subtype H3N8; and (c2) assistant identification of that whether a to-be-detected sample contains the American canine influenza virus subtype H3N8. The primer set has good specificity and high sensitivity when used for detection and can detect a clinical sample within 4 to 5 h; and the primer set is simple to operate, easy to promote and convenient for basic-level operation and application, and is of great application value to diagnosis and epidemiological investigation of diseases caused by the American canine influenza virus subtype H3N8.
Owner:CHINA AGRI UNIV
Vaccines and Methods to Treat Canine influenza
The present invention relates to providing new vaccines and treatments for the diseases related to canine influenza virus. It discloses influenza viral antigens, and methods of presenting these antigens to canines, especially dogs. It relates to attenuated and killed vaccines. The present invention relates to experimentally generated canine and equine influenza viruses. invention also includes influenza A, including H3, N8, H3N8, H7N7 and viruses which contain at least one genome segment from an canine or equine influenza virus. The present invention also relates to the use of these viruses in therapeutic compositions to protect canines, dogs in particular, from diseases caused by influenza viruses.
Owner:ZOETIS SERVICE LLC
PCR (Polymerase Chain Reaction) detection kit for cat and/or dog pathogens, detection method and application
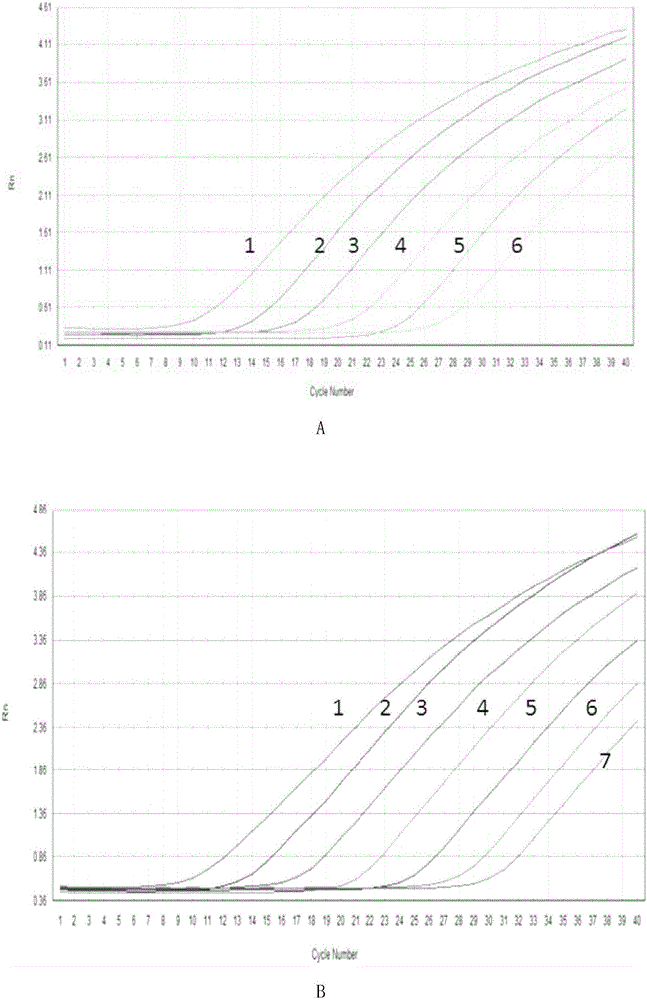
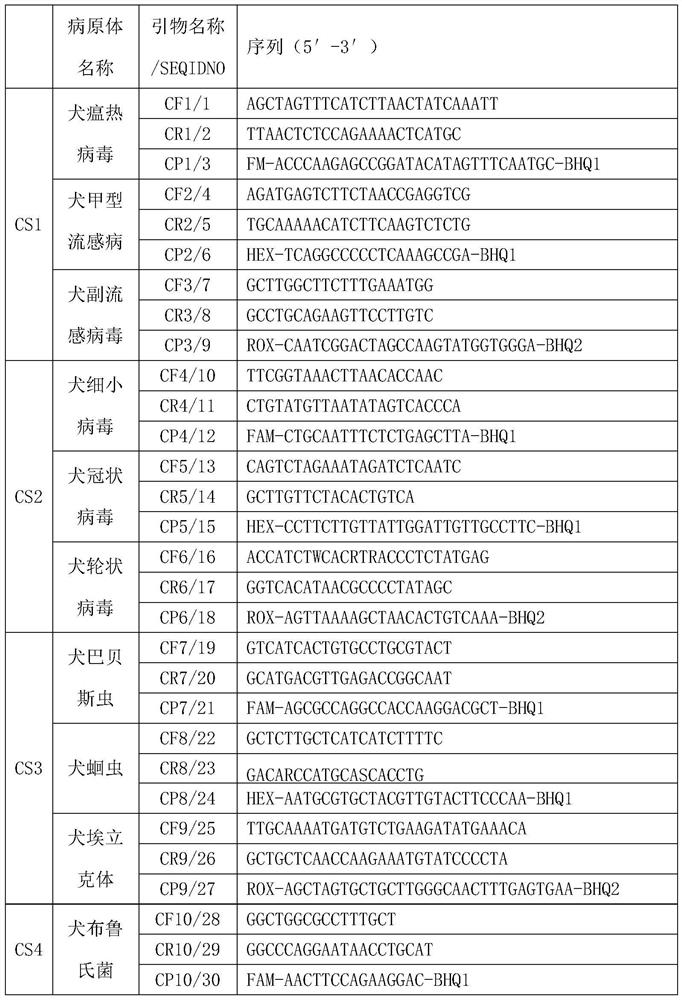
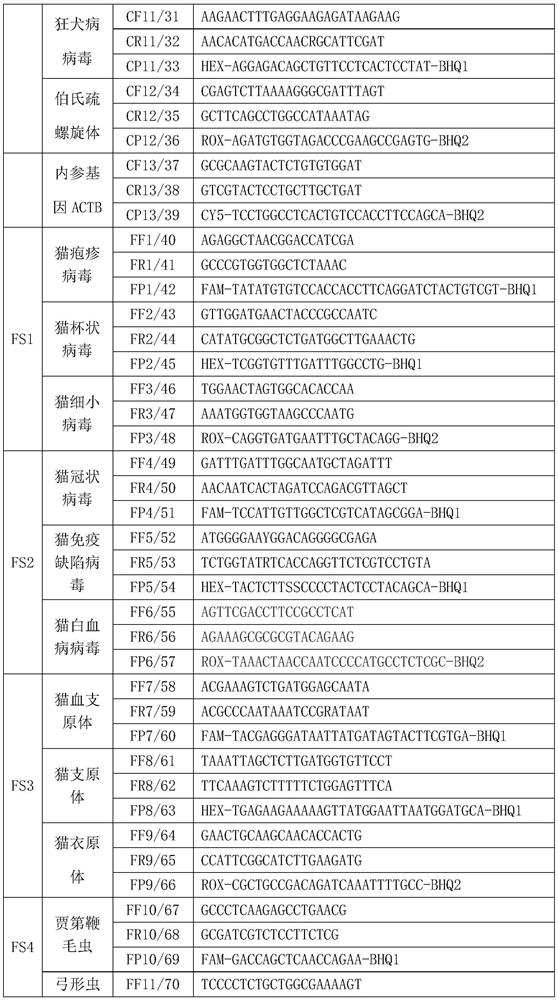
PendingCN114277189AShorten detection timeSave human resourcesMicrobiological testing/measurementAgainst vector-borne diseasesFeline parvovirusLeucosis
The invention relates to the technical field of molecular biomedicine, in particular to a PCR (Polymerase Chain Reaction) detection kit for cat and / or dog pathogens, a detection method and application. The kit is used for detecting canine distemper virus, canine influenza A virus, canine parainfluenza virus, canine parvovirus, canine coronavirus, canine rotavirus, canine babesia, canine ascaris, canine Ehrlichia, canine brucella, rabies virus, borrelia burgdorferi, reference gene ACTB, feline herpes virus, feline calicivirus and feline parvovirus. The kit comprises primers and probes of feline coronavirus, feline immunodeficiency virus, feline leukemia virus, feline mycoplasma, feline mycoplasma, feline chlamydia, giardia, toxoplasma, bartonella and reference gene GAPDH, collected DNA and RNA are added into the kit, a real-time fluorescence PCR instrument is adopted for PCR reaction, FAM, HEX, ROX and CY5 fluorescence signals are collected in each cycle, analysis of related pathogens is carried out, and the kit can be used for detecting the feline and the canine. Compared with a traditional detection method, the method has the advantages of higher specificity and higher sensitivity.
Owner:北京迈基诺基因科技股份有限公司
A kit for detecting h3n2 subtype canine influenza virus
ActiveCN104965083BHigh sensitivityImprove the detection rateBiological material analysisImmunologic functionRepeatability
The invention relates to the technical field of immunology, and concretely discloses a kit for detecting an H3N2 subtype canine influenza virus. The kit includes a monoclonal antibody generated by a hybridoma cell strain H3N2-CIV-HA-2C5, and the hybridoma cell strain is preserved in China Center for Type Culture Collection on May 13, 2015 with the preservation number of CCTCC NO:C201555. The detection kit has the advantages of high sensitivity, strong specificity, very good repeatability and stability, clinic sample detection, realization of a detection rate being greatly higher than that of routine detection, and practical clinic application significance.
Owner:SOUTH CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com