H3N2 and H3N8 subgenetype dog influenza divalent inactivated vaccine and preparation method and application thereof
A technology of H3N2 and H3N8, which is applied in the field of H3N2 and H3N8 subtype canine influenza bivalent inactivated vaccine and its preparation, and can solve the problems of vaccine marketing and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1, the discovery of H3N2 subtype canine influenza virus candidate vaccine strain
[0055] 1. Discovery of new branch H3N2 subtype canine influenza virus strain
[0056] New branch H3N2 subtype canine influenza virus strain A / canine / Beijing / 0528-147 / 2017 Molecular identification results of 147: the sequence has been uploaded to GenBank, sequence number: PB2 (MK212678, 2017 / 05 / 28), PB1 (MK212679, 2017 / 05 / 28), PA(MK212680, 2017 / 05 / 28), HA(MK212685, 2017 / 05 / 28), NP(MK212682, 2017 / 05 / 28), NA(MK212683, 2017 / 05 / 28), M (MK212684, 2017 / 05 / 28), NS (MK212681, 2017 / 05 / 28).
[0057] The virus strain A / canine / Beijing / 0528-147 / 2017 147 was preserved in the Culture Collection Center of the Institute of Microbiology, Chinese Academy of Sciences on April 29, 2019 (address No. 3, Courtyard No. 1, Beichen West Road, Chaoyang District, Beijing) , the preservation number is CGMCC NO.17589, and the classification is named H3N2 subtype canine influenza virus.
[0058] The molecu...
Embodiment 2
[0078] The preparation of embodiment 2, H3N2 and H3N8 canine influenza virus bivalent inactivated vaccine
[0079] (1) Preparation of H3N2 and H3N8 Canine Influenza Virus Whole Virus Bivalent Inactivated Vaccine
[0080] 1. Screening of vaccine virus strains
[0081] According to the titer detected in Example 1 above, the HA titer of the candidate strain for preparing the whole virus vaccine is too low, so the following passages are carried out:
[0082] The vaccine candidate virus strains A / canine / Beijing / 0528-147 / 2017 147 (H3N2), A / canine / Quanzhou / 1224-1109 / 2018 (H3N2) chicken embryo allantoic fluid and A / canine / Iowa / 13628 / 2005 (H3N8) chicken embryo allantoic fluid were made 10 times -1 ~10 -3 Dilute, inoculate 9-day-old to 11-day-old SPF chicken embryos through the allantoic cavity, the inoculation volume is 0.2mL / embryo, and incubate at 37°C. Aseptically collect the allantoic fluid of chicken embryos from 48h to 96h, measure the hemagglutination titer (HA) with 1% chi...
Embodiment 3
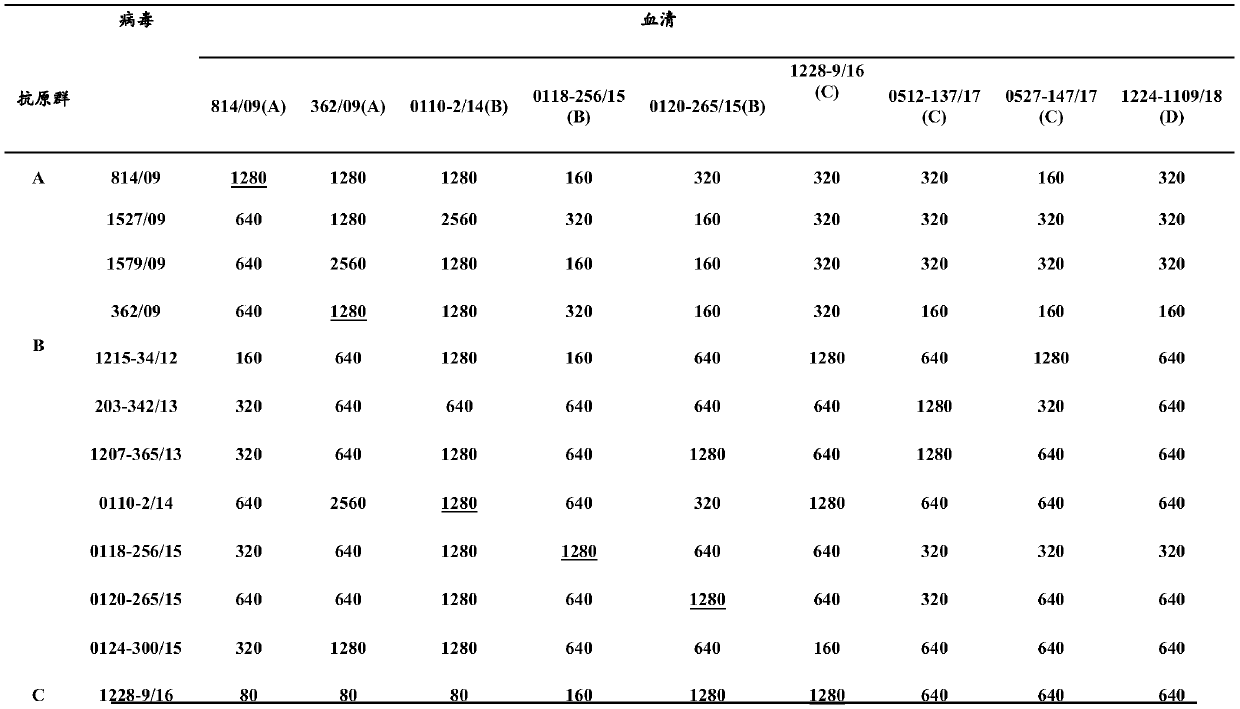
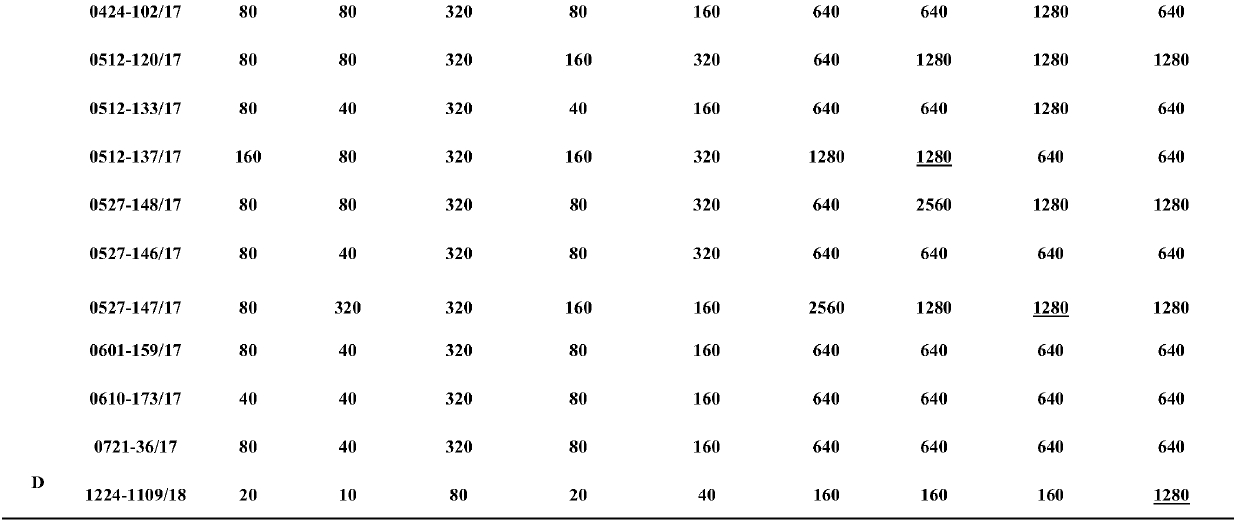
[0244] Example 3, Immunogenicity and protective evaluation of H3N2 canine influenza virus and H3N8 canine influenza virus bivalent inactivated vaccine
[0245] 1. Canine vaccine efficacy test
[0246] 1.1 Animal grouping
[0247] 93 8-week-old Beagle dogs were randomly divided into 31 groups, all of which were negative for H3 subtype influenza virus antibody in HI test: Group 1-1, Group 1-2, Group 1-3, Group 1-4, Group 1- Groups 5 and 1-6 are H3N2 (A / canine / Beijing / 0528-147 / 2017 147) and H3N8 canine influenza virus whole virus bivalent inactivated vaccine groups; Group 2-1, Group 2-2, Group 2- Group 3, Group 2-4, Group 2-5 and Group 2-6 are H3N2 (A / canine / Quanzhou / 1224-1109 / 2018) and H3N8 canine influenza virus whole virus bivalent inactivated vaccine group; Group 3-1 , Group 3-2, Group 3-3, Group 3-4 Group 3-5 and Group 3-6 are bivalent recombinant virus inactivated vaccine groups for H3N2-2017 and H3N8 canine influenza virus; Group 4-1, 4 -2 groups, 4-3 groups, 4-4 groups...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com