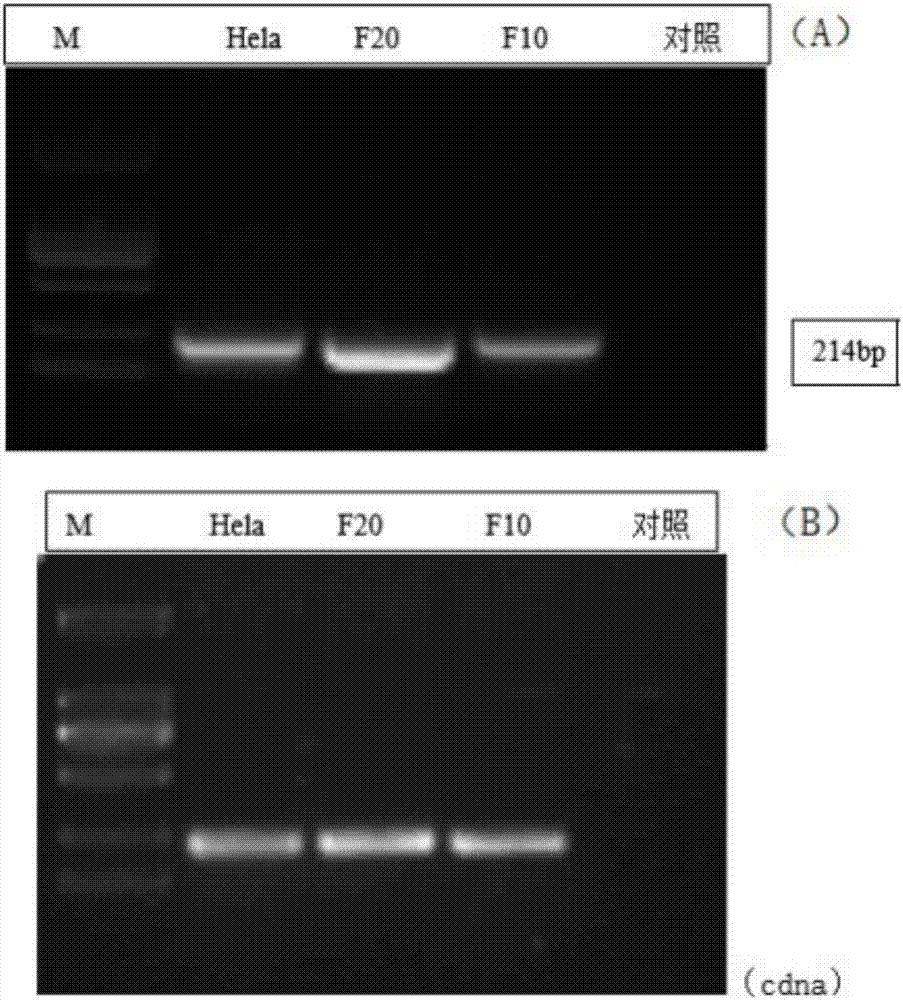
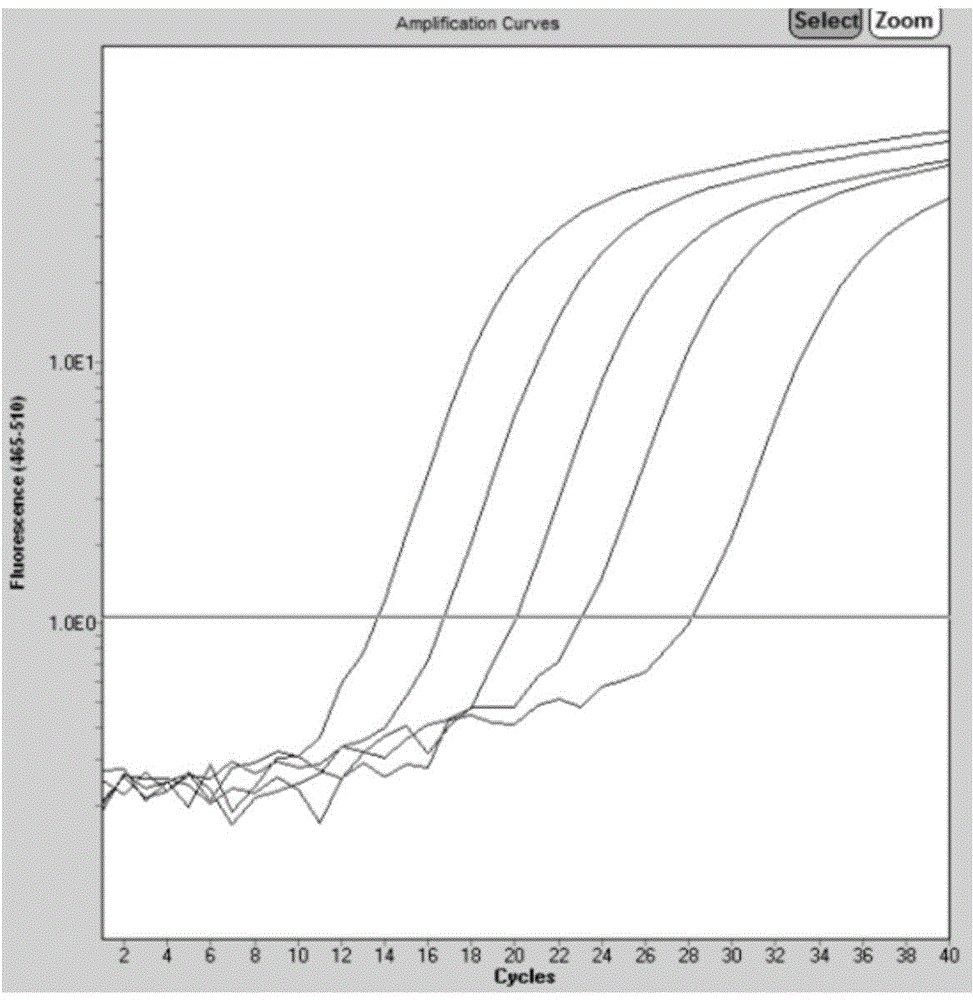
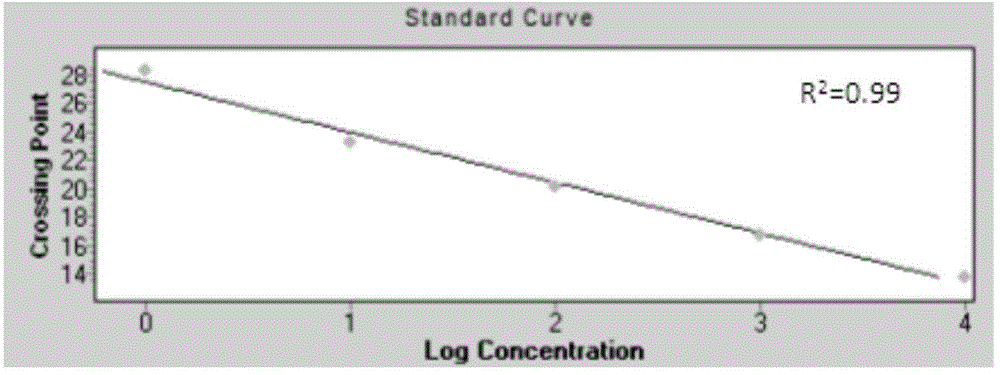
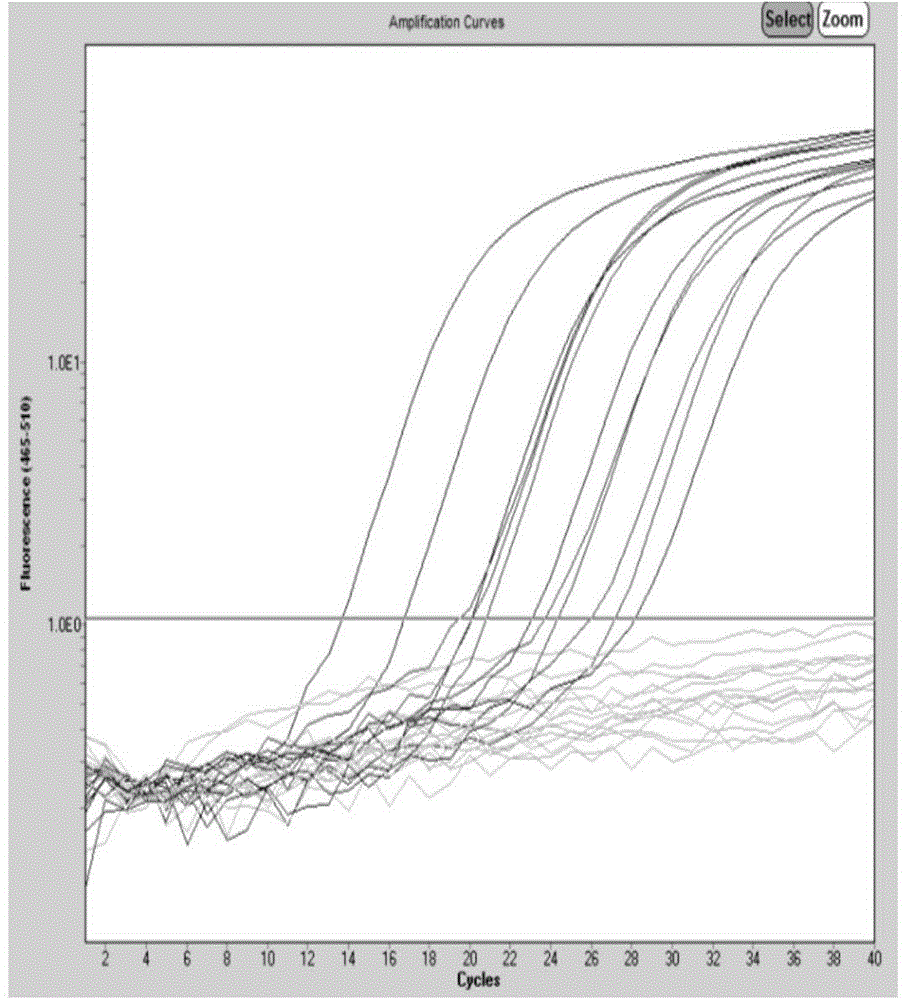
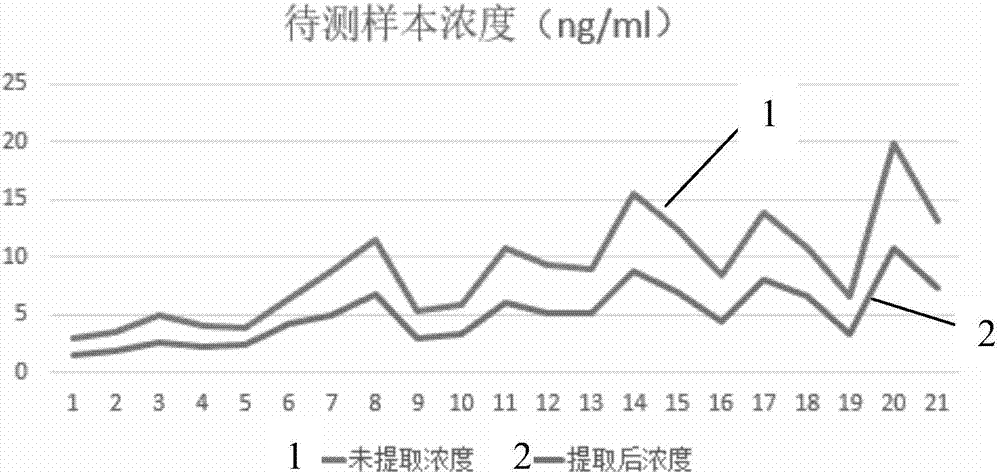
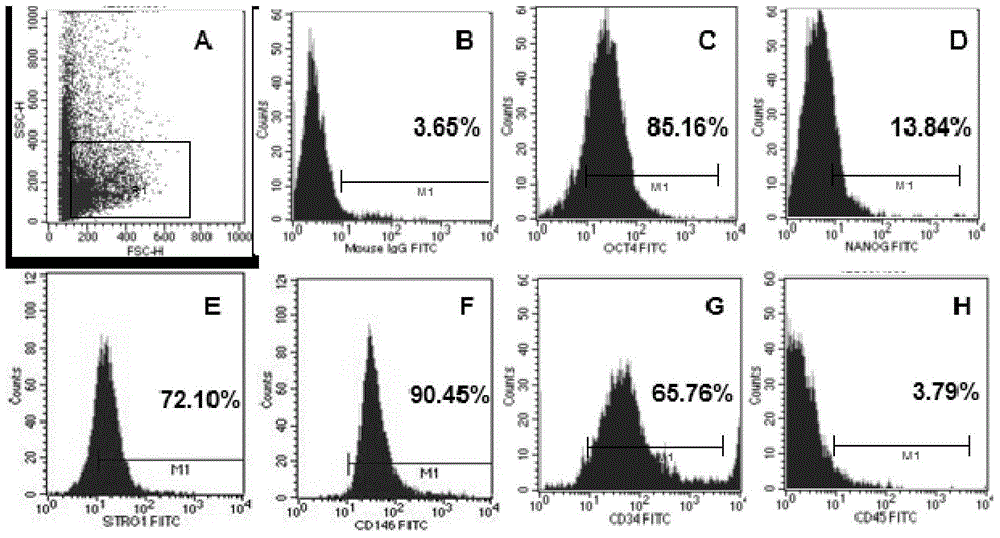
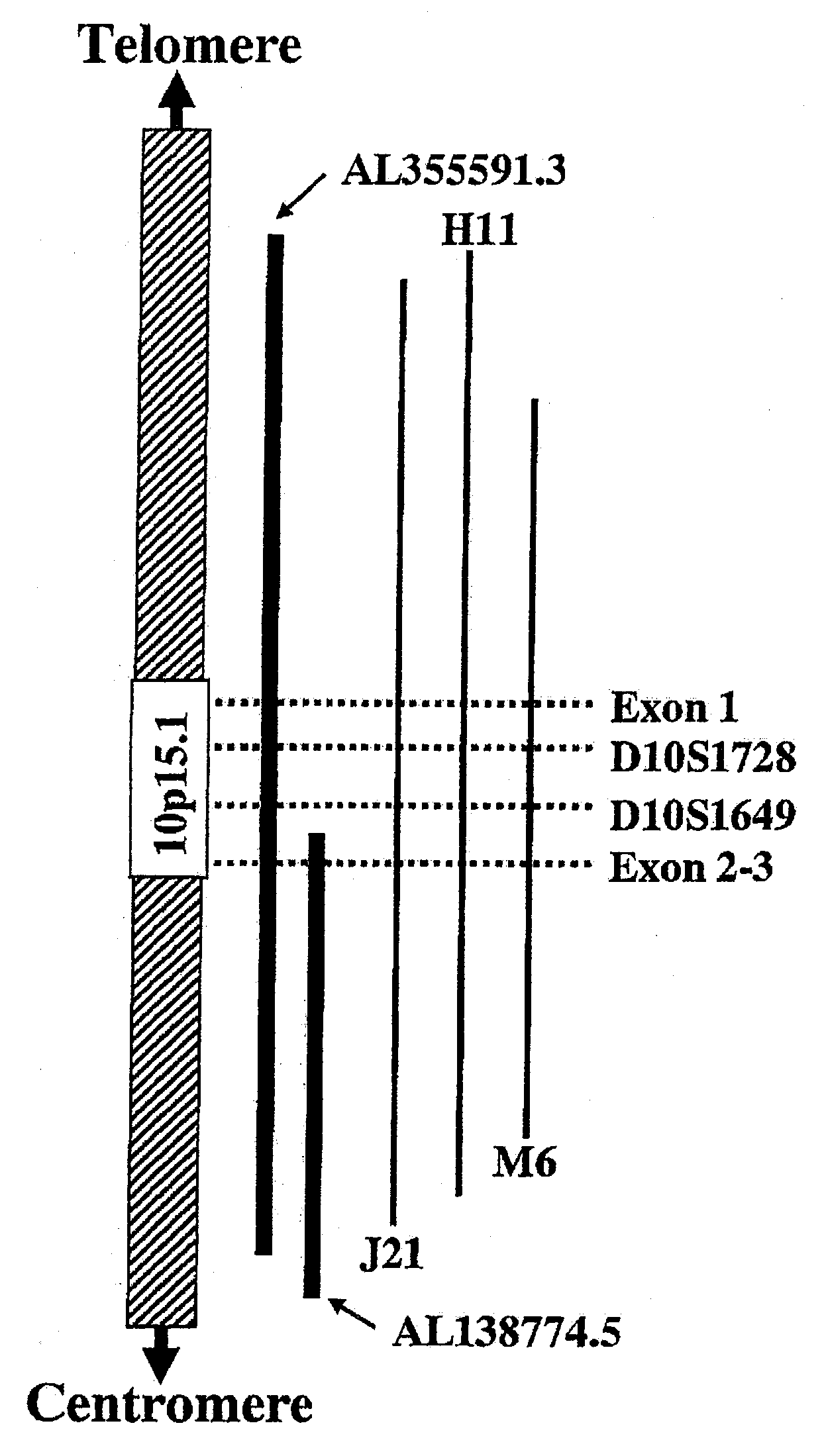
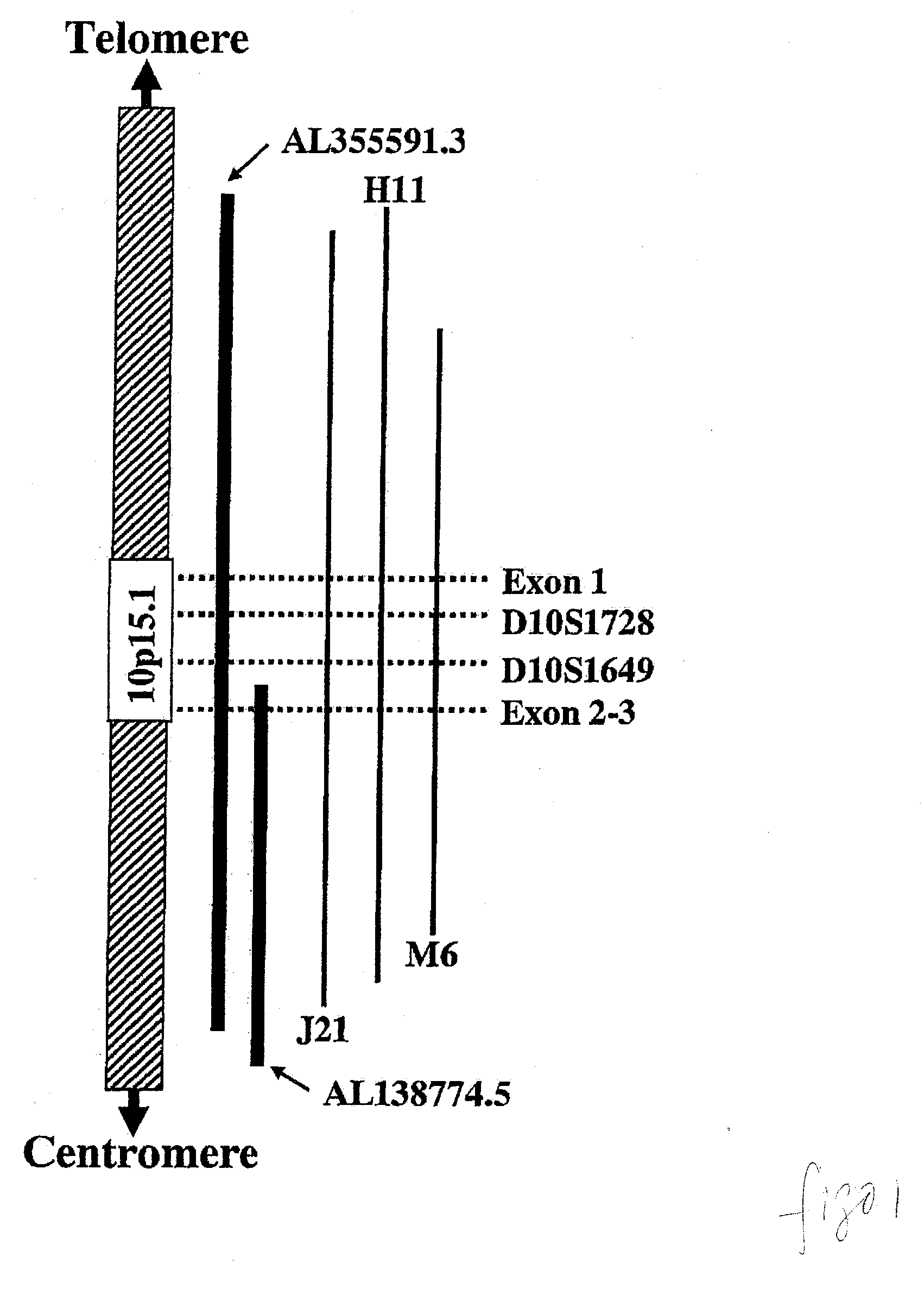
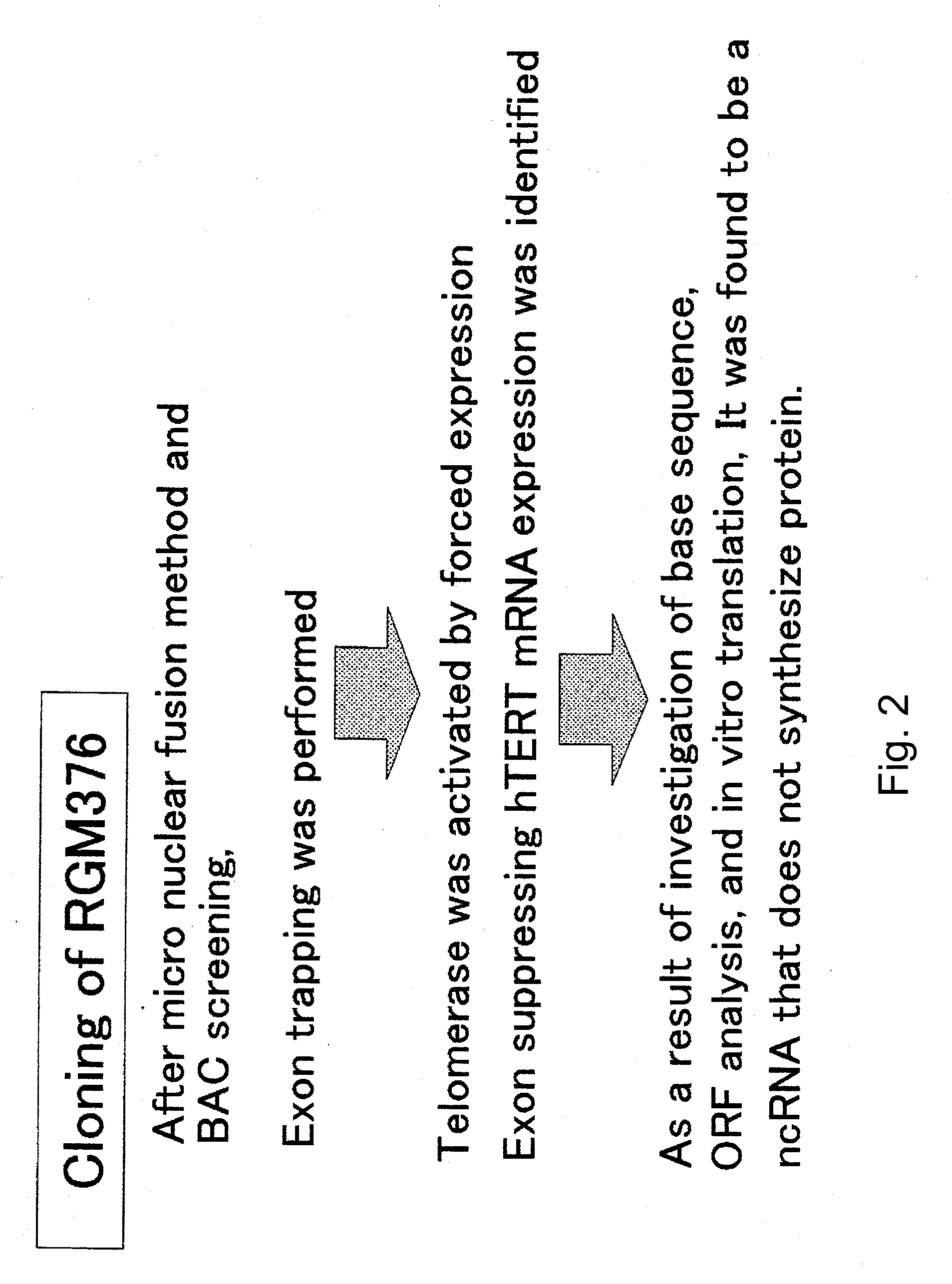
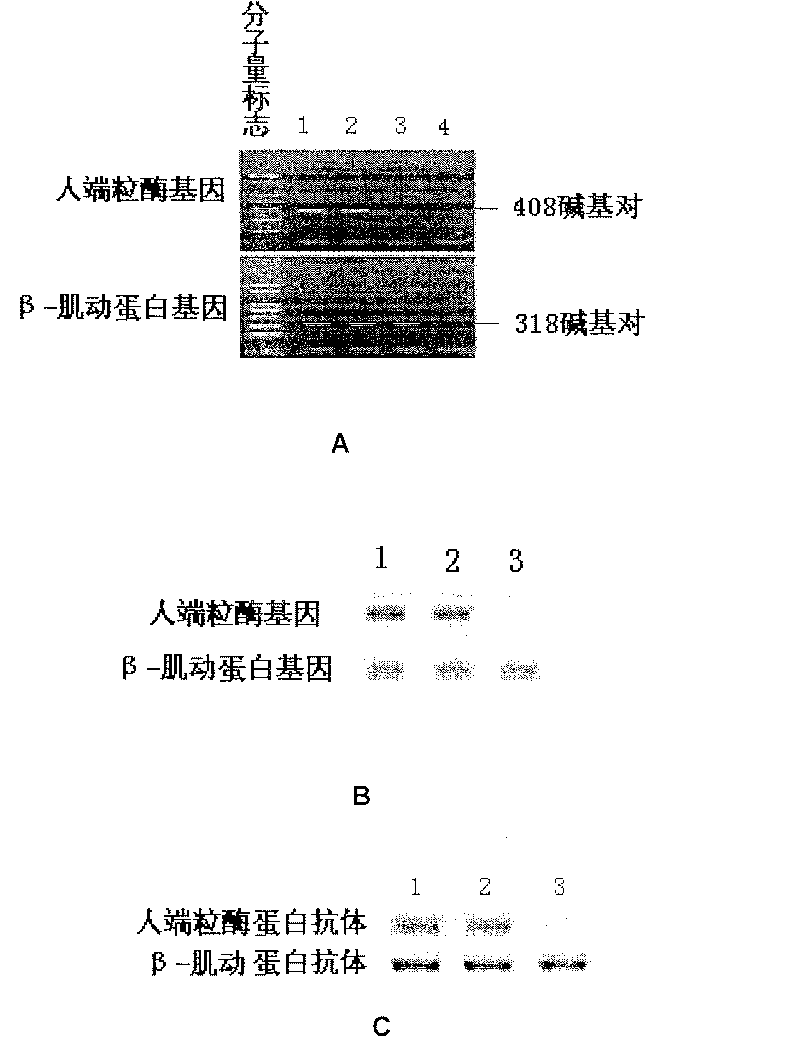
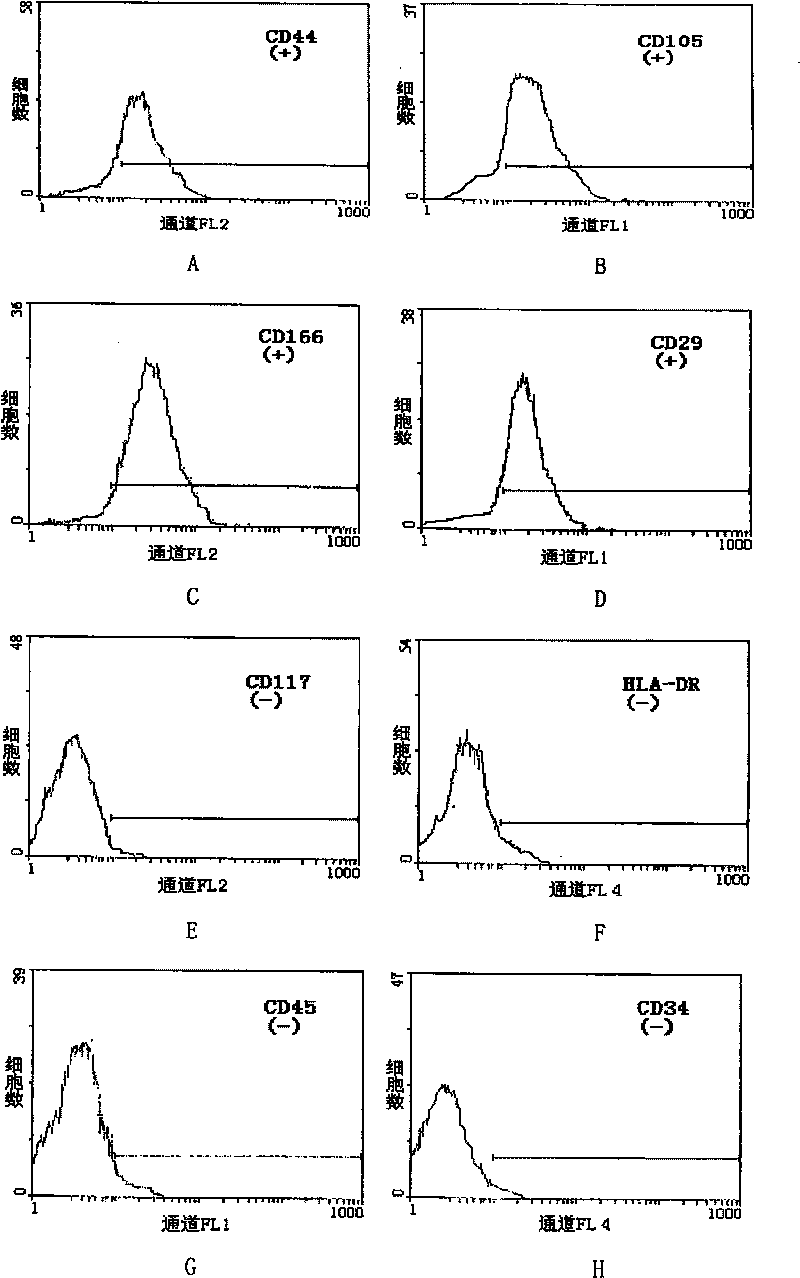
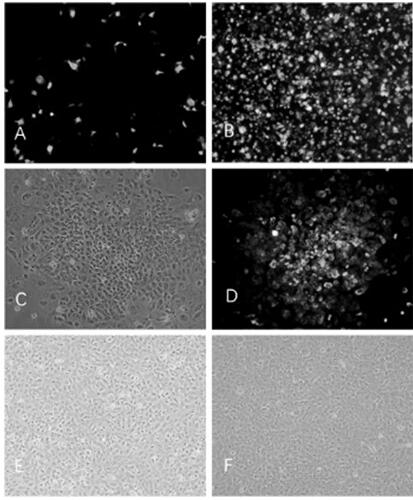
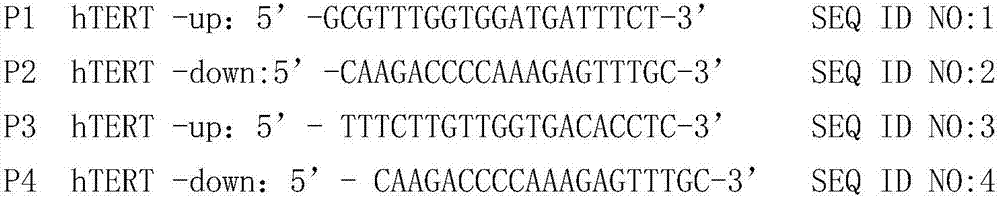Patents
Literature
41 results about "HTERT Gene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Porcine trachea epithelial cell line and building method thereof
InactiveCN102174573AEstablishment of Immortalized Cell LinesActivate telomerase activityMicroorganism based processesVector-based foreign material introductionAdhesive tissueDigestion
The invention discloses a building method of a porcine trachea epithelial cell line. The method comprises the following steps of: (1) primary culture: sterilely acquiring newborn piglet tracheas, clearing adhesive tissues, performing digestion and separation by adopting a pronase perfusion cold digestion method to obtain high-purity trachea epithelial cells, suspending the separated primary trachea epithelial cells in a DF12 culture medium, and culturing the cells at the temperature of 37 DEG C under the condition of 5 percent CO2; (2) transfection and screening: extracting and purifying eukaryotic expression plasmids pCI-neo-hTERT containing hTERT genes, and guiding the eukaryotic expression plasmids pCI-neo-hTERT for coding hTERT and neo genes to the porcine trachea epithelial cell primarily cultured in the step (1) by adopting a liposome transfection method to perform transfection; and (3) screening and amplified culture. The method is easy in culture, the growth speed is high, and the operating method is simple and convenient.
Owner:张彦明 +1
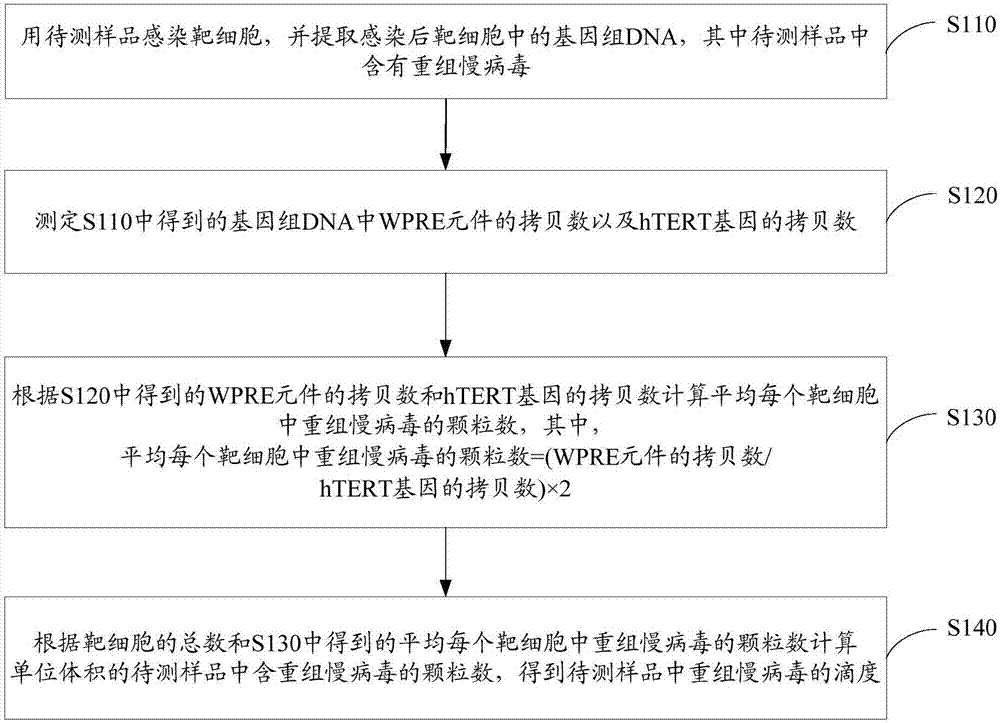
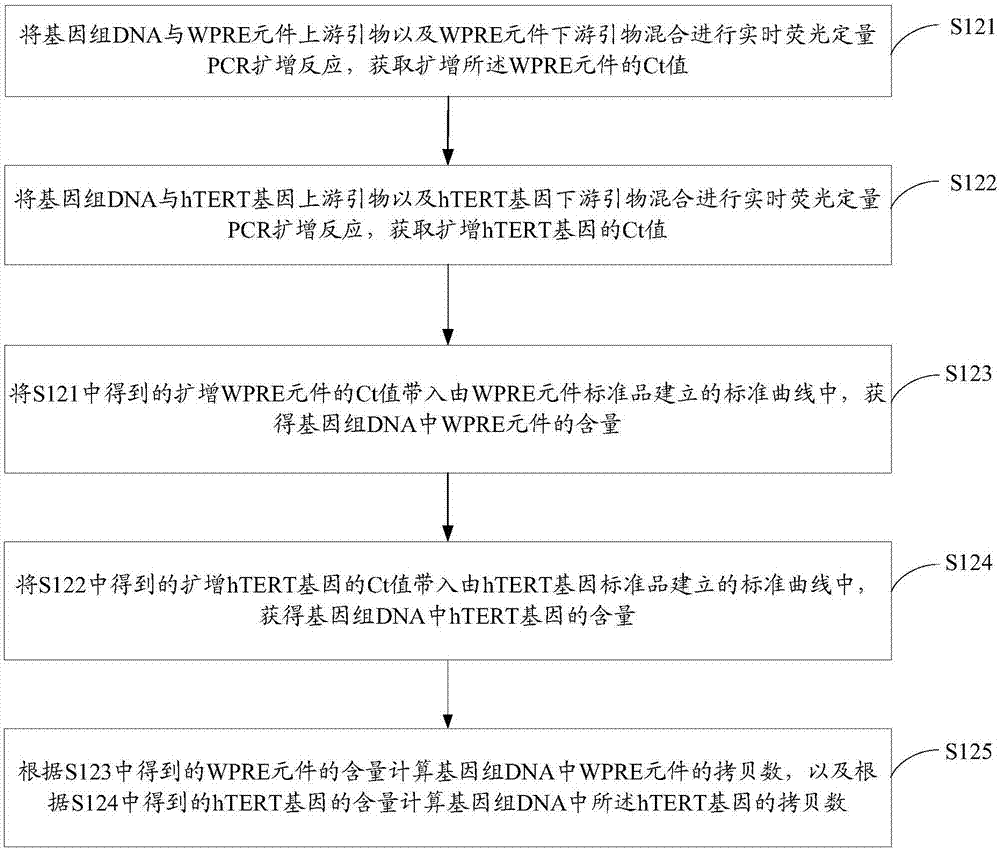
Method for quantitatively detecting recombinant lentivirus titers
ActiveCN107365875AAccurate titerAccurate measurement of titerMicrobiological testing/measurementMicroorganism based processesLentivirusTiter
The invention relates to a method for quantitatively detecting recombinant lentivirus titers. The method includes: infecting target cells with samples to be detected; extracting a genome DNA (deoxyribonucleic acid) in the infected target cells; then detecting the copy number of WPRE elements in the genome DNA and the copy number of hTERT genes; calculating the number of particles in recombinant lentivirus in the average target cells according to the copy number of the WPRE elements and the copy number of the tTERT genes; calculating the number of the particles of the recombinant lentivirus contained in the samples to be detected in unit volume according to the sum of the target cells and the number of the particles of the recombinant lentivirus in the average target cells to obtain the titers of the recombinant lentivirus in the samples to be detected. The method is simple in operation, and the recombinant lentivirus titers can be detected accurately under the condition of not using fluorescent markers.
Owner:SHENZHEN GENTARGET BIOTHERAPEUTICS CO LTD
Method for transduction of bone marrow mesenchyma stem cell by human telomerase gene
InactiveCN101063150AIncrease success rateVector-based foreign material introductionDNA/RNA fragmentationEnzyme GeneLiposome
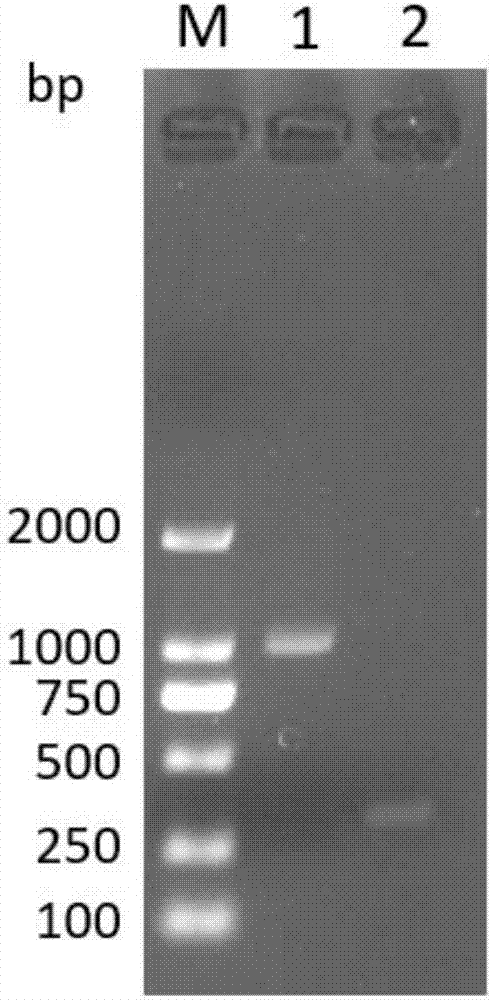
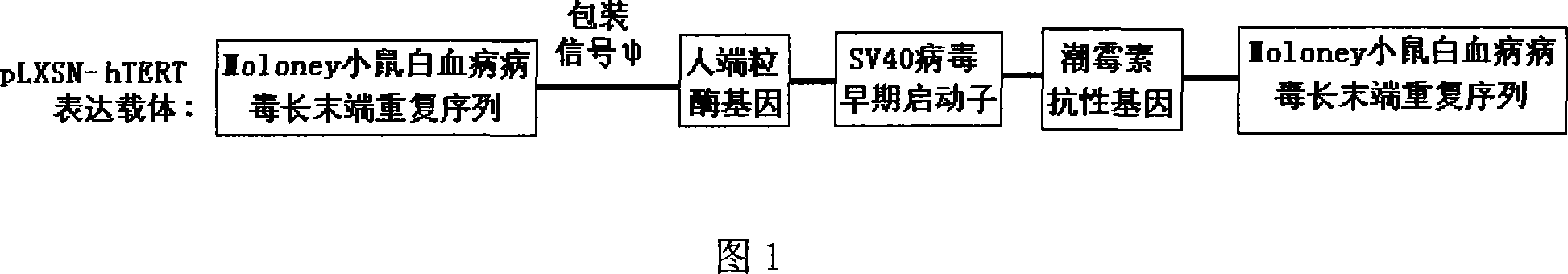
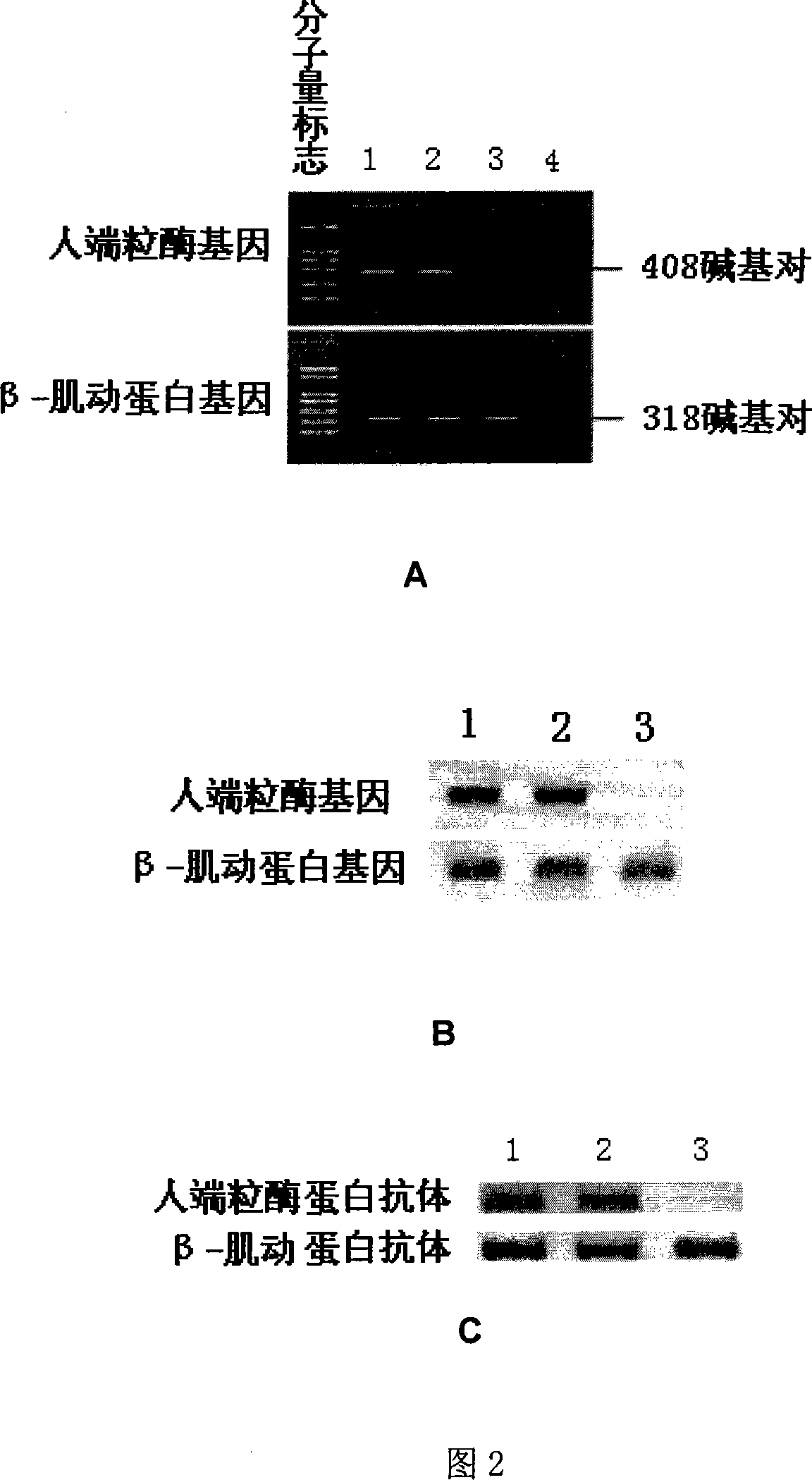
The invention discloses a method to transduce full material stem cell among marrow with human end particle enzyme gene, which comprises the following steps: enzyme-cutting pGRN145 plasmid with Hpa I and Not I restriction enzymes; getting hTERT cDNA fragment; connecting to pLXSN carrier with the same restriction enzymes; constructing pLXSN-hTERT retooling expressing carrier; using LipofectAMINE liposome transduction agent box; preparing pLXSN-hTERT retooling expressing carrier floated liquid through compatible eating property incasing line PA317; proceeding transduction for full material stem cell among marrow; constructing the stem cell with high effective expressing exogenesis hTERT gene; double-sieving with low density homomycin; getting the stem cell. This invention can reach the goal to increase life cycle of full cytoplasma stem cell among marrow.
Owner:ZHEJIANG UNIV
Construction and application of tumour targeting gonad correlation viral vectors
InactiveCN101173299ATreatment safetyEffective treatmentGenetic material ingredientsAntineoplastic agentsTumor targetVector system
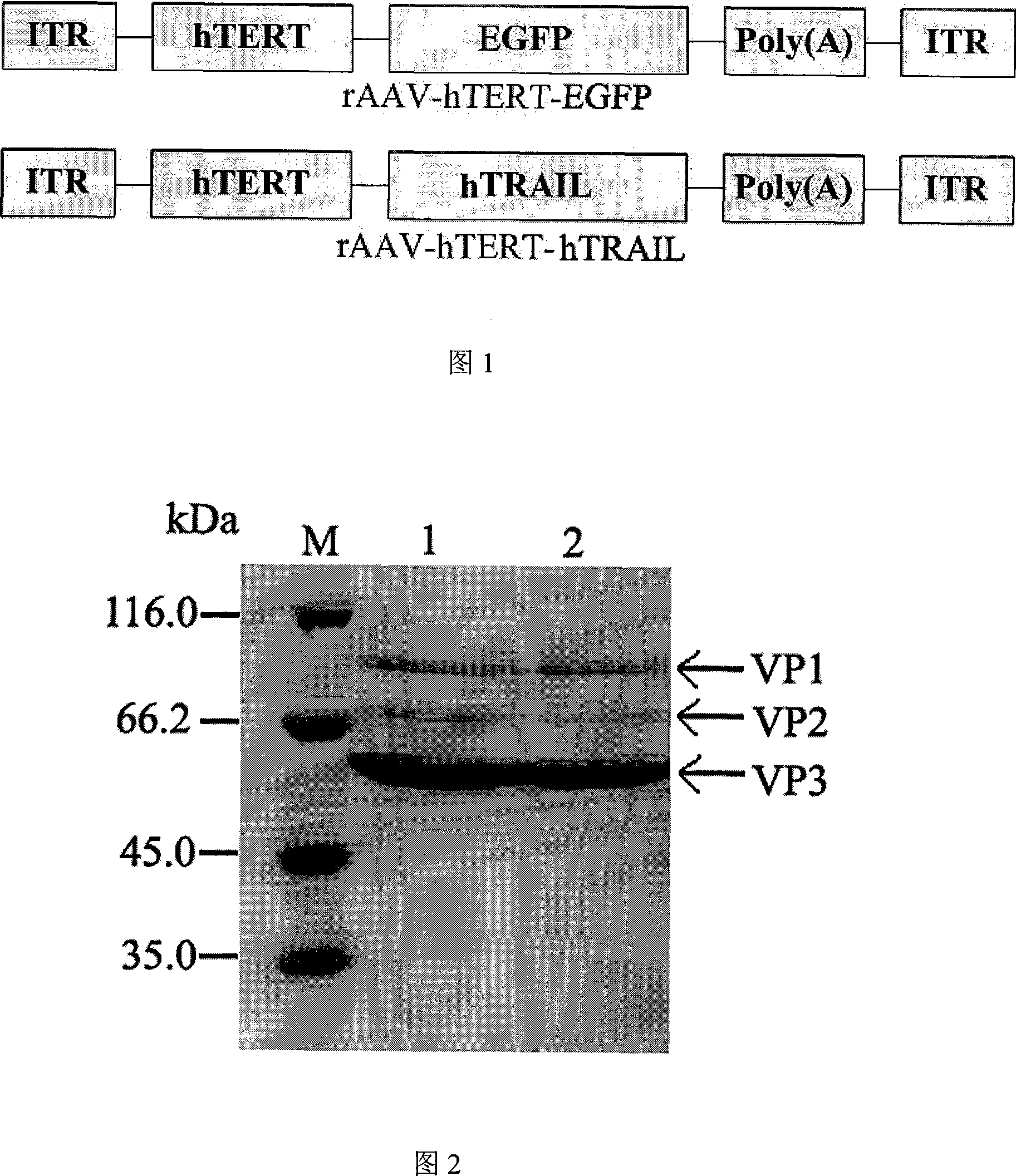
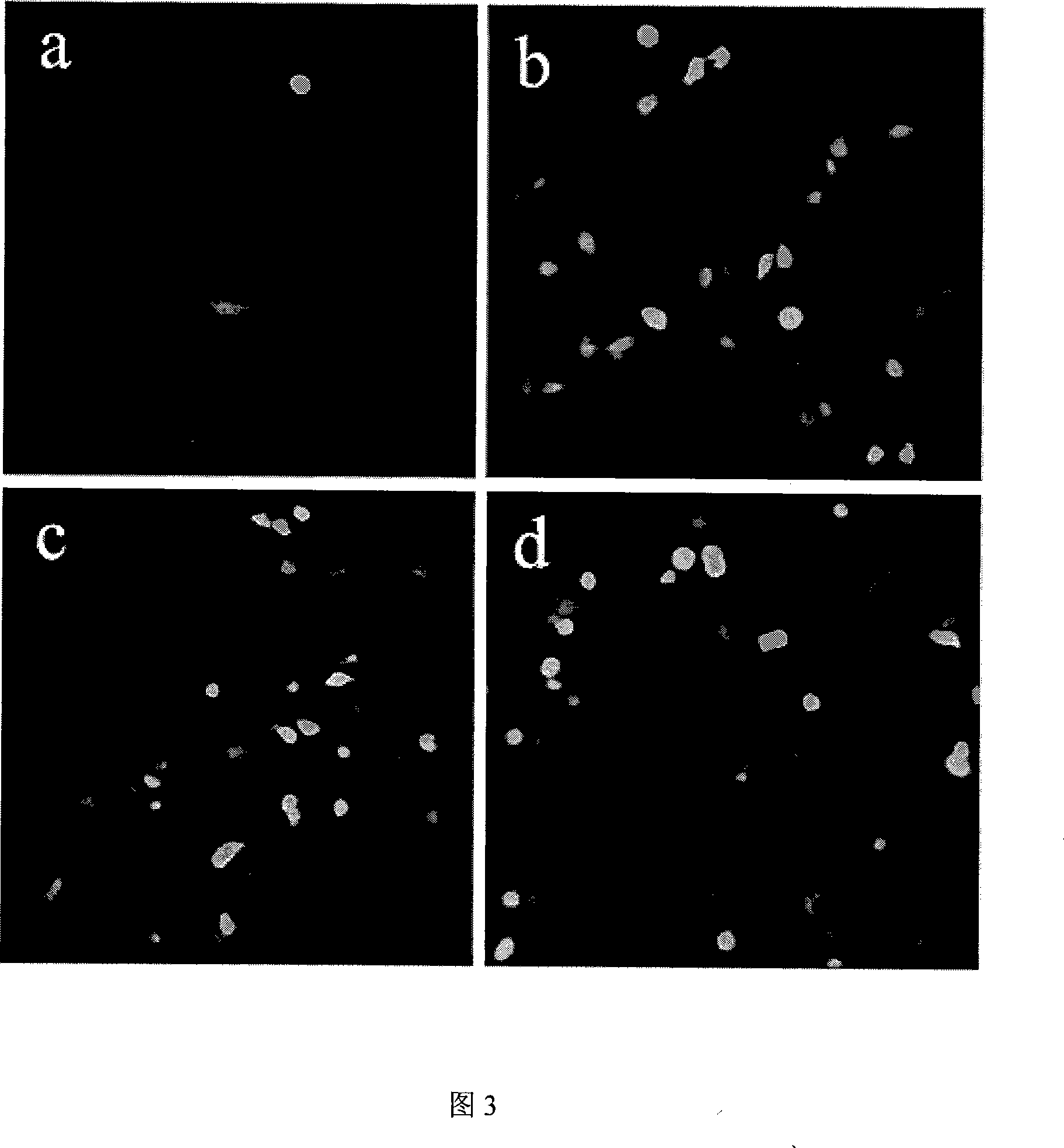
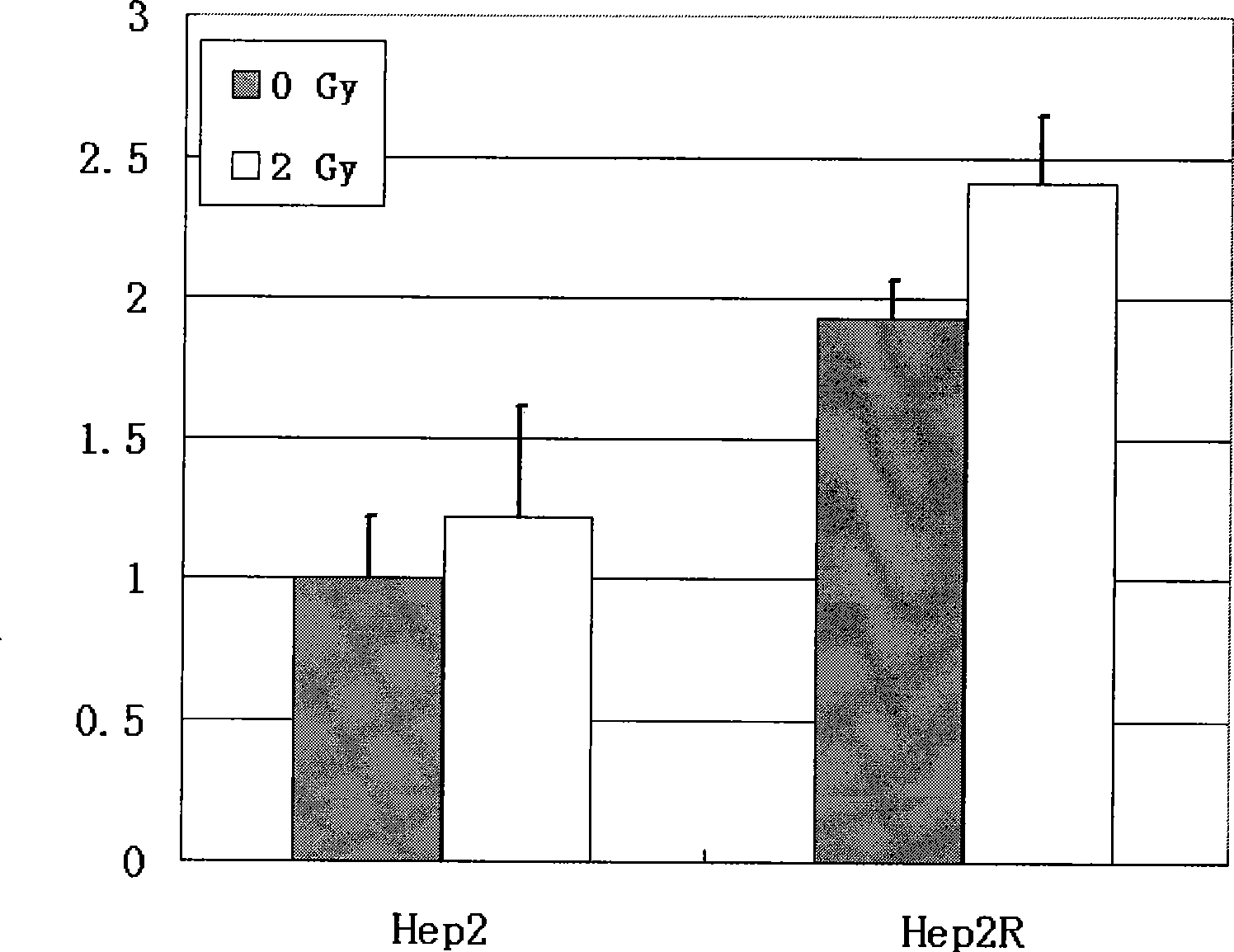
The invention discloses a construction method of a tumor targeting adeno-associated virus vector system rAAV-hTERT-gene: a tumor specific hTERT is used to start the foreign genes carried by a sub-control-gland-associated virus to express to construct an adeno-associated virus vector system rAAV-hTERT-gene which has tumor targeting and can be inserted into a therapeutic gene with anticancer effect at will. The invention also discloses the tumor targeting adeno-associated virus rAAV-hTERT-hTRAIL constructed by the above method: the virus is an anti-tumor active hTRAIL gene with wide selectivity carried by a tumor targeting adeno-associated virus vector system rAAV-hTERT-gene. The invention has the advantages of constructing safe, high efficient tumor targeting adeno-associated viruses.
Owner:ZHEJIANG SCI-TECH UNIV
Uses of gamma-ray combined phTERT-HRP/IAA in medicament for treating cancer of larynx
InactiveCN101366950AIncrease enzyme activityHigh activityGenetic material ingredientsAntineoplastic agentsCancer cellPromoter activity
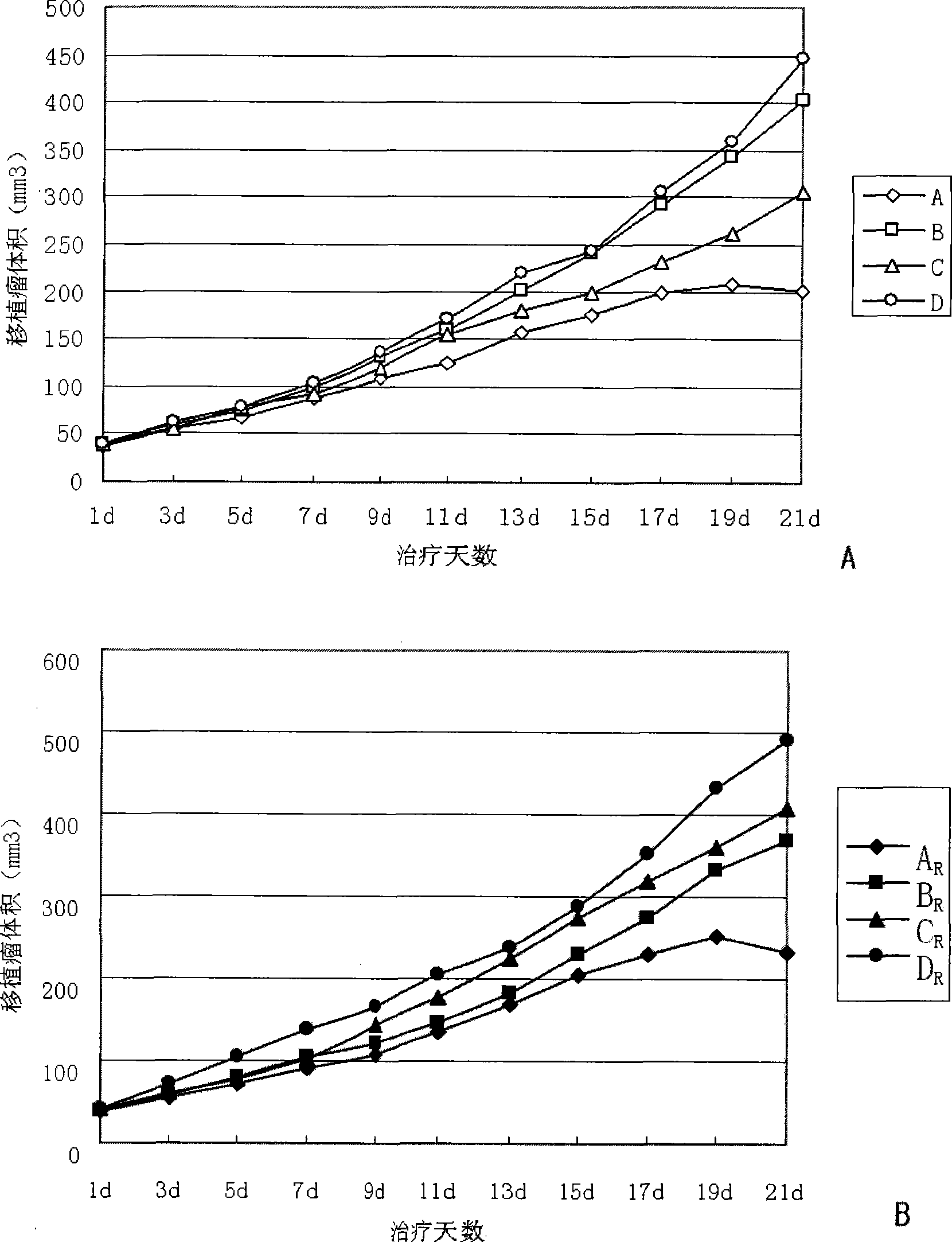
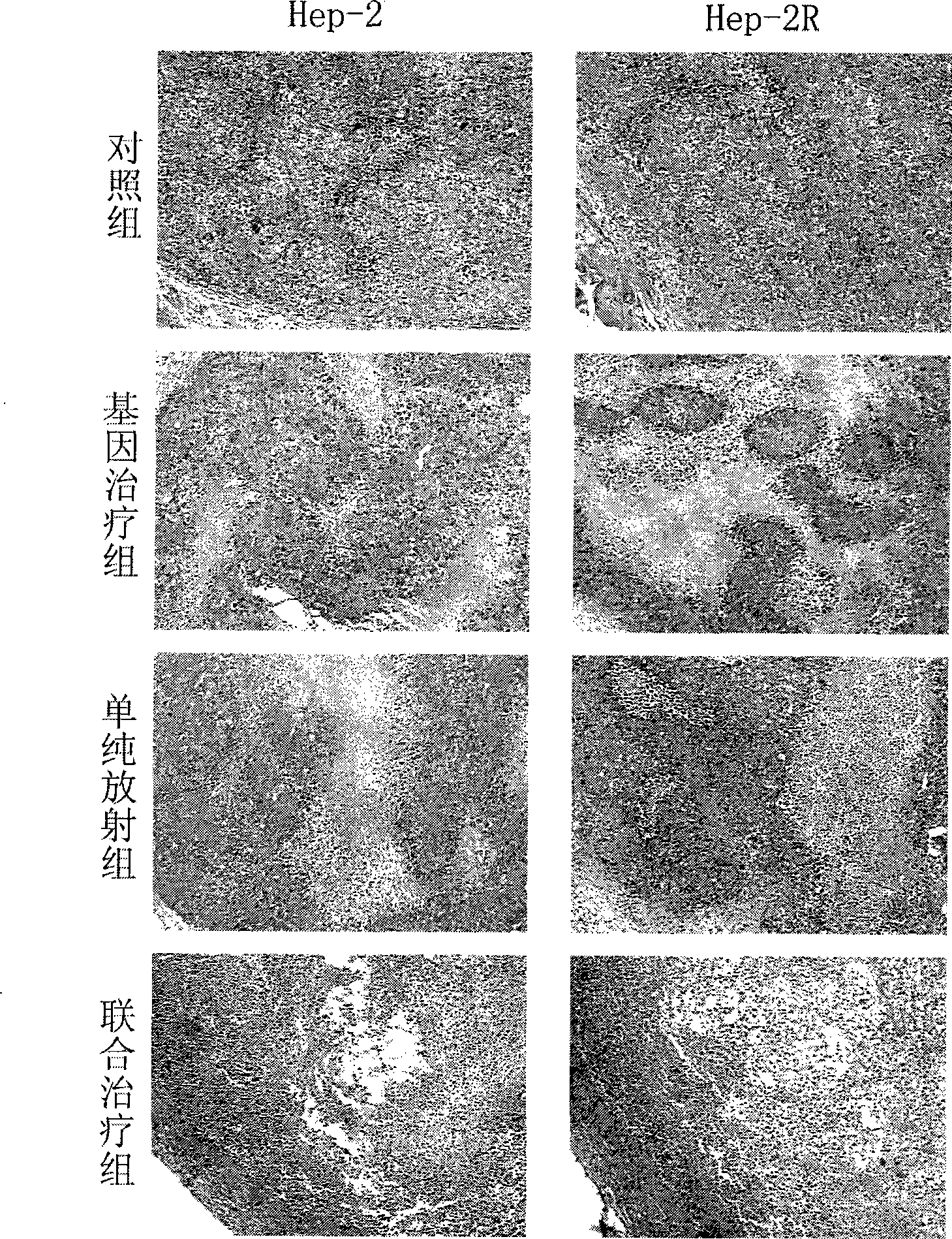
The invention discloses application of the combination of gamma ray and phTERTp-HRP / IA in medicines for killing larynx cancer cells, and in particular relates to the enhancement of the gamma ray in promoter activity of hTERT gene and the synergetic effect of the combination of the gamma ray and the phTERTp-HRP / IA for killing the human larynx cancer cells. The invention studies levels of different radiation sensitivity cancer cells in cells and animals and detects the promoter activity and enzymatic activity, volume, weight and tumor inhibiting rate of transplanted tumor, tumor tissue HE coloration and apoptosis of lower-reach gene expression products to evaluate the synergetic killing effect when the gamma ray treatment and the hTERT promoter regulating-controlling HRP / IAA system gene treatment are jointly used. The result shows that when the gamma ray treatment and the phTERTp-HRP / IAA treatment are jointly used, the human larynx cancer cells are obviously killed.
Owner:WUHAN UNIV
Immortalized piglet oral mucosa epithelial cell line and establishment method and application thereof
InactiveCN105483089AUniform shapeClear borderGenetically modified cellsEpidermal cells/skin cellsPrimary cellCulture mediums
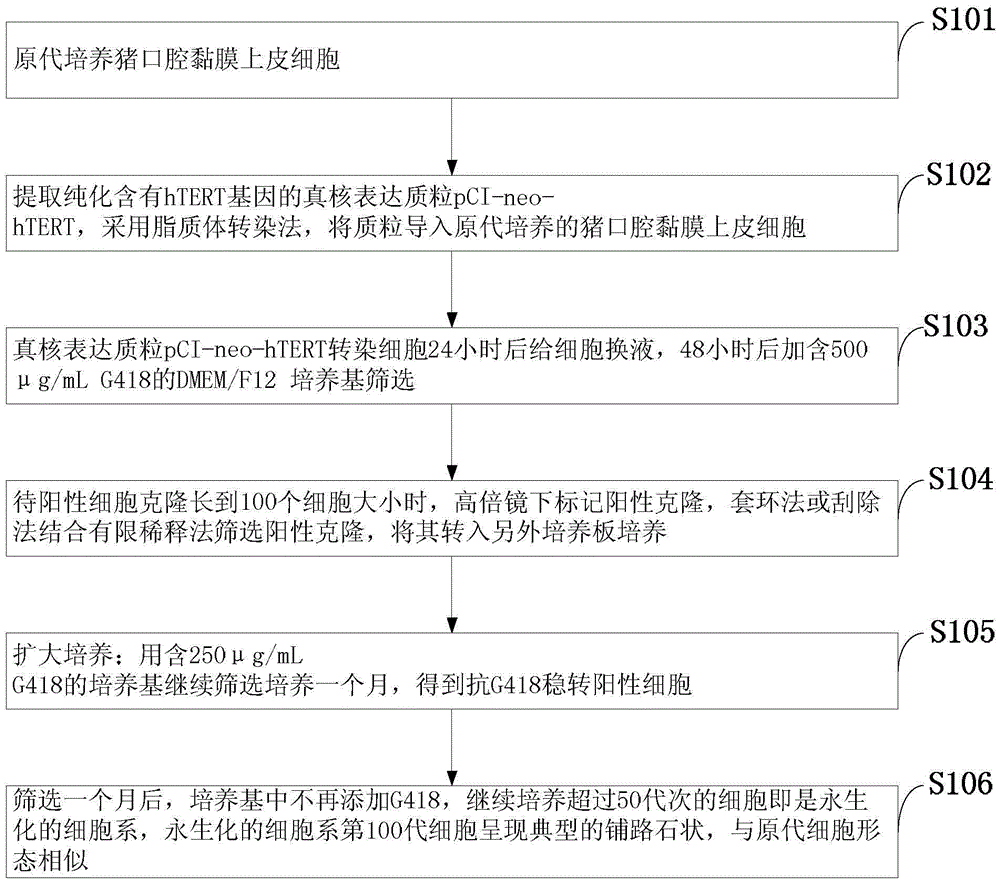
The invention discloses an immortalized piglet oral mucosa epithelial cell line and an establishment method and application thereof. The establishment method comprises the following steps: carrying out primary culture of piglet oral mucosa epithelial cells; extracting and purifying eukaryotic expression plasmids pCI-neo-hTERT containing hTERT genes; replacing the culture medium of the cells 24 hours after the cells are transfected with the eukaryotic expression plasmids pCI-neo-hTERT; after 48 hours, adding a DMEM / F12 culture medium containing 500 mu g / ml of G418 for screening; marking positive clones under a high power lens, and transferring into a culture plate for culture; carrying out enlarged culture; screening for 1 month and not adding G418 into the culture medium any more, so that the cells obtained after culturing for over 50 generations are the immortalized cell line. The immortalized piglet oral mucosa epithelial primary cells are consistent and uniform in morphology.
Owner:张彦明
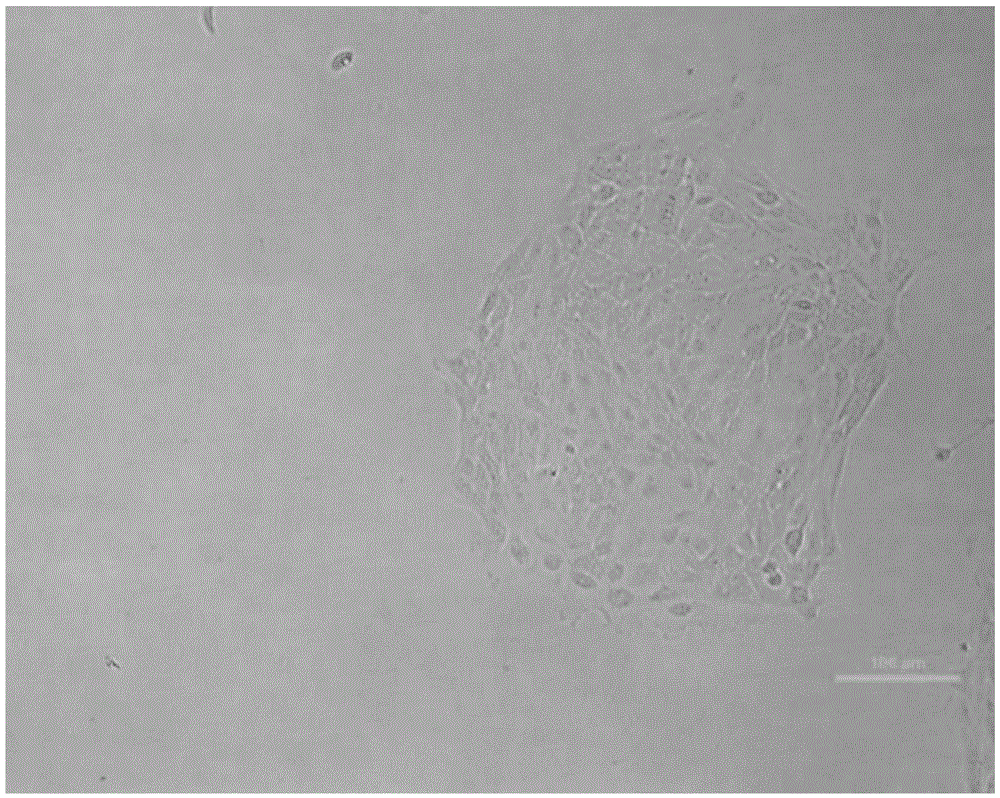
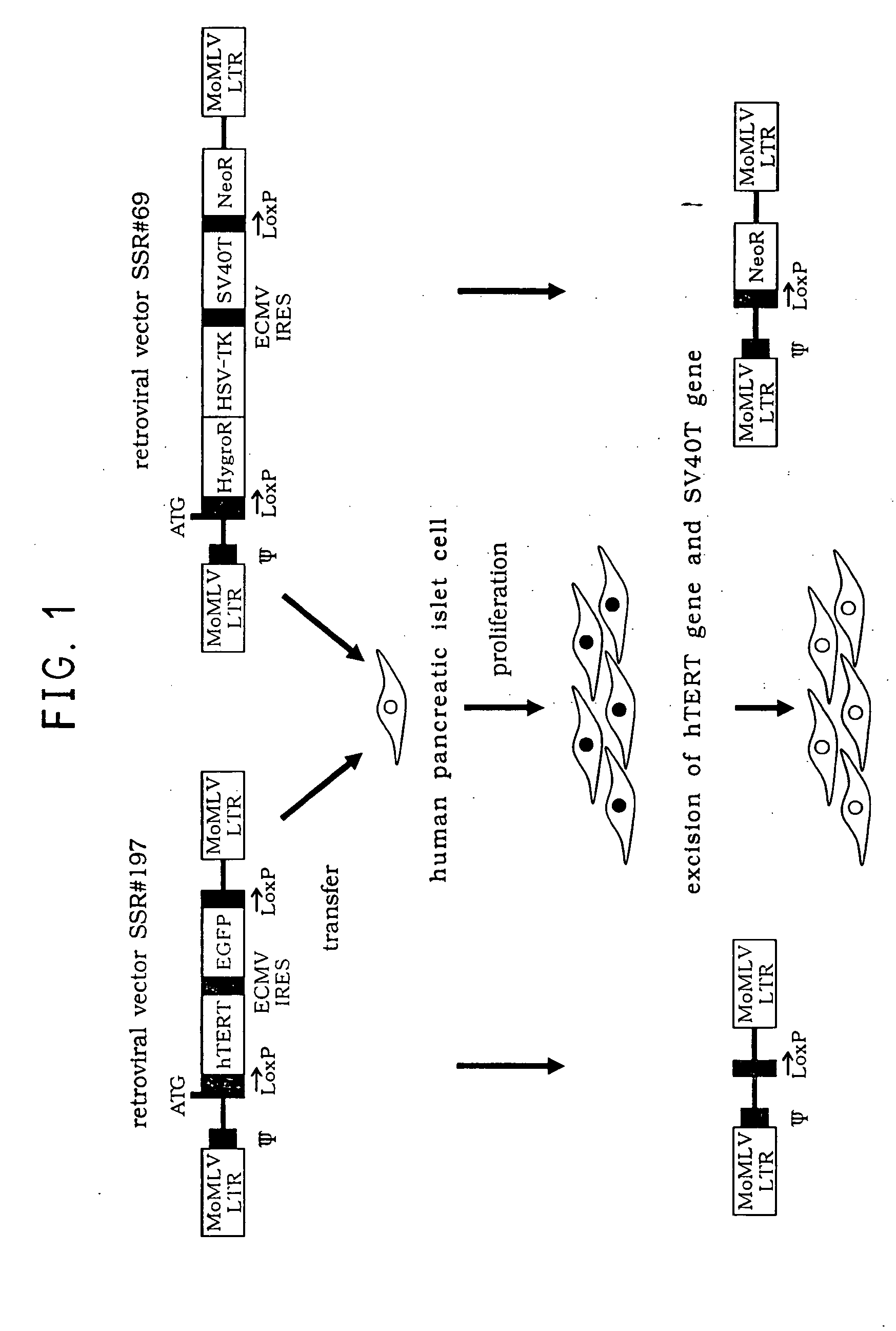
Insulin expressing human pancreatic islet cell line capable of reversibly proliferating and use thereof
InactiveUS20070086988A1BiocideGenetic material ingredientsInsulin producing cellSimian Vacuolating Virus 40
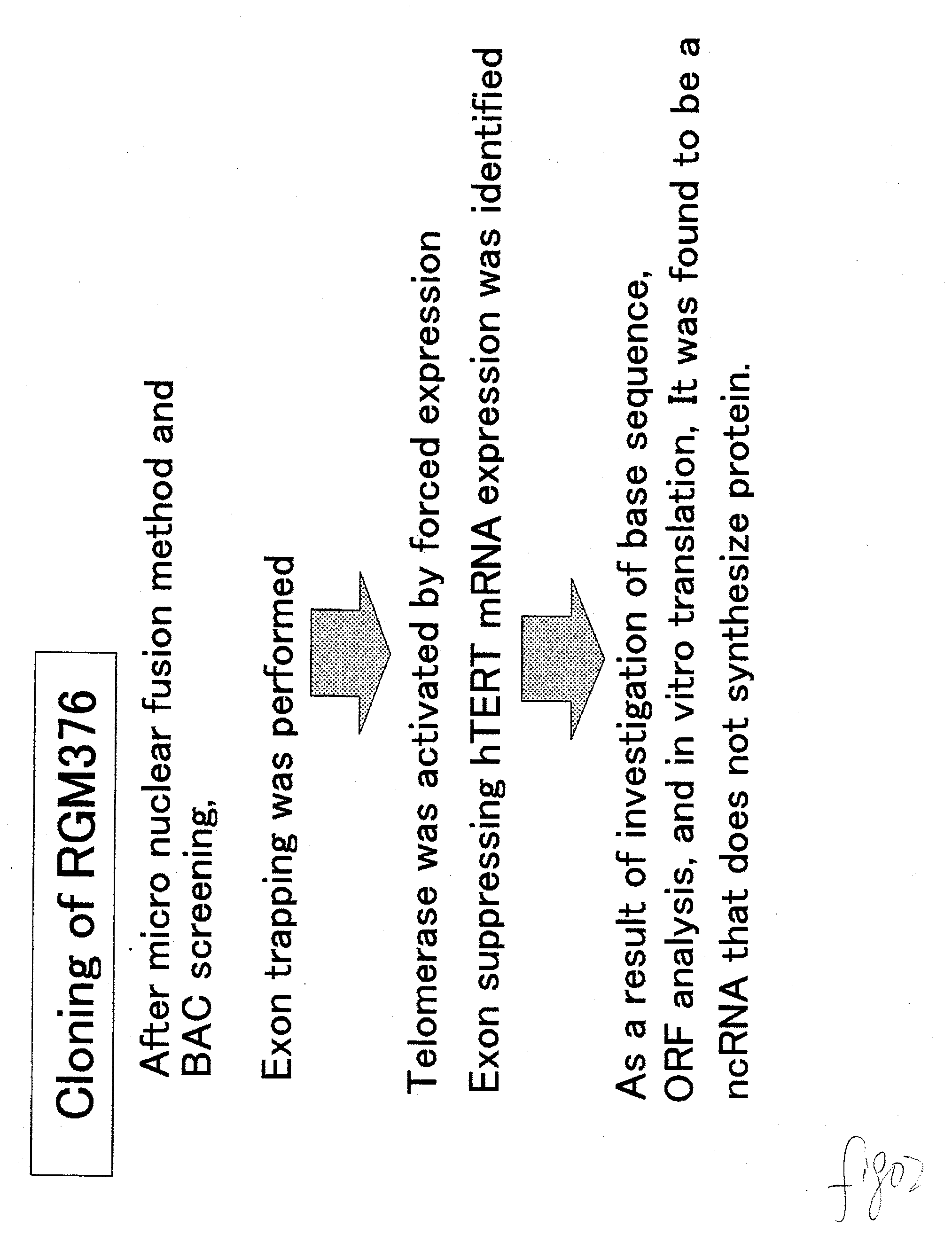
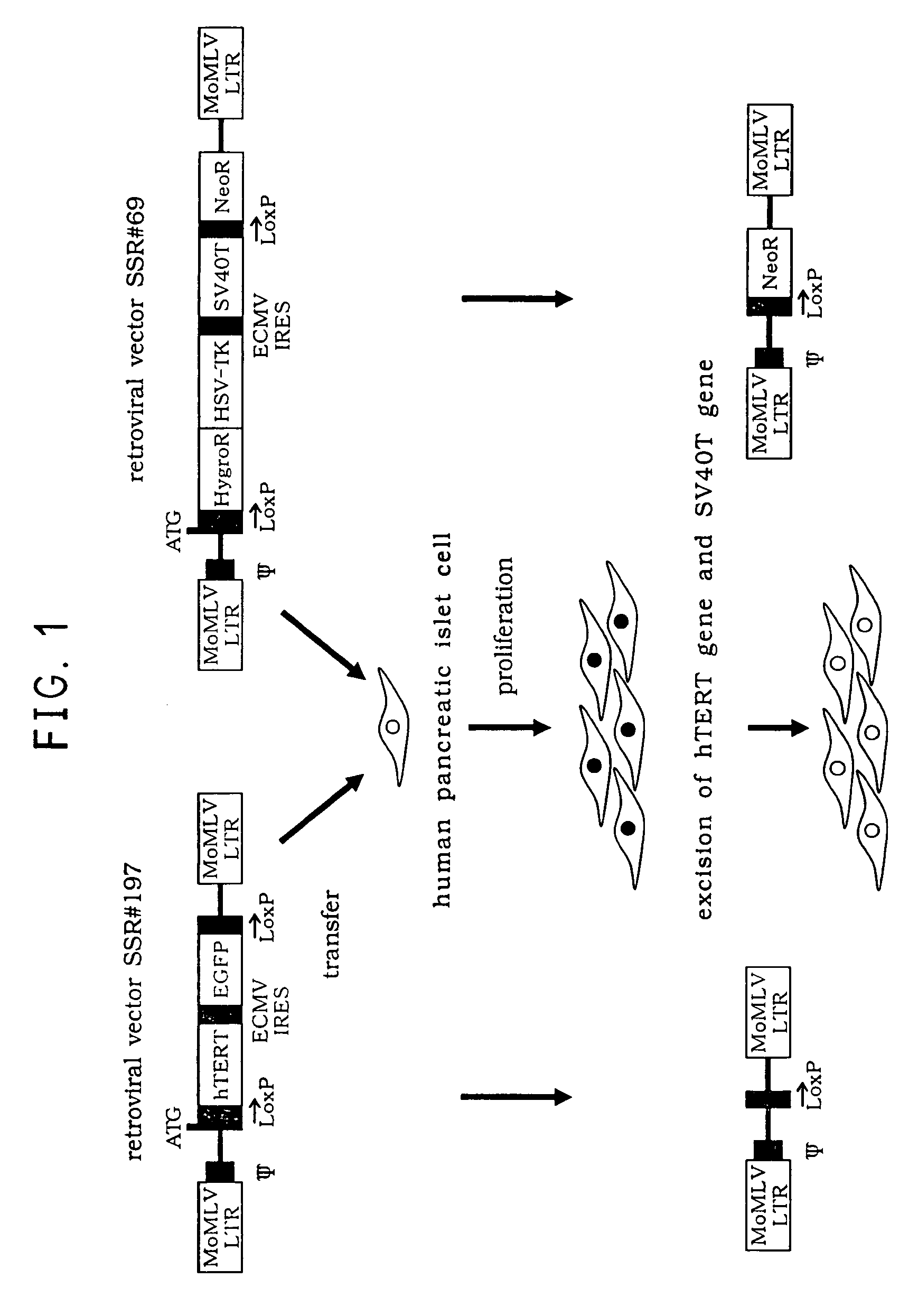
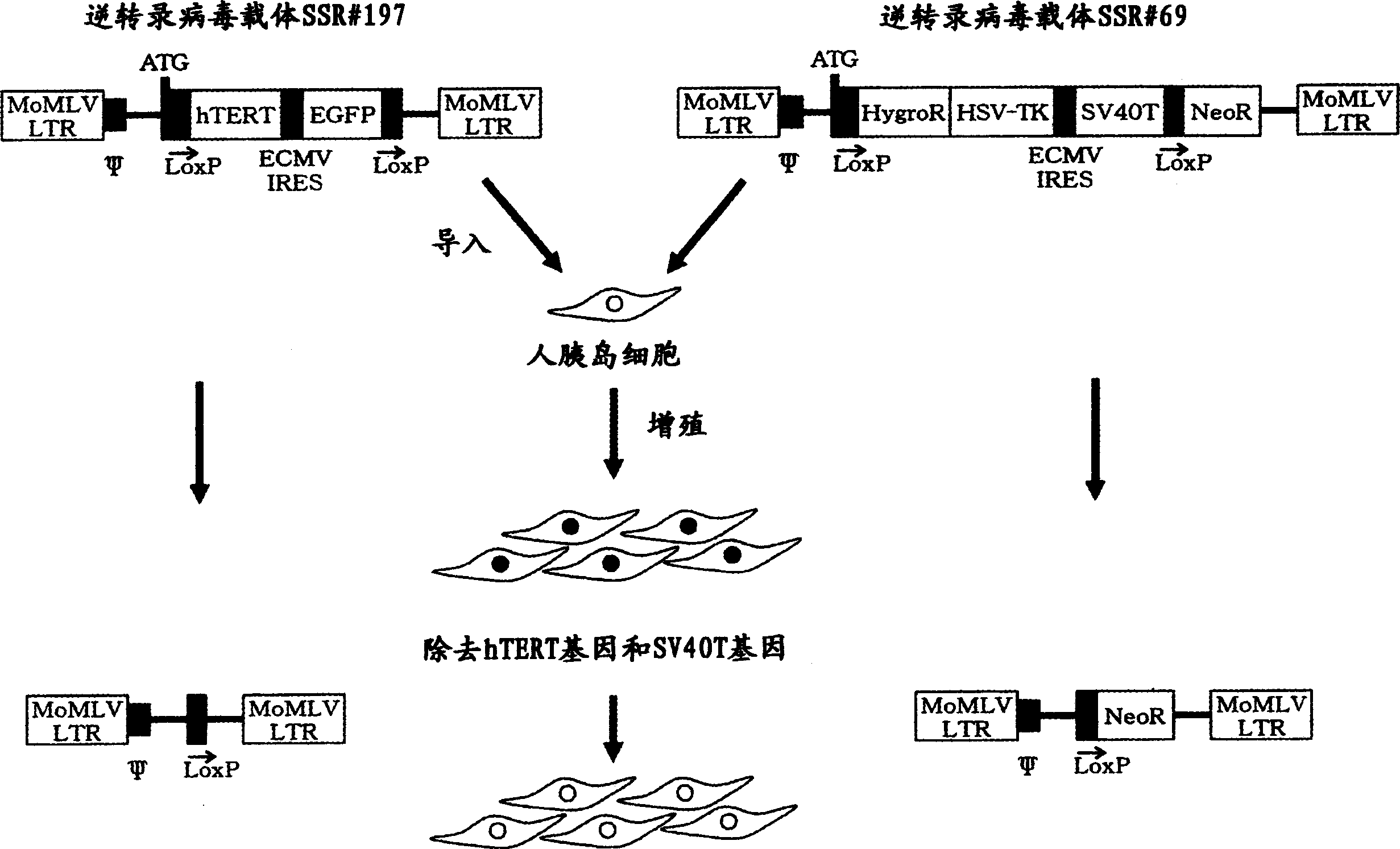
A reversibly immortalized human pancreatic islet cell line containing an hTERT gene and an SV40T gene each interposed between a pair of LoxP sequences, characterized in that it is capable of producing insulin and enhancing expression of insulin after excising the hTERT gene and the SV40T gene, in particular, NAKT-13 (deposited with International Patent Organism Depository, National Institute of Advanced Industrial Science and Technology, address: AIST Tsukuba Central 6, 1-1, Higashi 1-Chome, Tsukuba-shi, Ibaraki-ken, 305-8566 Japan, deposited date: Sep. 4, 2003, accession number: FERM BP-08461) or a passage cell line thereof; a human pancreatic islet cell obtained by excising the hTERT gene and the SV40T gene from the reversibly immortalized human pancreatic islet cell line or passage cell line thereof; and use of these cells. By using the reversibly immortalized human pancreatic islet cell line of the invention insulin-producing cells can be easily and surely obtained in a number enough to meet the demand.
Owner:NORIAKI TANAKA +2
Immortal human bone marrow matrix stem cell line and its establishing process
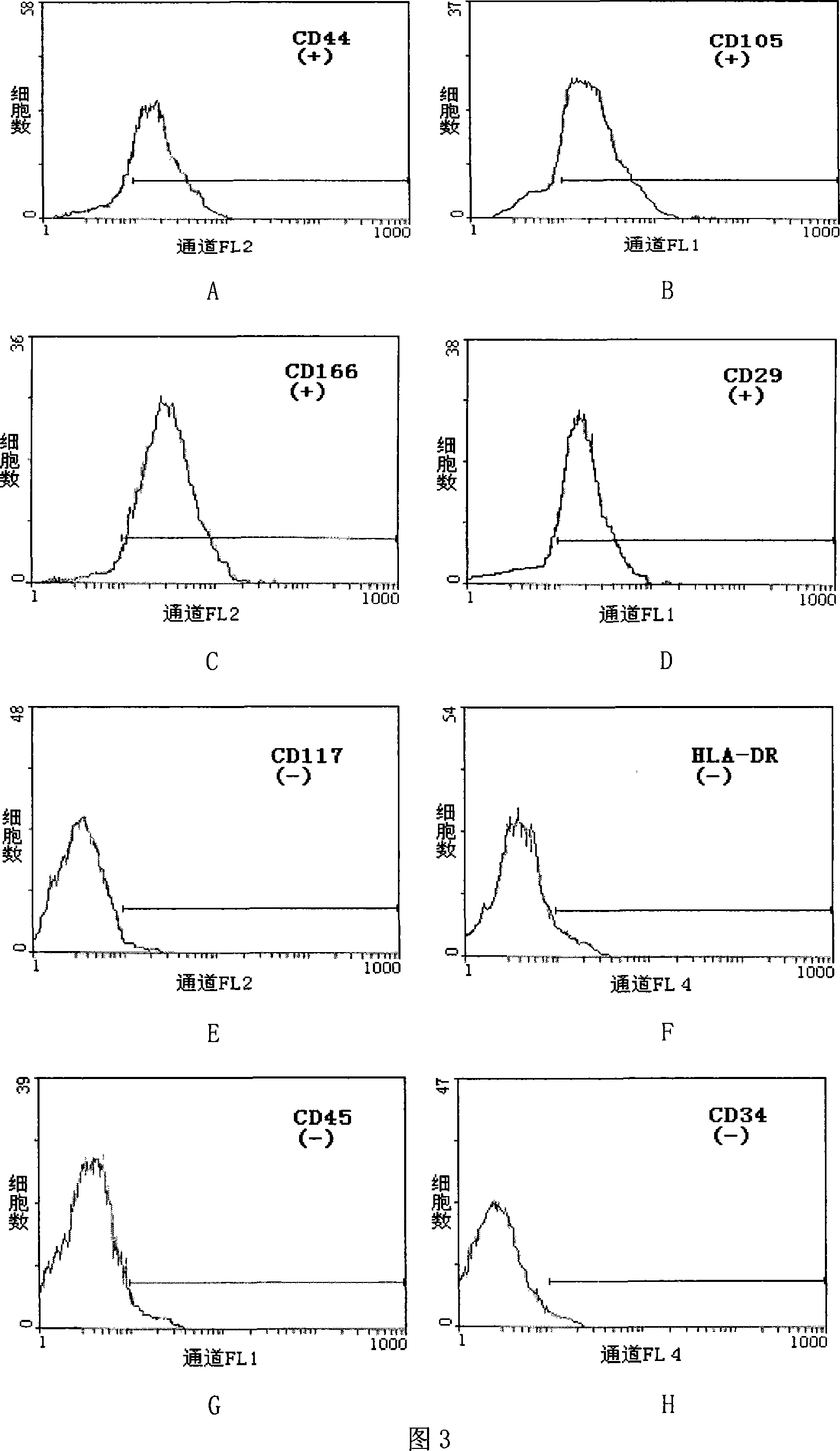
The present invention is immortal human bone marrow matrix stem cell line and its establishing process. The primary human bone marrow matrix stem cell from normally aborted fetus bone marrow tissue is single cell cloned and cultured in a finite dilution process to purify primary cell and transfected with pCIneo-hTERT gene for immortalizing. The human bone marrow matrix stem cell line is extracorporeal cultured, may be infinitely cloned and proliferated and is normal cytologic cell without conversion cell or cancer cell characteristic. The cell may be directionally induced and differentiated into bone cell, cartilage cell or cardiac muscle-like cell and has multiple direction differentiating latent and adult stem cell characteristic. It may be applied in biological engineering field. The present invention provides standard cell line for the biological research of biological behavior of human bone marrow matrix stem cell, platform for basic tissue engineering research, enough seed cells for clinical application and effective method for relevant research.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Canine primary bronchial epithelial cell and application thereof in preparation of immortalized cells
ActiveCN104531607AHigh purityOvercome the disadvantage of very limited proliferative abilityArtificial cell constructsVertebrate cellsTelomeraseBeagle
The invention provides a canine primary bronchial epithelial cell and an application thereof in preparation of immortalized cells, and belongs to the technical field of biology. The canine primary bronchial epithelial cell is prepared by taking 45-day-old clean male beagles; separating canine tracheal tissues, cutting into pieces, adding collagenase and trypsin to digest, so as to obtain intact bronchiole tissues; cultivating the obtained cells, and further carrying out indirect immunofluorescence assay to detect keratin 18 expression, wherein an experiment proves that the canine primary bronchial epithelial cell is successfully obtained. A eukaryotic expression vector pEGFP-hTERT containing telomerase genes (hTERT) is built; after the canine primary bronchial epithelial cell is transfected, the hTERT gene is highly expressed; the bronchial epithelial cell can be stably subcultured for at least 20 generations. According to the canine primary bronchial epithelial cell provided by the invention, important biological materials are provided for research of infection mechanism of canine pathogens such as canine influenza viruses.
Owner:NANJING AGRICULTURAL UNIVERSITY
Method for obtaining Chinese population individual age
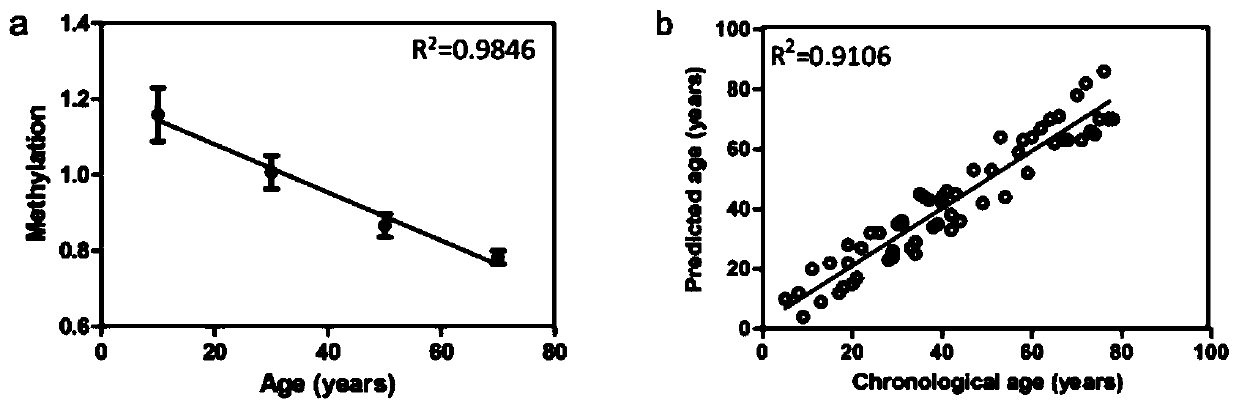
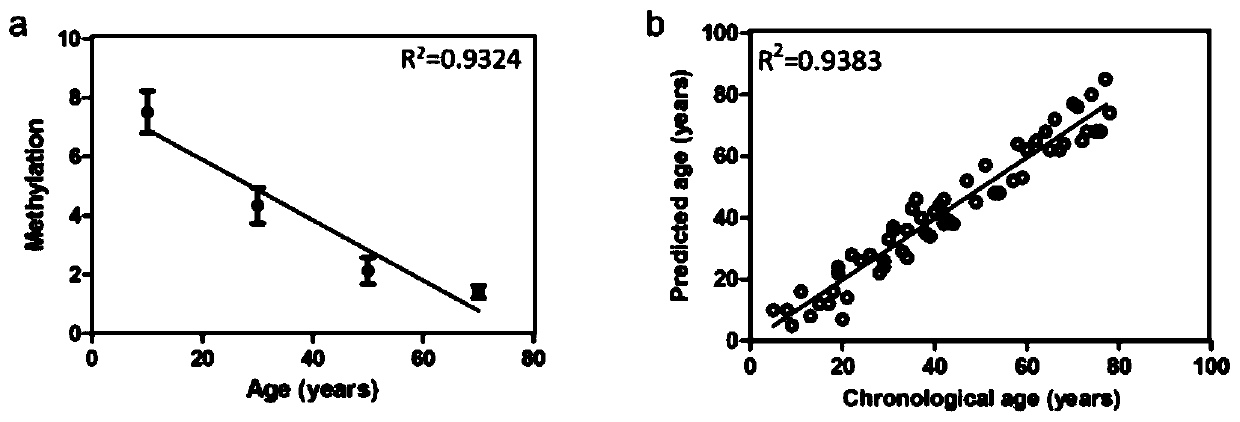
ActiveCN109943643ANarrow your searchImprove the efficiency of solving crimesMicrobiological testing/measurementDNA methylationBlood stained
The invention relates to a method for obtaining a Chinese population individual age. The method comprises the steps that DNA of a sample individual is extracted, hydrosulphite modification of DNA methylation is carried out; the methylation degree of an hTERT gene of the DNA is obtained, a regression model of the methylation degree and the age is established, and the Chinese population individual age can be obtained. The method for obtaining the Chinese population individual age achieves the inference of the individual age by using the blood or blood stain through a reliable method.
Owner:DONGHUA UNIV +1
Construction and application of immortalized primary generation sheep lung cell line
InactiveCN107384868AThe effect of stable transfectionLow toxicityGenetically modified cellsTransferasesCulture expansionBiology
The invention discloses construction and application of an immortalized primary generation sheep lung cell line. Construction comprises the following steps that fresh primary generation sheep lung fibroblasts with the high viability are obtained through a Dispase-collagenase perfusion method; after being cultured for 24 h, under the condition that polybrene with the concentration being 5 ug / ml exists, the primary generation sheep lung fibroblasts are infected through concentrated and purified cell supernatant of recombinant lentivirus containing hTERT genes; after one week, puromycin with the concentration being 2 ug / ml is used for drug pressure screening, and under the condition that the polybrene with the concentration being 5 ug / ml exists, repeated infection is conducted one time; and after one week, the primary generation sheep lung fibroblasts are cultured through puromycin maintenance media with the concentration being 1 ug / ml till monoclonal cells appear and grow to be about 2.0 cm, the monoclonal cells are selected for culture expansion, and thus the immortalized sheep lung fibroblasts with a passage characteristic can be obtained.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Kit for detecting activity degree of telomerase in whole blood or serum sample
InactiveCN104593505AHigh sensitivityAchieving absolute quantificationMicrobiological testing/measurementDNA/RNA fragmentationTelomeraseSerum samples
The invention provides a kit for detecting activity degree of telomerase in a whole blood or serum sample, belonging to the field of molecular biology detection. The kit comprises the following primers: (1) primer sequences of telomerase hTERT genes: hTERT-F: 5'-GGCCGATTGTGAACATGGA-3'; hTERT-R: 5'-CCTCTTTTCTCTGCGGAACGT-3'; and (2) probe sequences of telomerase hTERT genes: hTERT-P: 5'-TACGTCGTGGGAGCCA-3', the 5' end is connected with fluorescence reporter genes, and the 3' end is connected with fluorescence quenching genes. The kit for detecting activity degree of telomerase in the whole blood or serum sample has the advantages of high detection sensitivity, short analysis time and high stability.
Owner:SHANGHAI DIAN CLINICAL TESTING CENT
Establishing method of sheep testicle cell immortalized cell line and application of method
PendingCN109652451ALow degree of differentiationPromote generationGenetically modified cellsTransferasesExperimental researchTesticle
The invention discloses an establishing method of a sheep testicle cell immortalized cell line. The method approximately includes the following steps of firstly, establishing recombinant plasmids; secondly, preparing slow virus sample particles; thirdly, establishing and screening an hTERT gene cell line; fourthly, expressing an hTERT gene and detecting the mRNA transcriptional level of the hTERTgene; fifthly, detecting the expression of hETRT protein. By means of the establishing method, the research status of the shortage of existing cell lines can be easily improved, and the resource use and popularization are facilitated. By establishing the sheep testicle cell immortalized cell line, the use amount of experimental animals is reduced, and a more stable experimental research object isprovided.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Application of hTERT (human Telomerase Reverse Transcriptase) gene serving as reference gene in direct detection of content of cfDNA (cell-free Deoxyribonucleic Acid) in blood plasma by utilizing qPCR (quantitative Polymerase Chain Reaction)
InactiveCN107974505AEfficient removalReduce background noiseMicrobiological testing/measurementReference genesReverse transcriptase
The invention discloses application of an hTERT (human Telomerase Reverse Transcriptase) gene serving as a reference gene in direct detection of the content of cfDNA (cell-free Deoxyribonucleic Acid)in blood plasma by utilizing qPCR (quantitative Polymerase Chain Reaction). According to the application disclosed by the invention, the aim of quantifying the cfDNA is realized through detecting a house-keeping gene in DNA or certain non-coding repeated sequences which appear stably. Before the qPCR is carried out, the blood plasma is treated by utilizing a prepared buffering solution and prepared proteinase K to remove protein; special polymerase (velocity polymerase) which is used for difficult template amplification is added into a reaction mixture and the polymerase can be used for extending 1kb of basic group every 10S; after the blood plasma is diluted according to the ratio of 1 to 40, other treatment is not needed; then the blood plasma is directly added into a reaction system andis subjected to the qPCR to determine the concentration of the cfDNA in the blood plasma; the cfDNA is quantified through amplifying target sequences with two lengths. By adopting the application, error mutation caused by PCR and sequencing can be effectively removed and background noises in a detection process are reduced. With regard to 0.1 percent of detection limit, the sensitivity can reach90 percent or more and the specificity reaches 99.9 percent.
Owner:北京中源维康基因科技有限公司
Method for immortalizing human periodontal ligament stem cell line by using hTERT (human telomerase reverse transcriptase) lentivirus recombinant
ActiveCN104928319AStable in natureExtended service lifeGenetic engineeringFermentationReverse transcriptasePeriodontal ligament stem cells
The invention relates to a method for immortalizing a human periodontal ligament stem cell line by using an hTERT (human telomerase reverse transcriptase) lentivirus recombinant. The method comprises the following steps: 1, culturing primary human periodontal ligament stem cells (hPDLSC); 2, establishing an immortalized genetic engineering cell line hTERT-PDLSC stably expressing hTERT genes, to be specific, infecting the immortalized genetic engineering cell line with primarily cultured PDLSC by using a lentivirus carrier expressing the hTERT genes under a certain condition, and carrying out resistance screening of puromycin to obtain the immortalized cell line stably expressing the hTERT genes, wherein the service life of the cells is prolonged to 35 generations from 3-8 generations, and thus an immortalized cell model having stable properties, favorable functions and normal phenotype is obtained. The method disclosed by the invention has a beneficial effect on research of periodontal ligament stem cells to periodontal tissue engineering as well as research and development of medicaments for effectively treating periodontitis.
Owner:XI AN JIAOTONG UNIV
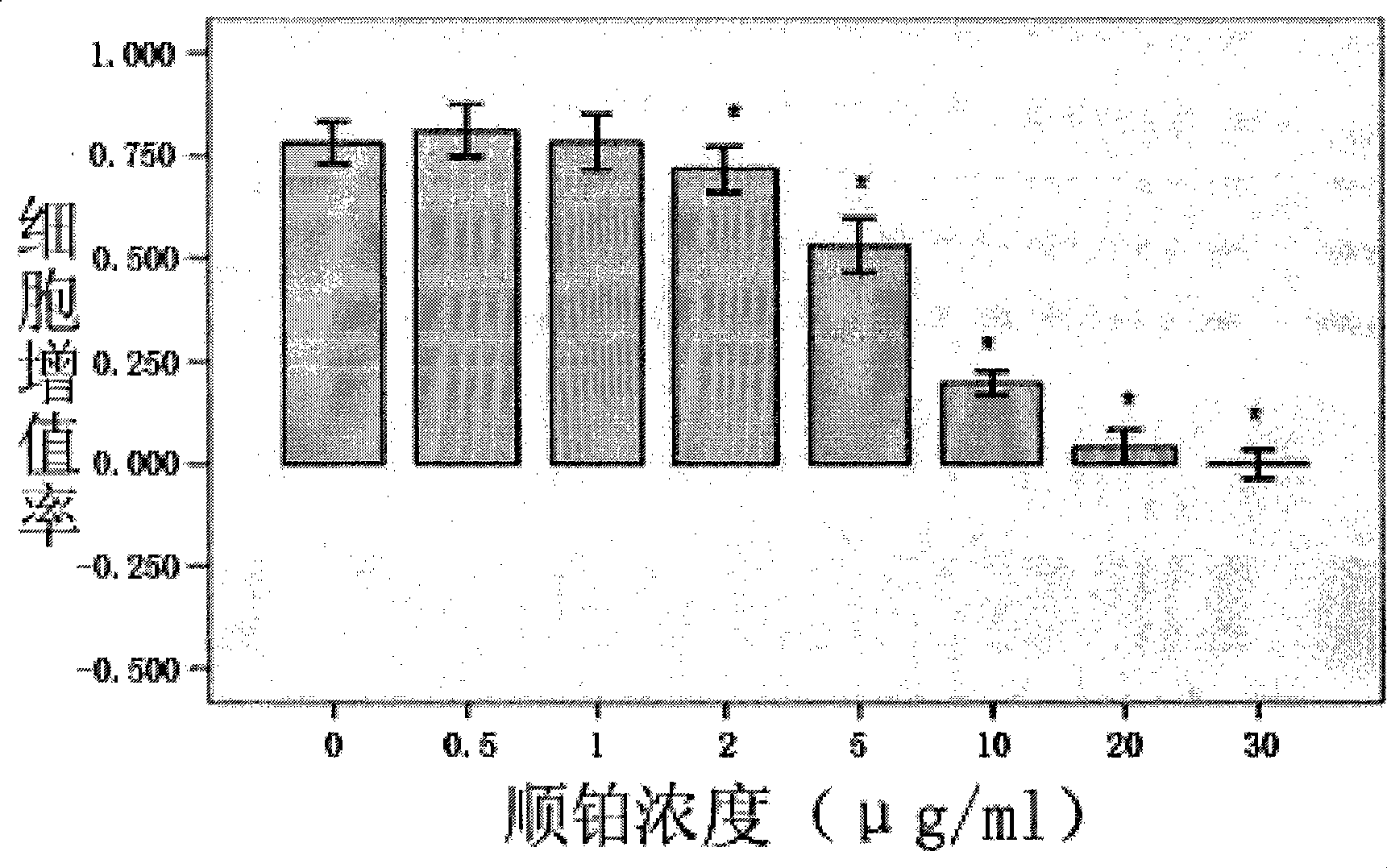
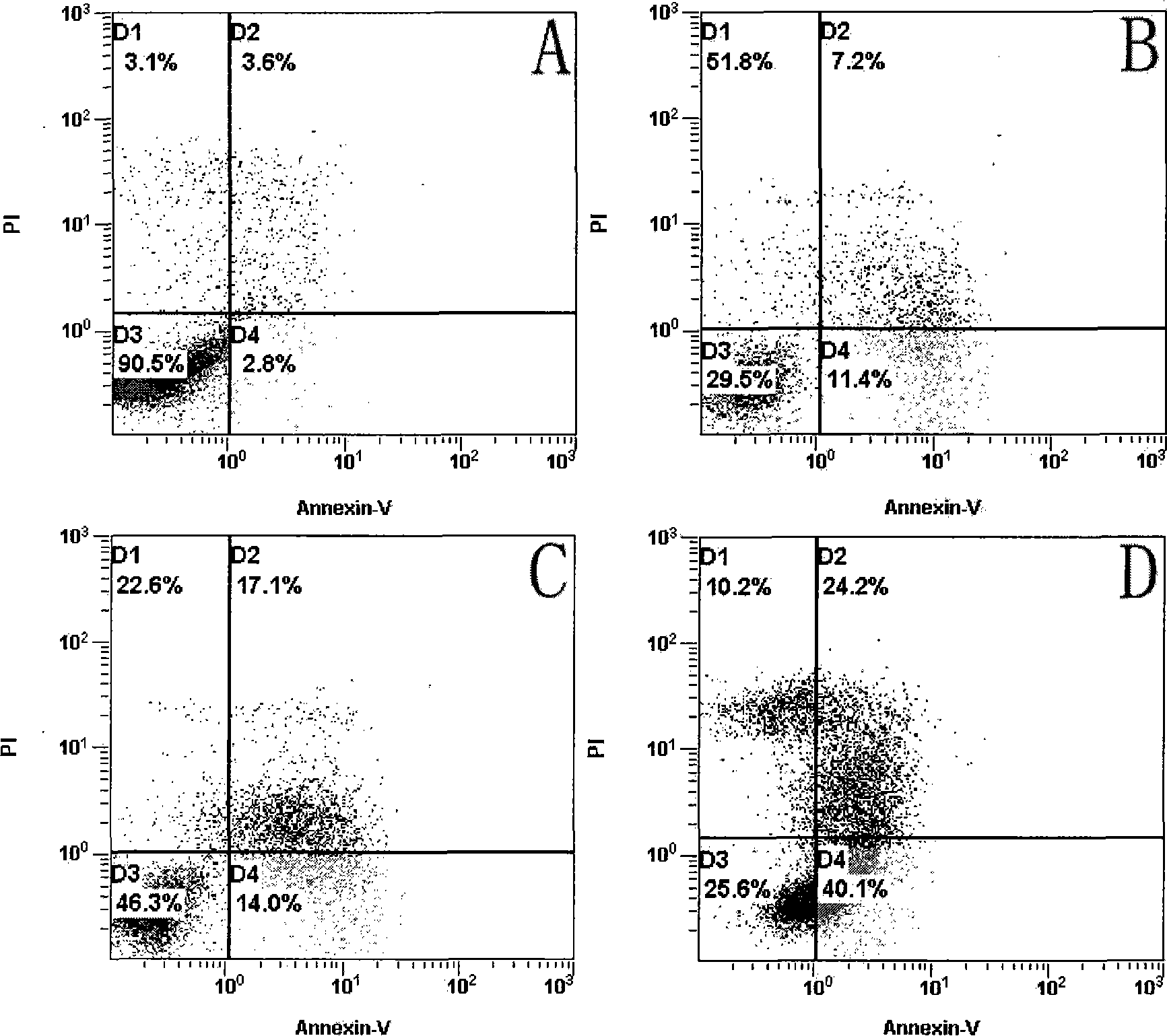
Application of cisplatin combined with hTERT-HRP/IAA in medicament for treating cervical cancer cell
InactiveCN101375860AHigh activityIncrease lethalityHeavy metal active ingredientsAntineoplastic agentsCisplatinMechanism of action
The invention discloses an application of cisplatin combined with phTERTp-HRP / IAA in drugs for the treatment of cervical cancer cells, in particular to the strengthening role of the cisplatin on the activity of a hTERT gene promoter. The cisplatin and the phTERTp-HRP / IAA are commonly used in the synergy for killing the human cervical cancer cells, and the action mechanism thereof is further explored. The application adopts Hela cells for carrying out the study and evaluates the impacts of the cisplatin on the activity of the hTERT promoter through the expression of downstream luciferase reporter gene regulated by the hTERT promoter. The synergic killing role and the mechanism of the cisplatin and the hTERT promoter during the combined use for regulating the gene therapy of an HRP / IAA system are evaluated through the detection of cell proliferation rate, cell apoptosis rate and cell cycle. The results confirm that the cisplatin can strengthen the activity of the hTERT gene promoter; and when in combined use with the phTERTp-HRP / IAA, the killing role of the human cervical cancer cells is significant.
Owner:WUHAN UNIV
Construction of down's syndrome cell model and cell bank of down's syndrome cell by employing hTERT gene recombination
InactiveCN104419676AReduce injuriesIncrease success rateMicroorganism librariesVector-based foreign material introductionEnzyme digestionAmpicillin
The invention relates to construction of a down's syndrome cell model and a cell bank of a down's syndrome cell by employing hTERT gene recombination applied to the field of medicines. The construction is mainly characterized by comprising the following processes: performing double-enzyme digestion on a plasmid pCIneo-hTERT and a carrier pLXSNneo by incision enzyme EcoR I and Xho I, and connecting hTERT and pLXSNneo enzyme-digested products which are processed by PCR amplification and gel electrophoresis separation with Ligation Mix; constructing a pLXSNneo-hTERT recombinant, and converting a DH5a competent cell for purifying amplifying and choosing ampicillin-resistant colonies to extract plasmids; transfecting down's syndrome cells which are subjected to in vitro passage by lipidosome and are in logarithmic growth; integrating the recombinants with the cell DNA, carrying out enlarging circulation, and screening cells containing positive recombinants by G418; and screening the cell of which cell morphology, growth curve, chromosome karyotype, nude mouse carcinogenicity test, transfected cell telomerase activity, hTERT mRNA expression, immumohistochemistry, cell generation cycle and cell apoptosis rate accord with the cell characteristics of an immortalized cell and are the same as or similar to those of a primary cell as an hTERT-mediated down's syndrome in-vitro study cell model and cryopreserved in liquid nitrogen, thus a foundation is laid for in vitro long-term research of pathogenesis from the cellular level.
Owner:翁炳焕
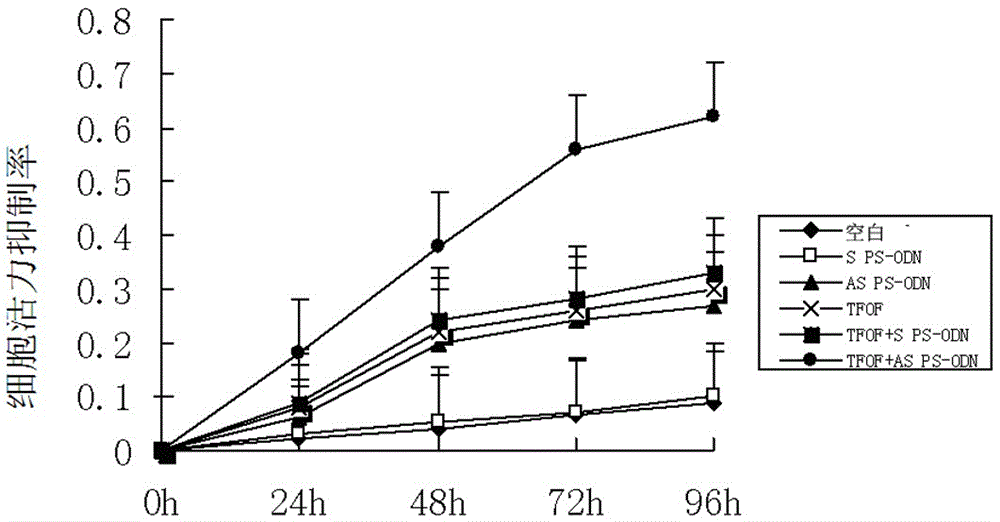
hTERT gene antisense oligodeoxynucleotide, drug composition and application
The invention provides hTERT gene antisense oligodeoxynucleotide, a drug composition and application, belonging to the technical field of gene drugs. The sequence of the antisense oligodeoxynucleotide is 5minute-GGAGCGCGCGGCATCGCGGG-3minute. After hTERT gene total phosphorothioate modified antisense oligodeoxynucleotide (AS PS-ODN) inhibits flutamide tolerance type prostate cancer cell LNCaP-flu telomerase activity, a Tibetan drug, i.e., dry whole grass of O. Falcata Bunge has a sensibilization effect on LNCaP-flu cell apoptosis.
Owner:甘肃省中医院
Method for immortalizing human exfoliated deciduous tooth endodontium stem cell line through hTERT recombinant lentivirus
InactiveCN105063089AStable in natureExtended service lifeGenetic engineeringFermentationTissue repairStem cell line
The invention relates to a method for immortalizing a human exfoliated deciduous tooth endodontium stem cell line through hTERT recombinant lentivirus. The method comprises: 1, carrying out primary culture on primary human exfoliated deciduous tooth endodontium stem cells SHED; and 2, constructing immortalized human exfoliated deciduous tooth endodontium stem cells hTERT-SHED stably expressing hTERT gene. According to the present invention, the constructed immortalized genetically engineered cells expressing the hTERT gene is adopted as the effective cell model for researching the biological characteristics of the human exfoliated deciduous tooth endodontium stem cells so as to easily and deeply research the applications of the constructed immortalized genetically engineered cells as the seed cells in the periodontal tissue engineering and the actions of different drugs on the cells and the signal transduction pathways of the related action mechanisms, and the constructed immortalized genetically engineered cells are used to screen the traditional Chinese medicine effective components for effectively treating chronic periodontitis so as to provide the powerful tool for the subsequent research of the periodontal tissue repair and reconstruction and the development of the novel effective drugs.
Owner:XI AN JIAOTONG UNIV
Construction of trisomy 21 syndrome cell model and cell bank thereof by using recombined SV40LT and hTERT genes
InactiveCN104419682AReduce injuriesIncrease success rateMicroorganism librariesVector-based foreign material introductionTrisomy X syndromePrimary cell
The invention relates to construction of a trisomy 21 syndrome cell model and a cell bank thereof by using recombined SV40LT and hTERT genes in the reproduction and genetics fields. The construction is mainly characterized in that T4DNA is used for connecting products obtained by carrying out digestion on plasmid SV40LTag DNA and a carrier pcDNA3.1(-) DNA with a BamHi enzyme to construct an SV40LTag-pcDNA3.1(-) recon; Ligation Mix is used for connecting products obtained by carrying out double digesion on plasmid pCIneo-hTERT and a carrier pLXSNneo with EcoR I and Xho I to construct a pLXSNneo-hTERT recon; the two recons are subjected to competent cell purification and identification and then are transfected into trisomy 21 syndrome cells, positive cells are subjected to G418 screening and amplification culture, and then cells, which meet the immortalized cell characters and are as same as or similar to primary cells, are selected as an SV40LT and hTERT combined mediated trisomy 21 syndrome cell model. Therefore, the foundation of in vitro long-term research of pathogenesis of trisomy 21 syndrome based on the cellular level is established.
Owner:翁炳焕
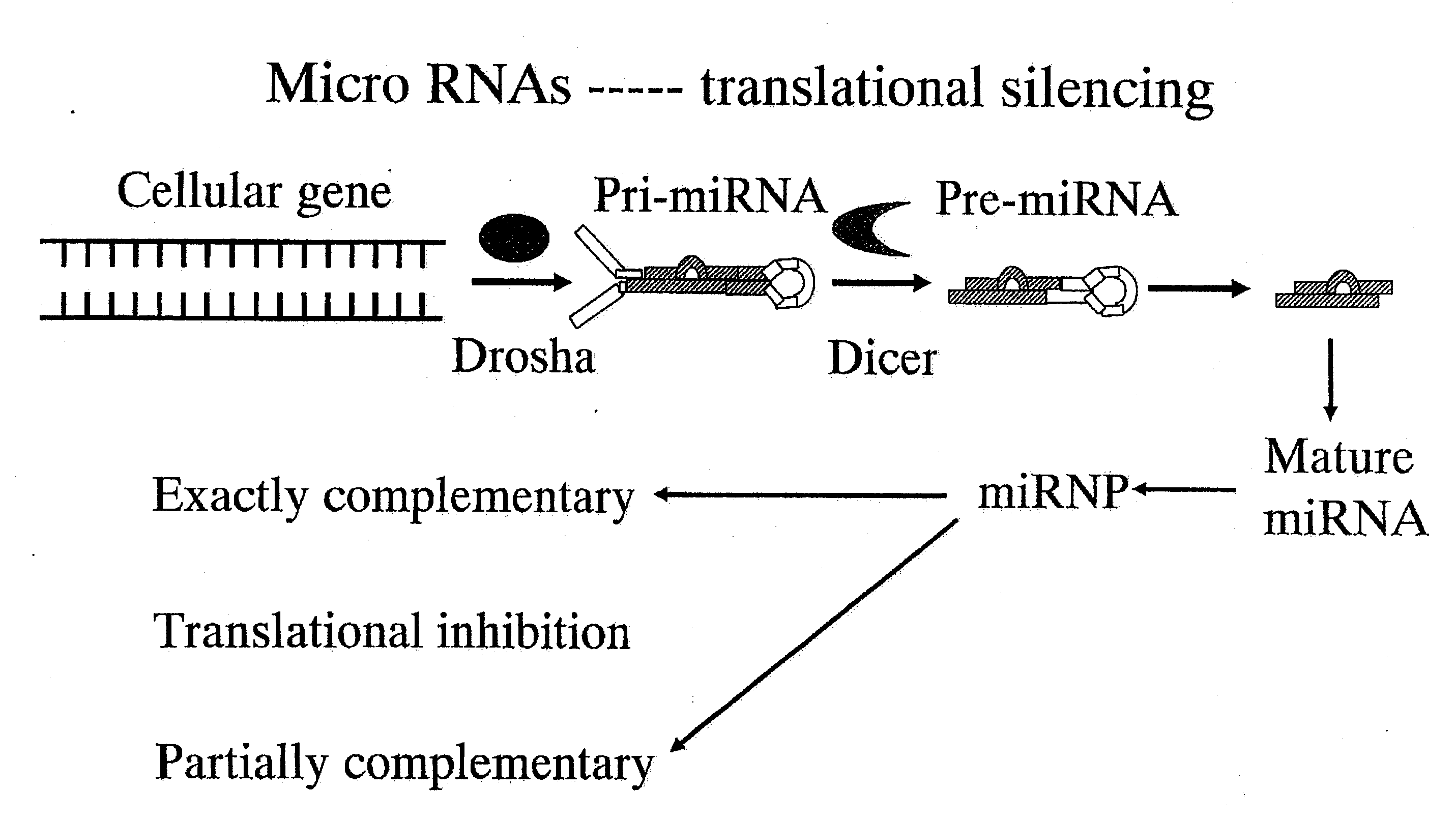
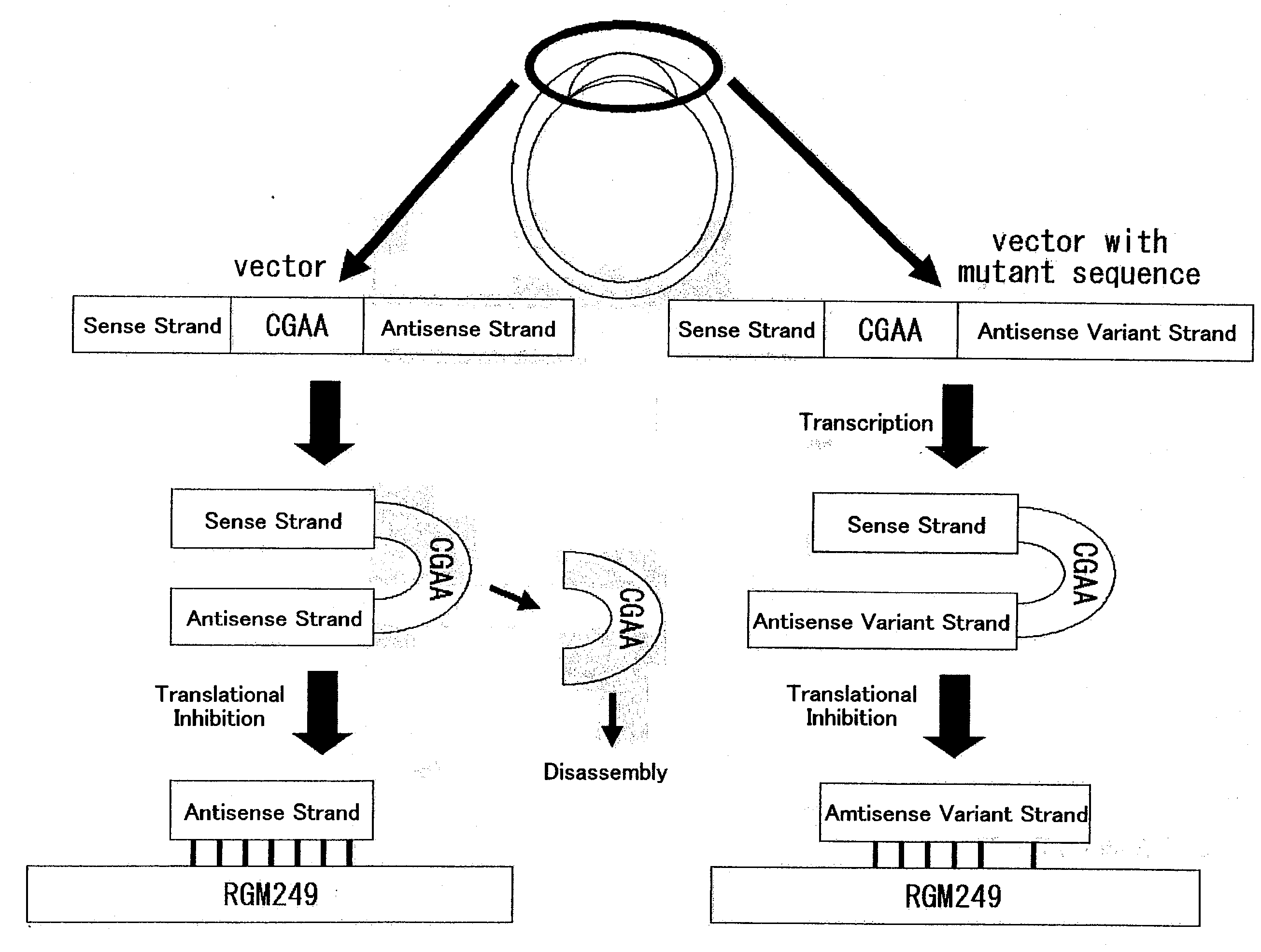
hTERT GENE EXPRESSION REGULATORY GENE
Disclosed is a novel substance capable of regulating the expression of a telomerase reverse transcriptase gene in a cell of a mammal. A gene capable of regulating the expression of hTERT, comprising a nucleotide sequence depicted in SEQ ID No: 1 or 2. The expression of a telomerase reverse transcriptase gene can be inhibited by inhibiting the expression of the gene. By utilizing this mechanism, the expression of a telomerase reverse transcriptase gene can be regulated.
Owner:TOTTORI UNIVERSITY
Insulin expressing human pancreatic islet cell line capable of reversibly proliferating and use thereof
A reversibly immortalized human pancreatic islet cell line containing an hTERT gene and an SV40T gene each interposed between a pair of LoxP sequences, characterized in that it is capable of producing insulin and enhancing expression of insulin after excising the hTERT gene and the SV40T gene, in particular, NAKT-13 (deposited with International Patent Organism Depository, National Institute of Advanced Industrial Science and Technology, address: AIST Tsukuba Central 6, 1-1, Higashi 1-Chome, Tsukuba-shi, Ibaraki-ken, 305-8566 Japan, deposited date: Sep. 4, 2003, accession number: FERM BP-08461) or a passage cell line thereof; a human pancreatic islet cell obtained by excising the hTERT gene and the SV40T gene from the reversibly immortalized human pancreatic islet cell line or passage cell line thereof; and use of these cells. By using the reversibly immortalized human pancreatic islet cell line of the invention insulin-producing cells can be easily and surely obtained in a number enough to meet the demand.
Owner:NORIAKI TANAKA +2
Recombinant replication-defective adenovirus for expressing hTERT (human telomerase reverse transcriptase) gene and application thereof
The invention provides a recombinant replication-defective adenovirus for expressing an hTERT (human telomerase reverse transcriptase) gene. A target gene hTERT is obtained by virtue of enzyme digestion from a plasmid pIRES2-EGFP(enhanced green fluorescent protein)-hTERT, the target gene hTERT is inserted to downstream of a cytomegalovirus promoter in a pSG218 shuttle vector to obtain a recombinant shuttle vector with the hTERT gene; and the recombinant shuttle vector and a virus framework vector pPE3-F11B are co-transferred to a human embryo kidney 293 cell to obtain replication-defective adenovirus with hTERT. The recombinant adenovirus infects a human multiple myeloma cell, so that the cell excessively expresses the hTERT gene, and therefore, drug resistance of the cell on pharmorubicin is strengthened. The recombinant replication-defective adenovirus disclosed by the invention is mainly used for establishing a multiple myeloma cell strain model for excessively expressing hTERT gene, and further researching a mechanism that the hTERT gene participates in drug resistance of the multiple myeloma cell based on the model.
Owner:ZHEJIANG UNIV
Method, sequences, compositions and kit for detection of mutations in the htert gene promoter
InactiveCN109790580AMicrobiological testing/measurementVector-based foreign material introductionDiseaseHepatocellular carcinoma
The present invention relates to a method for detecting c.-124 C>T and c.-146 C>T mutations in the Htert gene promoter. The method uses reaction compositions that comprise amplification primers and genotyping probes. Another aspect of this invention relates to the primers and probes used to implement the aforementioned method, with sequences, identified as SEQ ID no.1 to SEQ ID no.6, that displayhigh specificity for these mutations, and to the compositions containing these primers and probes. The present invention further relates to a kit comprising the above-mentioned compositions for detecting c.-124 C>T and c.-146 C>T mutations in the Htert gene promoter by carrying out the method according to the present invention. The method, gene sequences, compositions and kit of the present invention can be advantageously used for detecting trace amounts of c.-124 C>T and c.-146 C>T mutations in biological samples, due to their high sensitivity and specificity for such mutations. The present invention can thus be applied in early detection, identification, detection of recurrence or prediction and monitoring of diseases associated with this type of mutations, such as bladder carcinomas, thyroid carcinomas, squamous cell carcinomas, basal cell carcinomas, melanomas, gliomas and hepatocellular carcinomas, inter alia, and ultimately provide the basis for defining a suitable treatment. Thus, the present invention pertains to the technical fields of medicine, pharmaceutics, molecular biology, biochemistry, human genetics and the like.
Owner:IPATIMUP INST DE PATOLOGIA E IMUNOLOGIA MOLECULAR DA UNIV DO PORTO
hTERT GENE EXPRESSION REGULATORY GENE
Disclosed is a novel substance capable of regulating the expression of a telomerase reverse transcriptase gene in a cell of a mammal. A gene capable of regulating the expression of hTERT, comprising a nucleotide sequence depicted in SEQ ID No: 1 or 2. The expression of a telomerase reverse transcriptase gene can be inhibited by inhibiting the expression of the gene. By utilizing this mechanism, the expression of a telomerase reverse transcriptase gene can be regulated.
Owner:TOTTORI UNIVERSITY
Insulin-expressing human islet cell lines capable of reversibly proliferating and use thereof
Reversibly immortalized human islet cell lines containing an hTERT gene and an SV40T gene each located between a pair of LoxP sequences, characterized by being capable of producing insulin and the expression of insulin being enhanced after removing the hTERT gene and the SV40T gene, in particular, NAKT-13 (having been deposited with International Patent Organism Depositary, National Institute of Advanced Industrial Science and Technology, Address: Tsukuba Central 6, Higashi 1-1-1, Tsukuba, Ibaraki, 305-8566 JAPAN, Deposition date: 04 September, 2003, Deposition No.: FERM BP-08461) or passage cell lines thereof; human islet cells obtained by removing the hTERT gene and the SV40T gene from the reversibly immortalized human islet cell lines or passage cell lines thereof as described above; and use of these cells. By using the reversibly immortalized human islet cell lines as described above, insulin-producing cells can be easily and surely obtained in a number enough to meet demand.
Owner:KURARAY CO LTD +2
hTERT GENE EXPRESSION REGULATORY GENE
Disclosed is a novel substance capable of regulating the expression of a telomerase reverse transcriptase gene in a cell of a mammal A gene capable of regulating the expression of hTERT, comprising a nucleotide sequence depicted in SEQ ID No: 1 or 2. The expression of a telomerase reverse transcriptase gene can be inhibited by inhibiting the expression of the gene. By utilizing this mechanism, the expression of a telomerase reverse transcriptase gene can be regulated.
Owner:TOTTORI UNIVERSITY
Method for transduction of bone marrow mesenchyma stem cell by human telomerase gene
InactiveCN101063150BIncrease success rateVector-based foreign material introductionDNA/RNA fragmentationEnzyme GeneLiposome
Owner:ZHEJIANG UNIV
A kind of immortalized tree shrew intestinal epithelial cell line and its construction method and application
ActiveCN108486156BEasy to shapeAvoid efficiencyGastrointestinal cellsMicrobiological testing/measurementTelomeraseThelial cell
The invention relates to an immortalized tree shrew small intestinal epithelial cell line and its construction method and application, belonging to the field of cell technology. The present invention activates the telomerase activity of tree shrew intestinal epithelial cells by using lentivirus-mediated hTERT gene transfection to obtain the cell line, and can rapidly and effectively establish immortalized tree shrew intestinal epithelial cells, and the established immortalized Tree shrew intestinal epithelial cells have successfully overcome replicative senescence and acquired immortality, and the immortalized tree shrew intestinal epithelial cells have maintained a phenotype very similar to that of primary tree shrew intestinal epithelial cells, maintaining cell contact inhibition and cell proliferation. Density is limited, and CPE can appear after infection with reovirus, so it can be used for research on virus infection and the like.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
A kind of indolinone compound and its preparation method and application
ActiveCN109574907BInhibitory activityInhibit expressionOrganic chemistryAntineoplastic agentsPhenyl groupPromoter
Owner:TIANJIN UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com