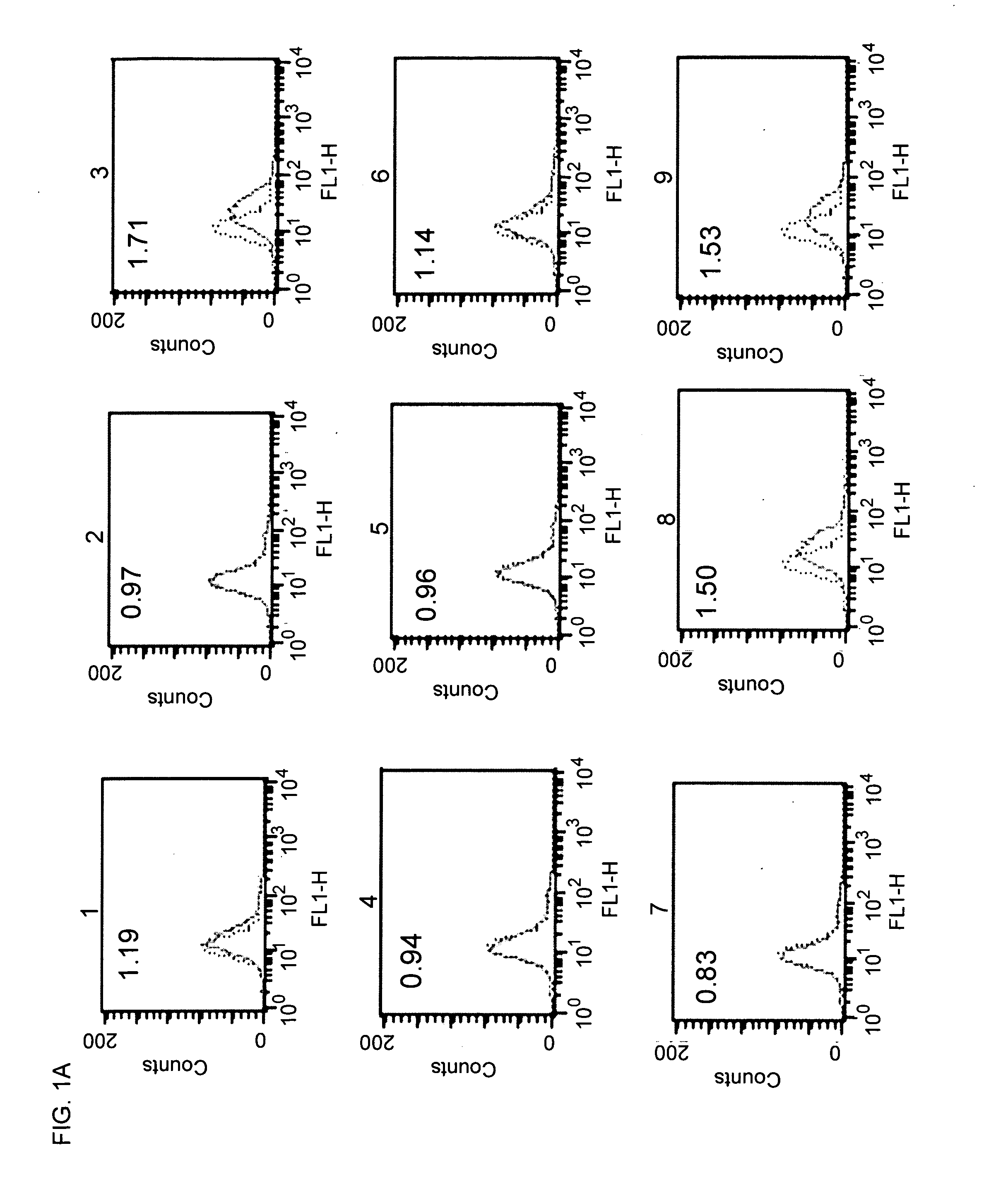
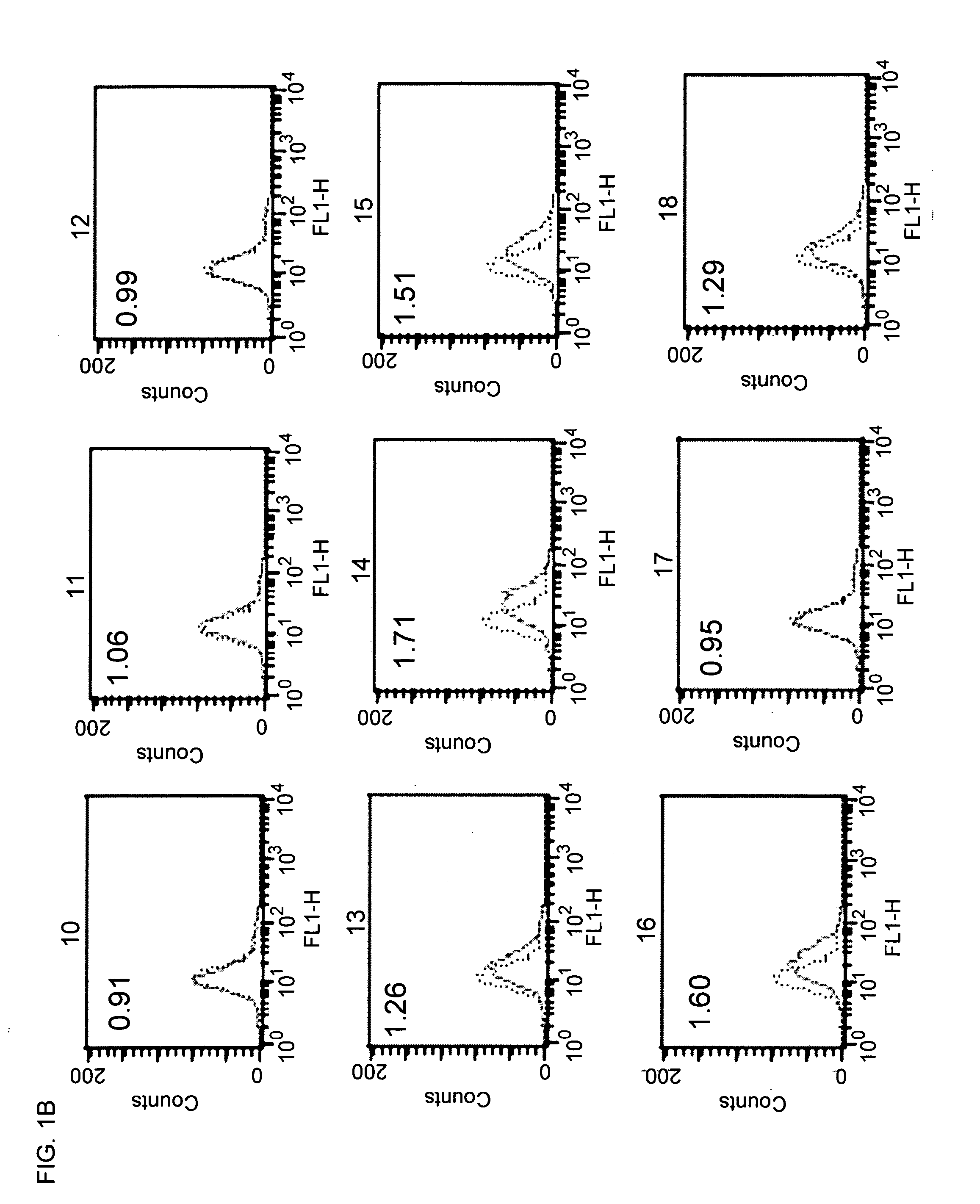
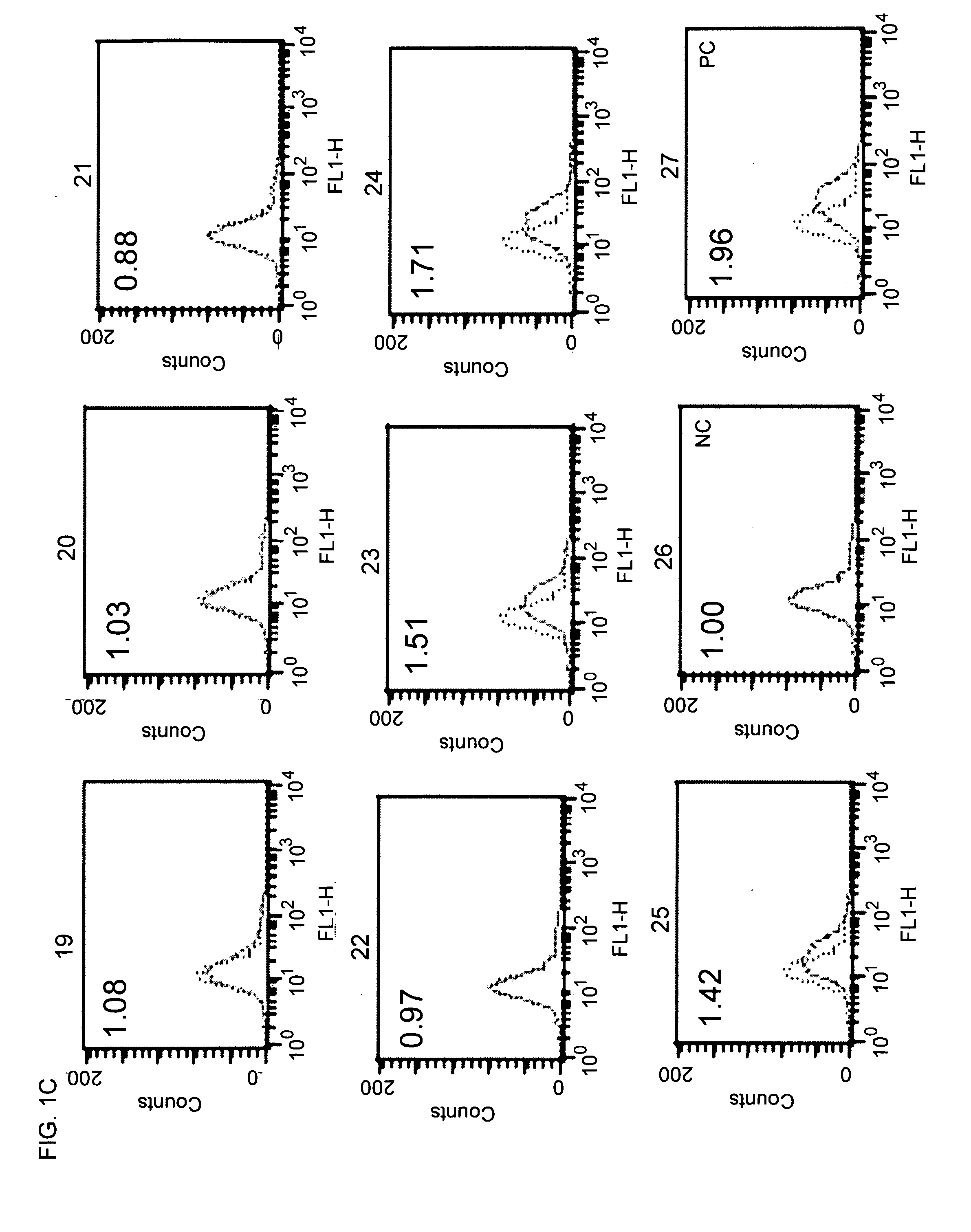
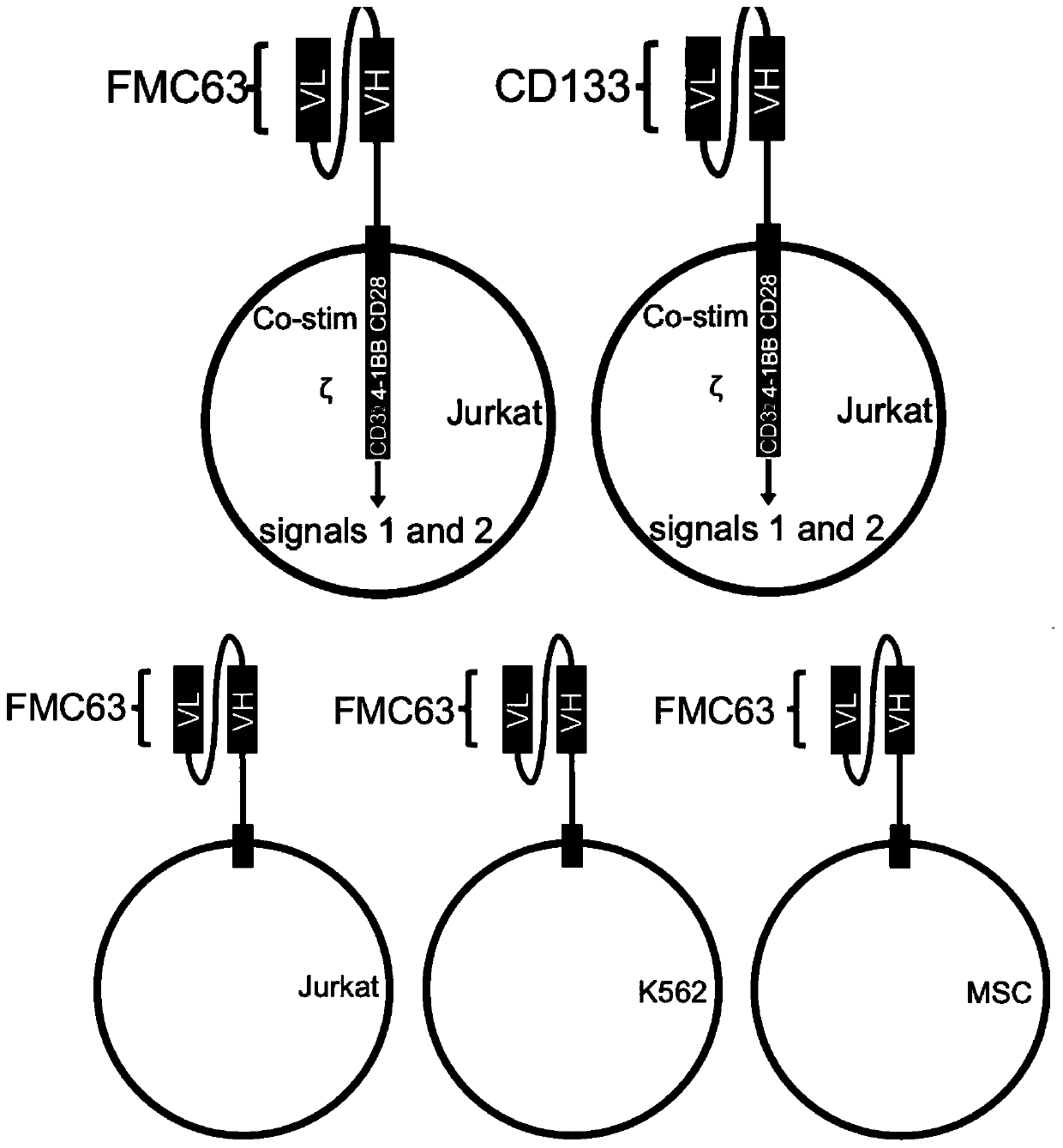
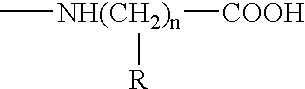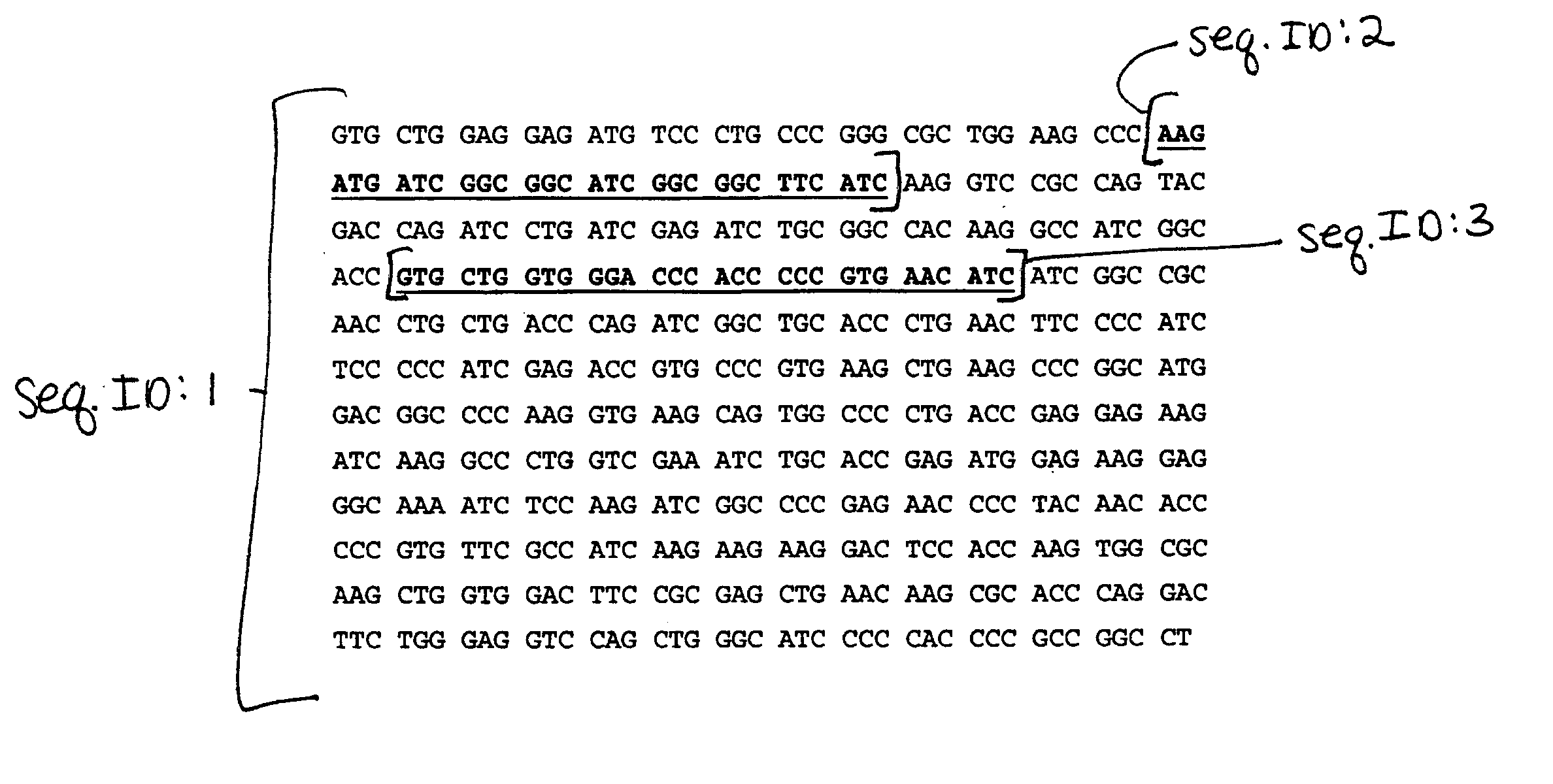Patents
Literature
40 results about "Hla restriction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for differentially quantifying naturally processed hla-restricted peptides for cancer, autoimmune and infectious diseases immunotherapy development
ActiveUS20130096016A1Efficient use ofBiological material analysisLibrary member identificationDiseaseAntigen
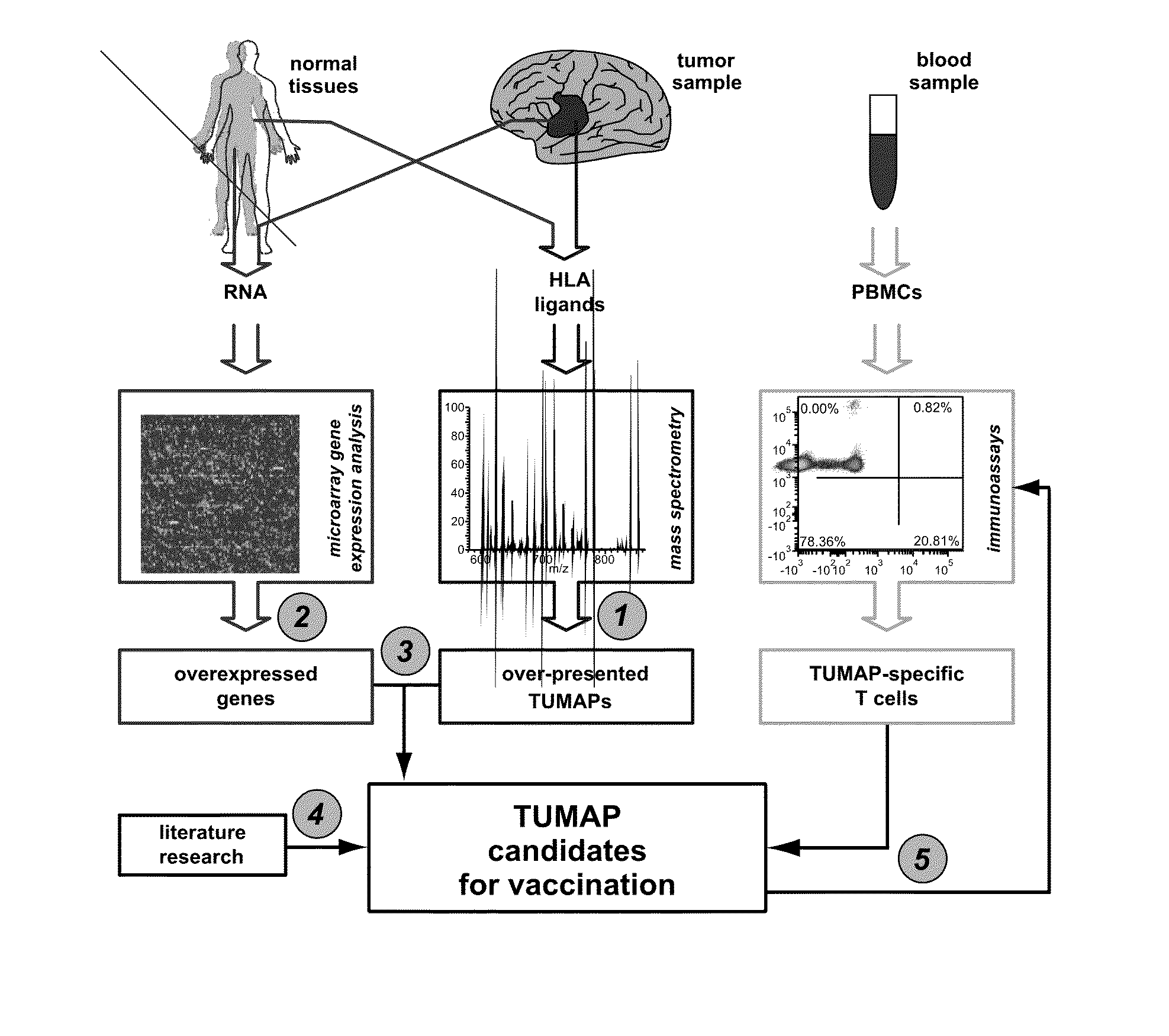
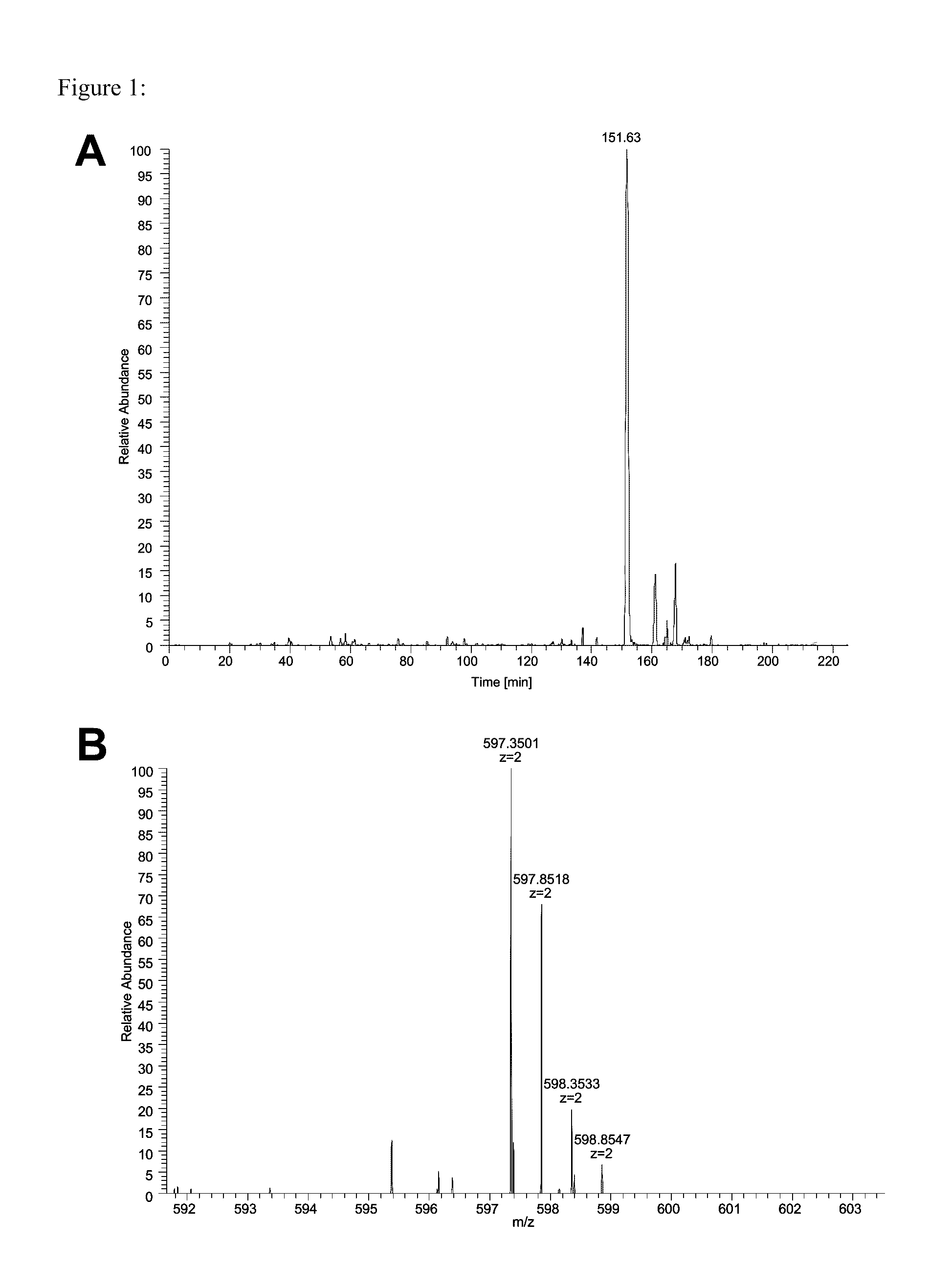
The invention relates to a method for quantitatively identifying relevant HLA-bound peptide antigens from primary tissue specimens on a large scale without labeling approaches. This method can not only be used for the development of peptide vaccines, but is also highly valuable for a molecularly defined immunomonitoring and the identification of new antigens for any immunotherapeutic strategy in which HLA-restricted antigenic determinants function as targets, such as a variety of subunit vaccines or adoptive T-cell transfer approaches in cancer, or infectious and autoimmune diseases.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Anti-cancer vaccines
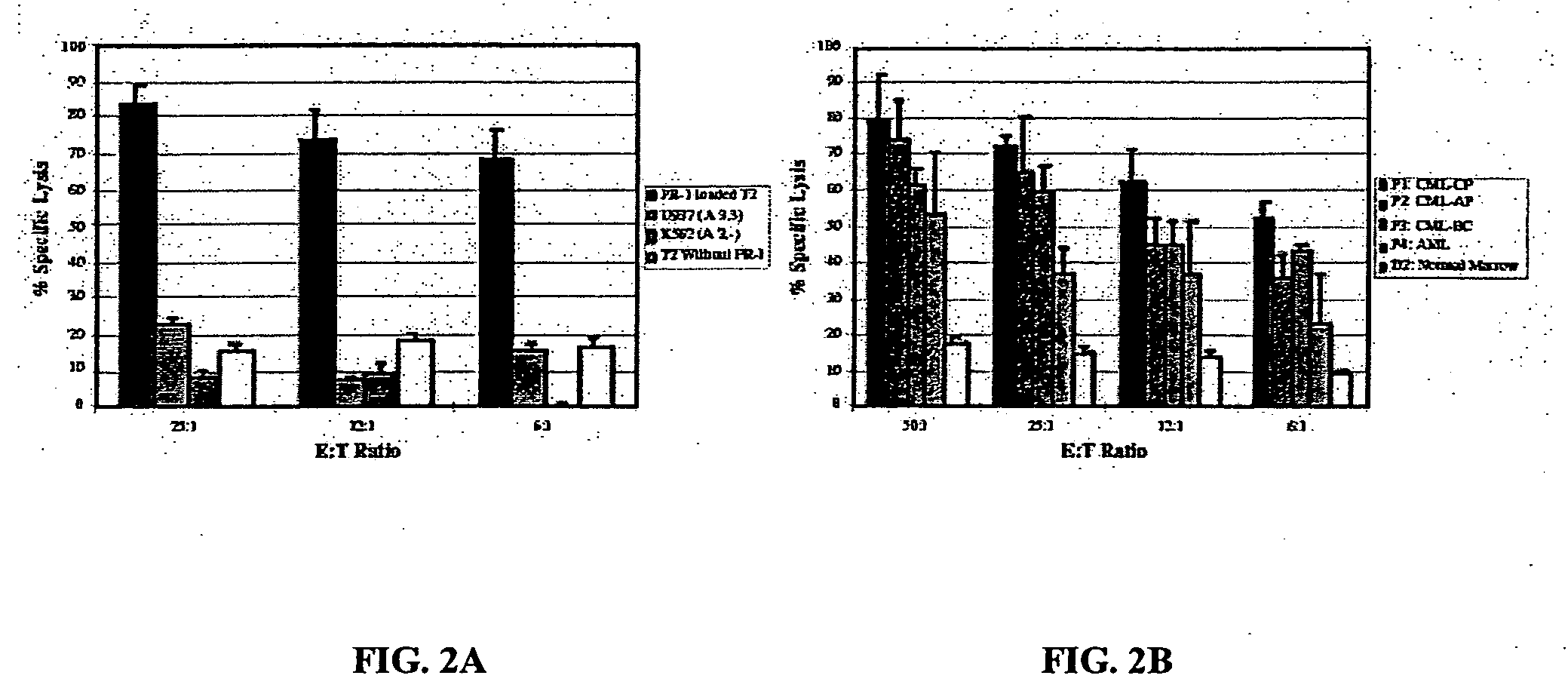
The present provides tumor-associated HLA-restricted antigens, and in particular HLA-A2 restricted antigens, as vaccines for treating or preventing cancers in a patient. In specific aspects, neutrophil elastase peptides other than PR1, cyclin E1 peptides, cyclin D peptides, or cyclin E2 peptides are provided. Such peptides can be used to elicit specific CTLs that preferentially attack tumor cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method for Differentially Quantifying Naturally Processed HLA-Restricted Peptides for Cancer, Autoimmune and Infectious Diseases Immunotherapy Development
The invention relates to a method for quantitatively identifying relevant HLA-bound peptide antigens from primary tissue specimens on a large scale without labeling approaches. This method can not only be used for the development of peptide vaccines, but is also highly valuable for a molecularly defined immunomonitoring and the identification of new antigens for any immunotherapeutic strategy in which HLA-restricted antigenic determinants function as targets, such as a variety of subunit vaccines or adoptive T-cell transfer approaches in cancer, or infectious and autoimmune diseases.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
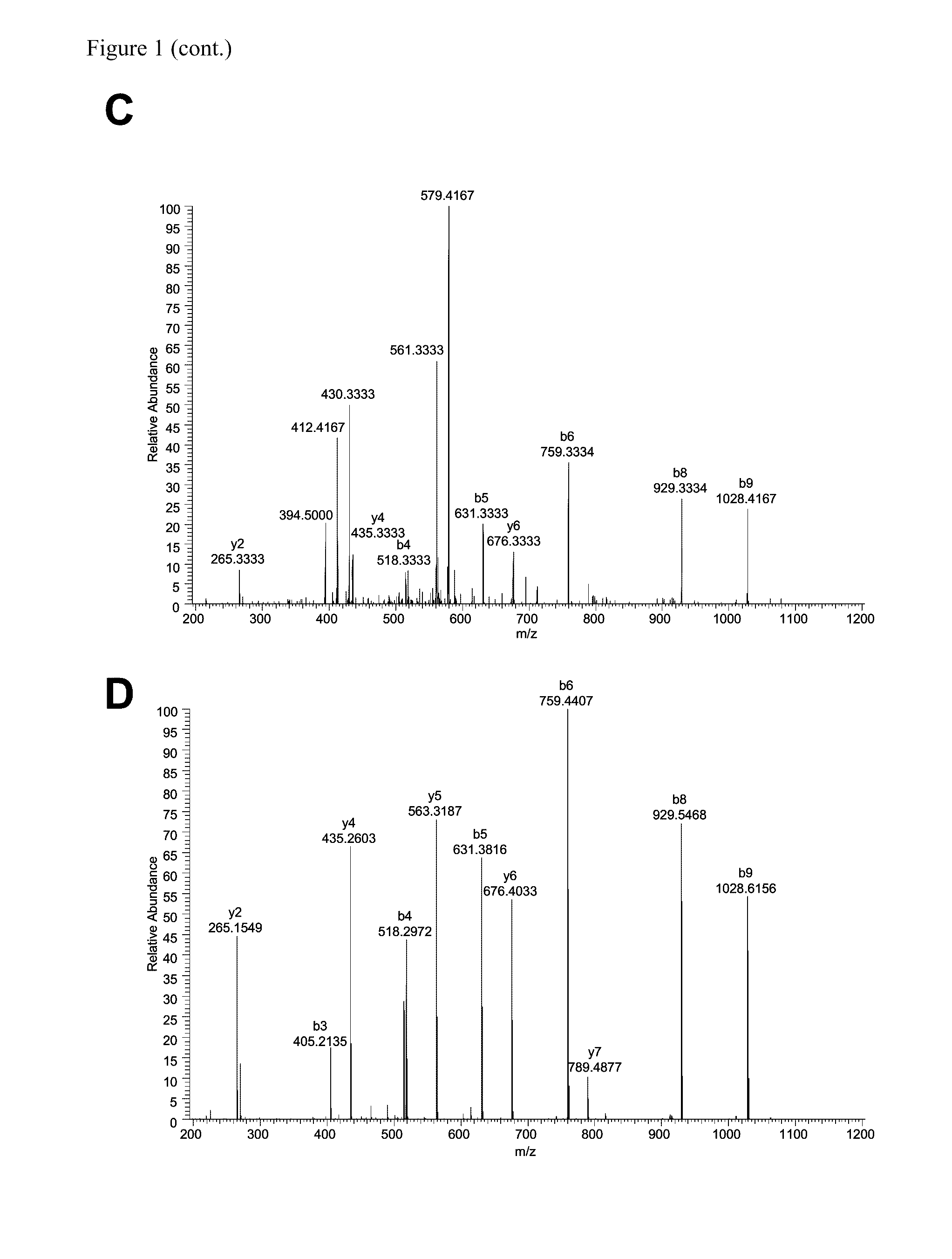
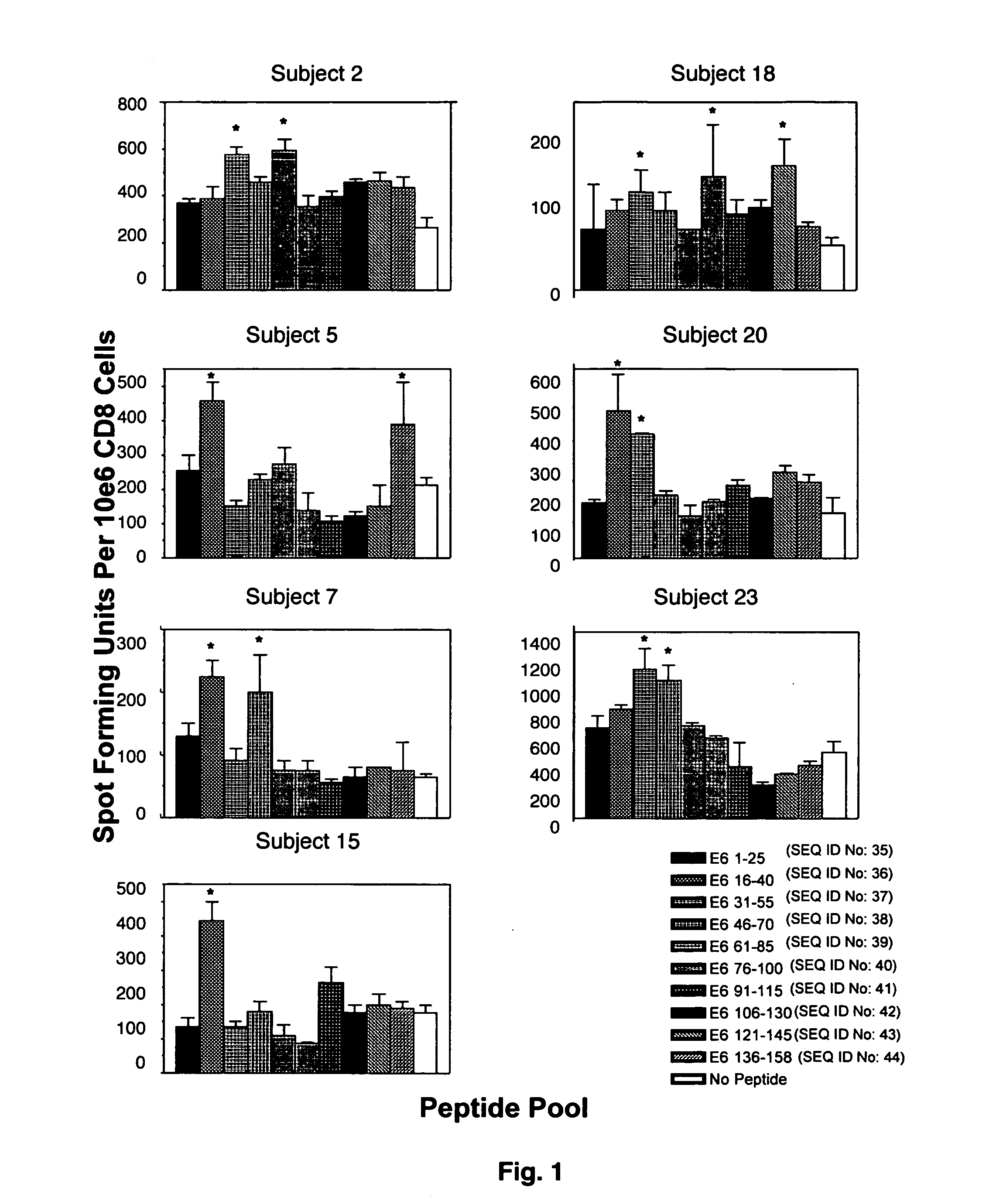
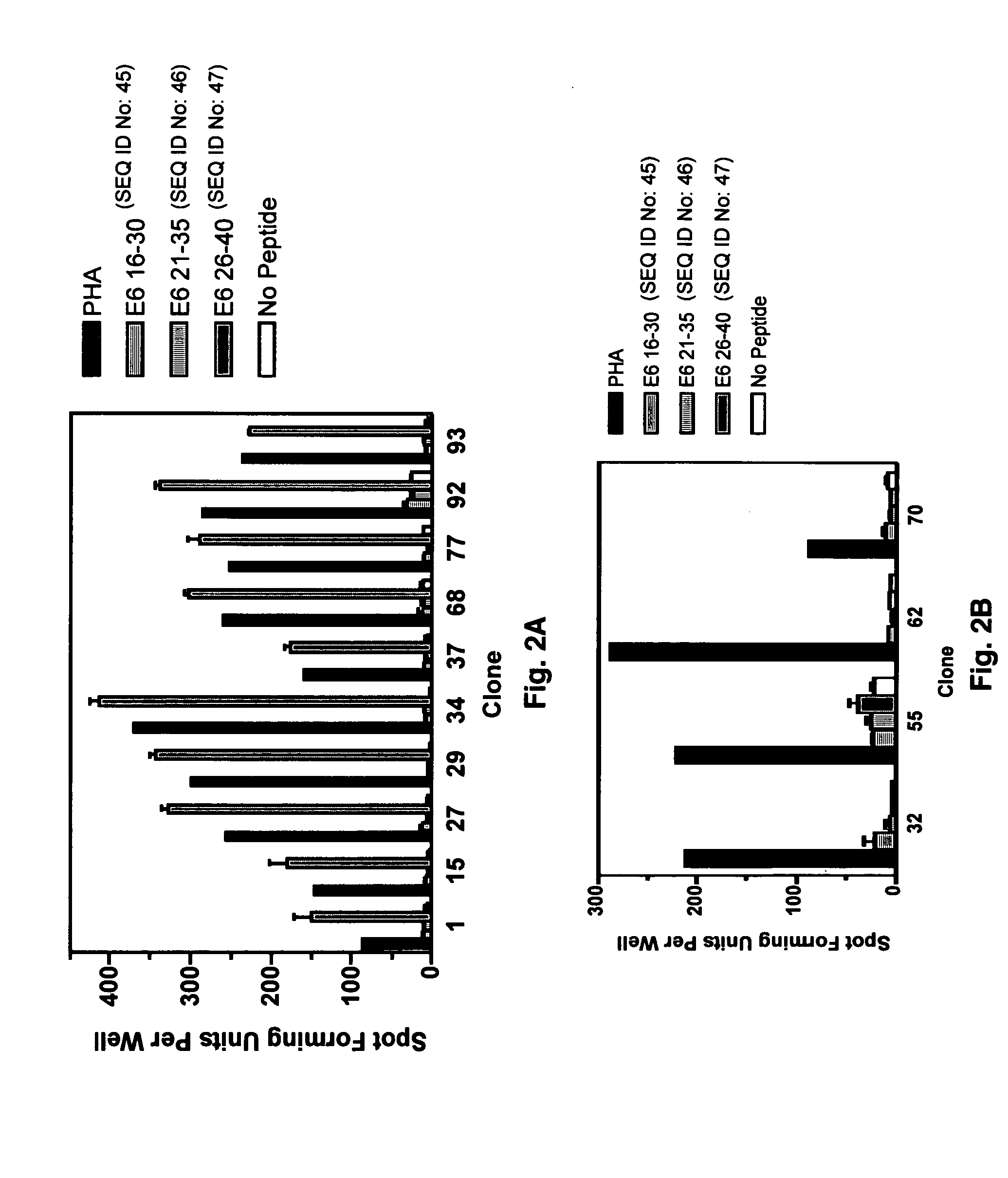
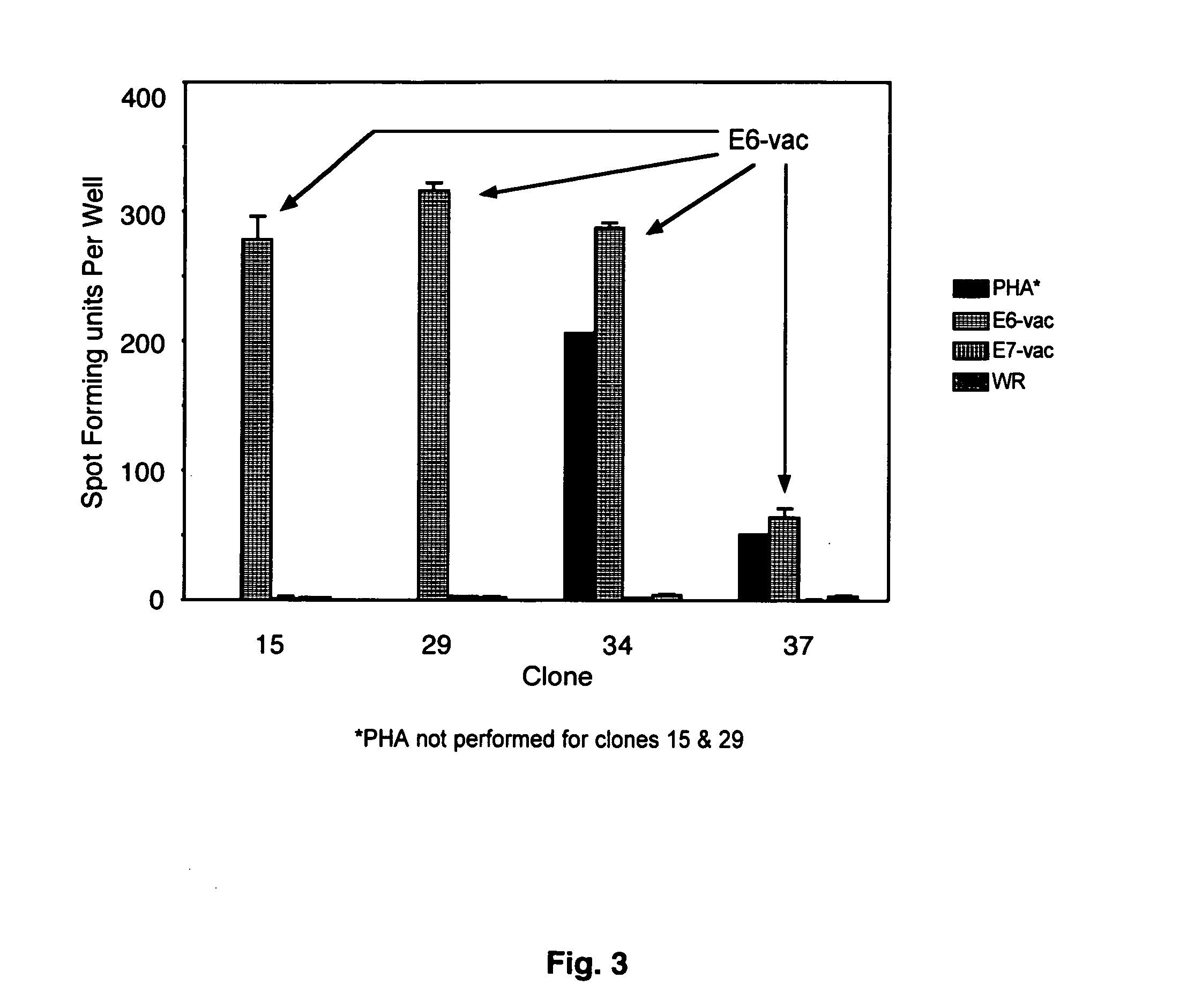
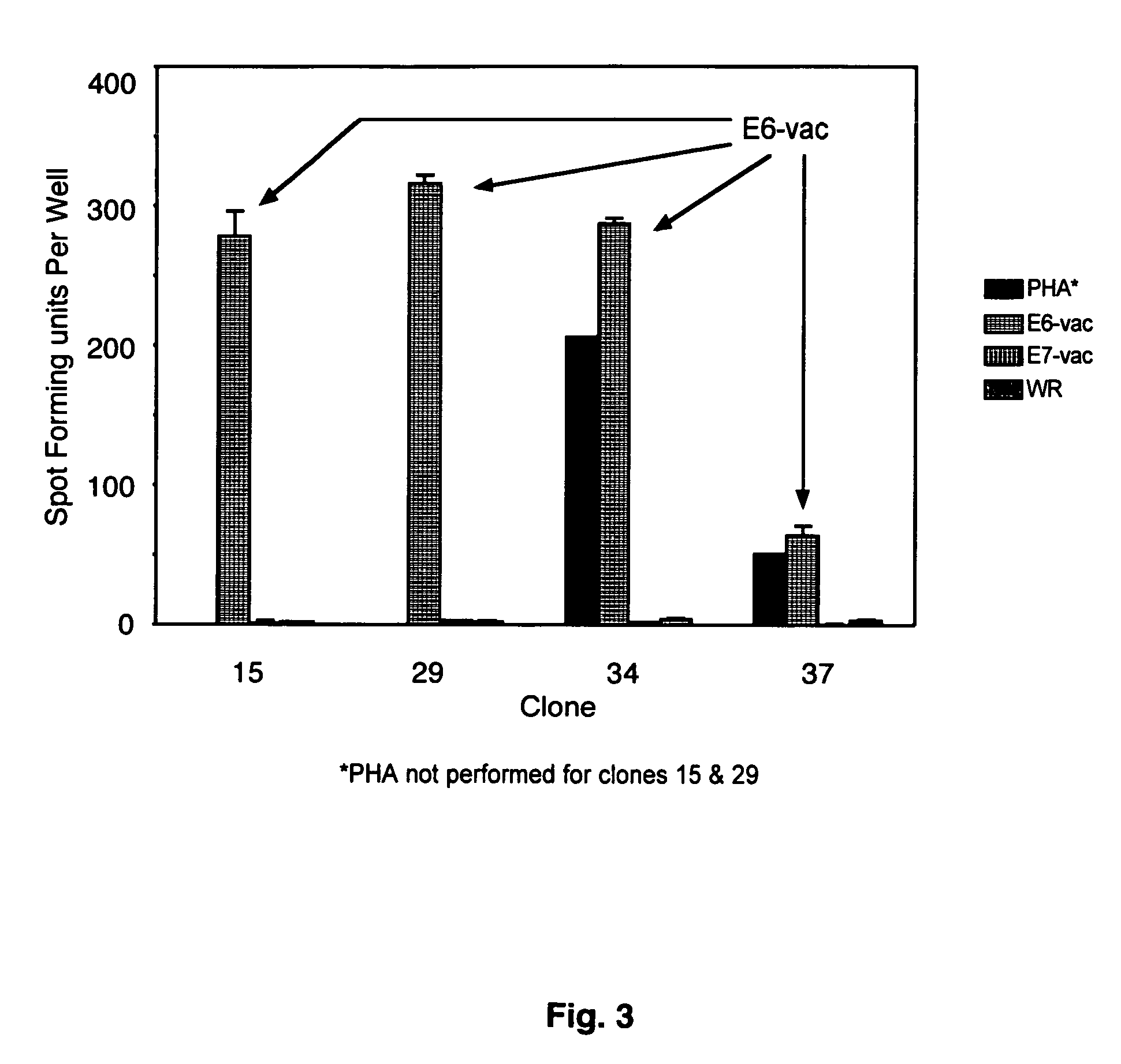
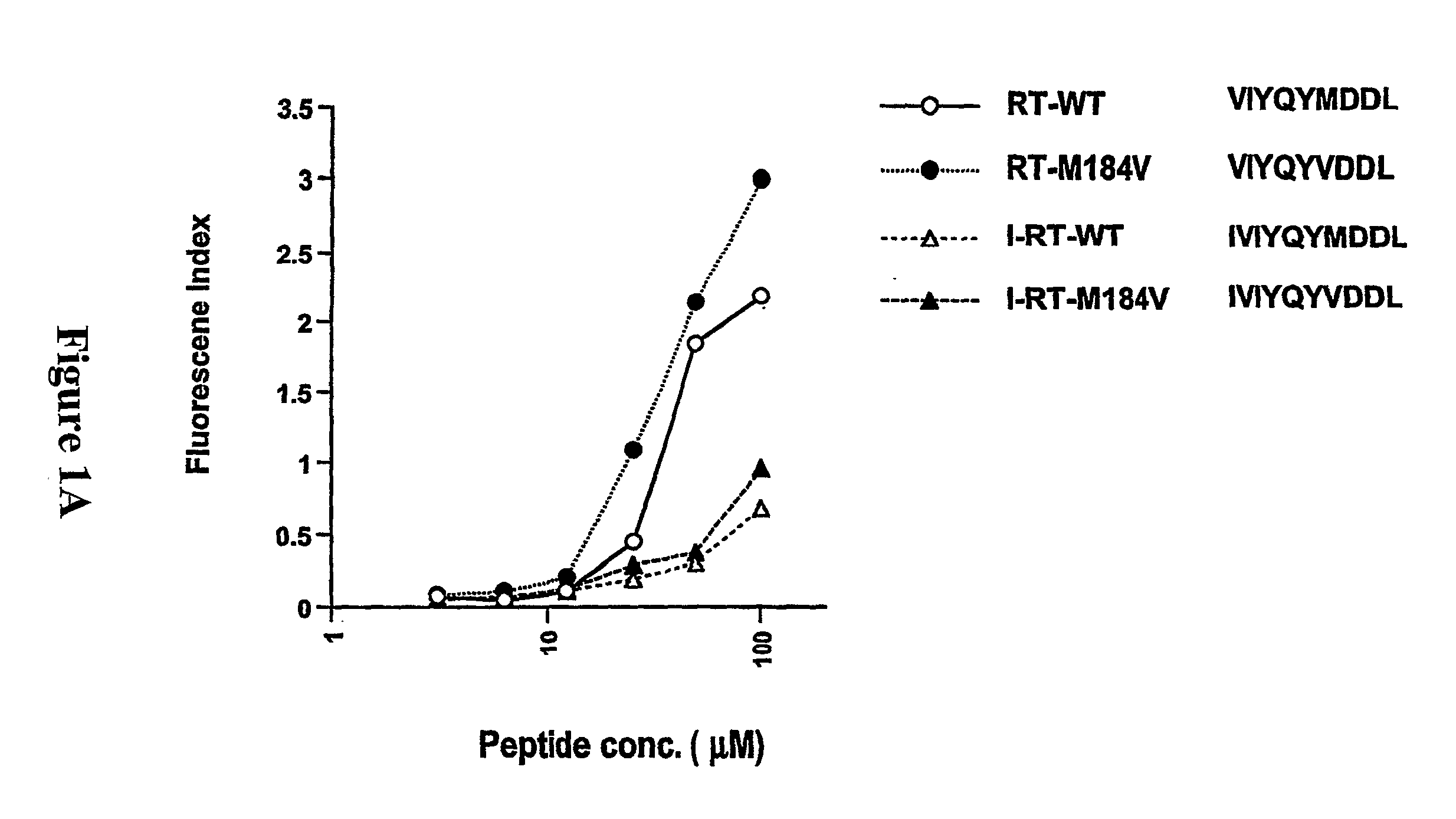
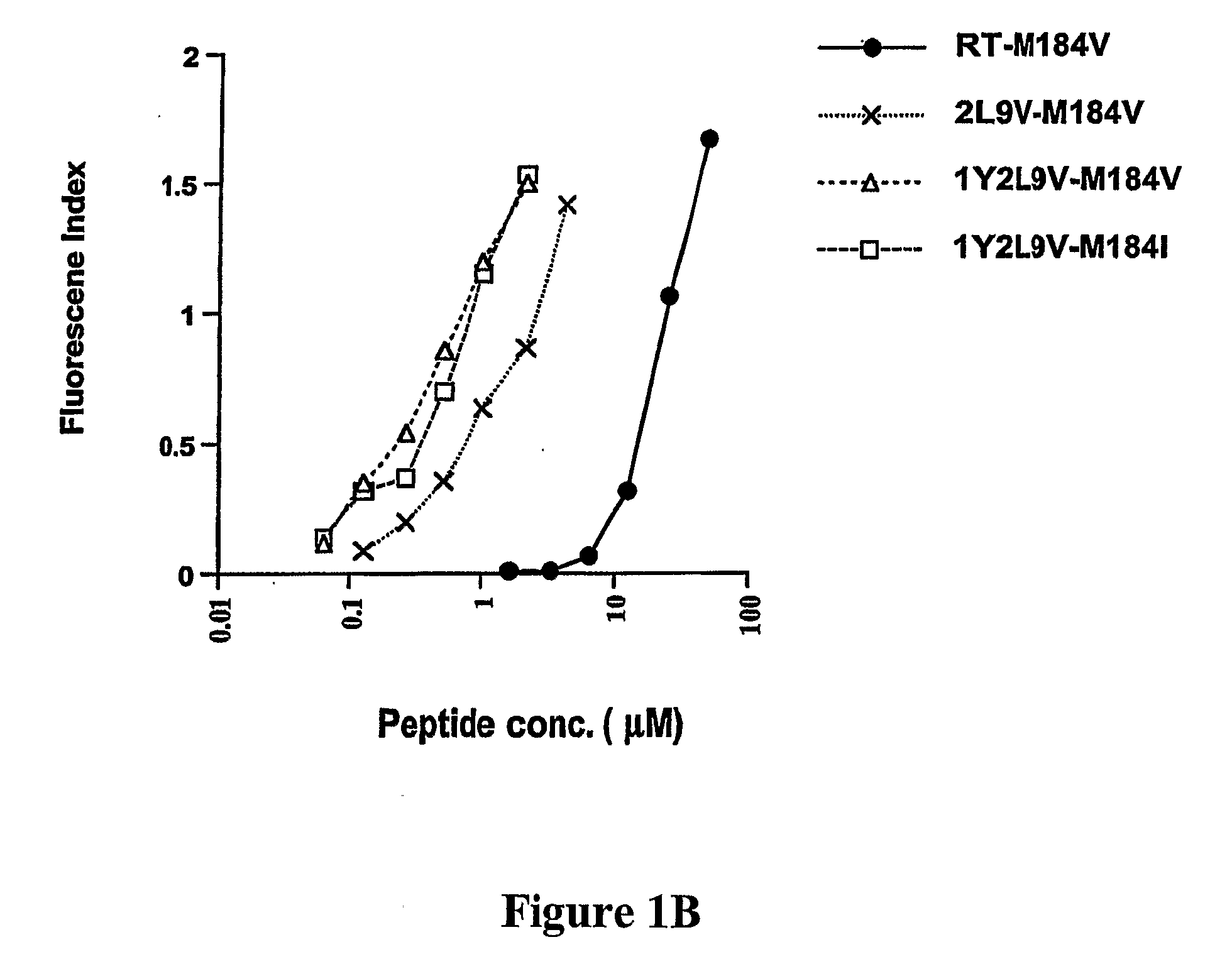
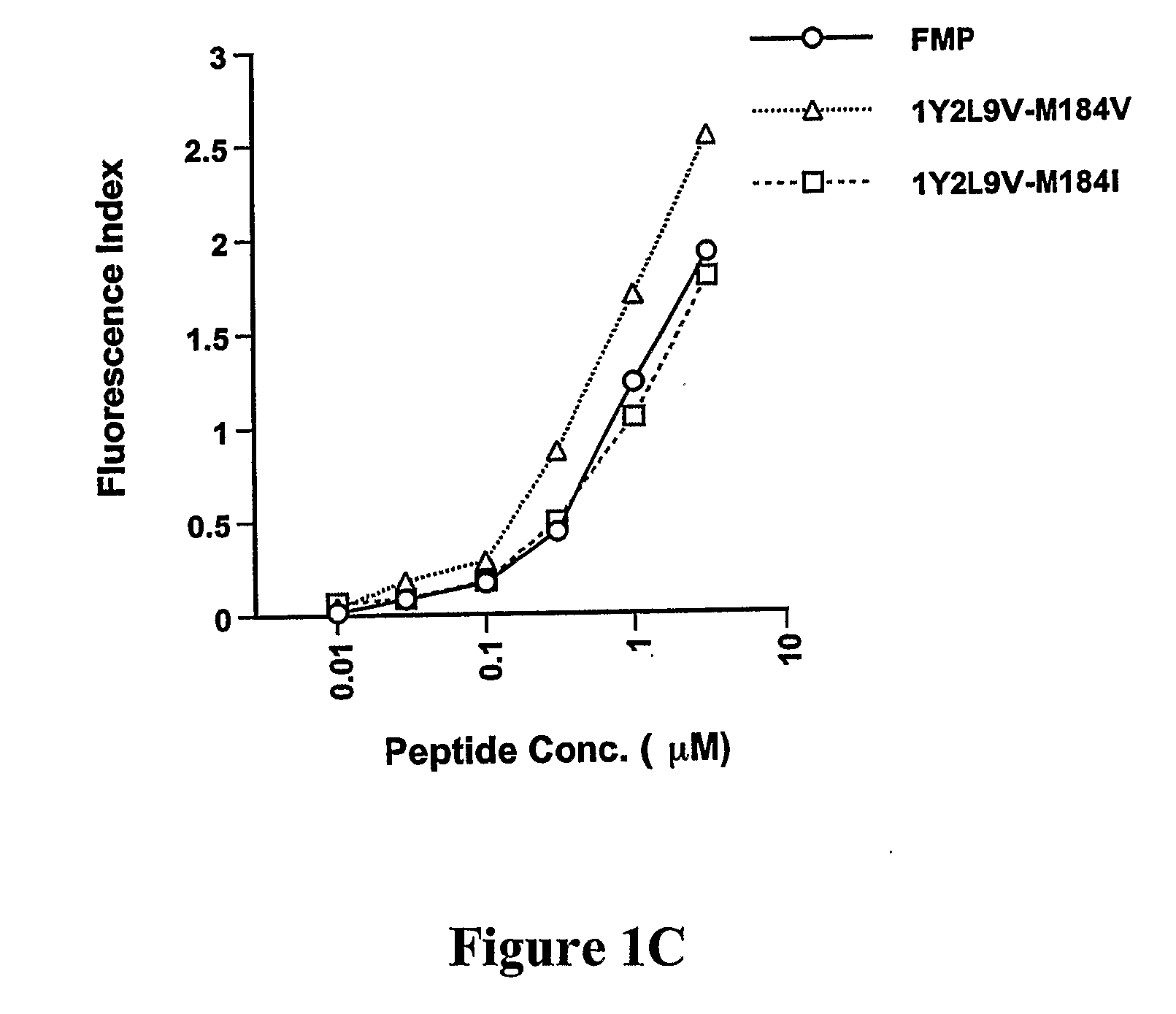
CD8 T cell epitopes in HPV 16 E6 and E7 proteins and uses thereof
InactiveUS20060182763A1Peptide/protein ingredientsViral antigen ingredientsHuman papillomavirusDendritic cell
The present invention is directed to the examination of the pattern of immunodominant CD8 T cell epitopes in the E6 and E7 protein of Human Papillomavirus (HPV) and its further characterization in terms of its amino acid sequence and HLA restriction. These epitopes are identified based on their ability to induce strong CD8 T cell response and therefore, are important as sources of antigens for dendritic cell immunotherapy to treat cervical cancer. The present invention contemplates identifying a number of similar epitopes restricted by a wide variety of HLA types so that they can be used in concert to develop a preventative vaccine, which can be used for general population.
Owner:BIOVENTURES LLC
Antibody having a T-cell receptor-like specificity, yet higher affinity, and the use of same in the detection and treatment of cancer, viral infection and autoimmune disease
InactiveUS6992176B2Antibody mimetics/scaffoldsMicrobiological testing/measurementDiseaseAutoimmune disease
An isolated molecule which comprises an antibody specifically bindable with a binding affinity below 20 nanomolar, preferably below 10 nanomolar, to a human major histocompatibility complex (MHC) class I being complexed with a HLA-restricted antigen and optionally further comprises an identifiable or therapeutic moiety conjugated to the antibody.
Owner:TECHNION RES & DEV FOUND LTD
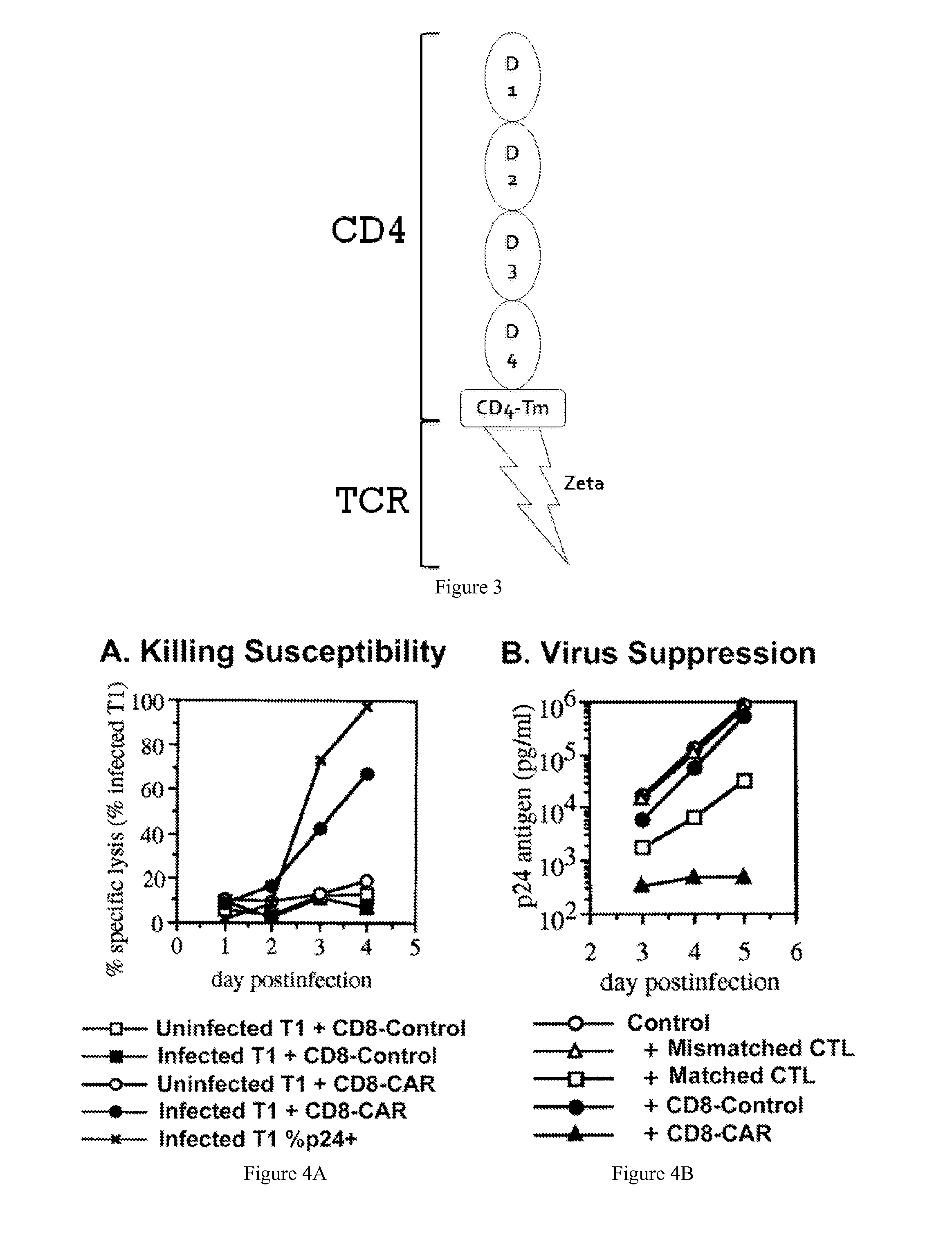
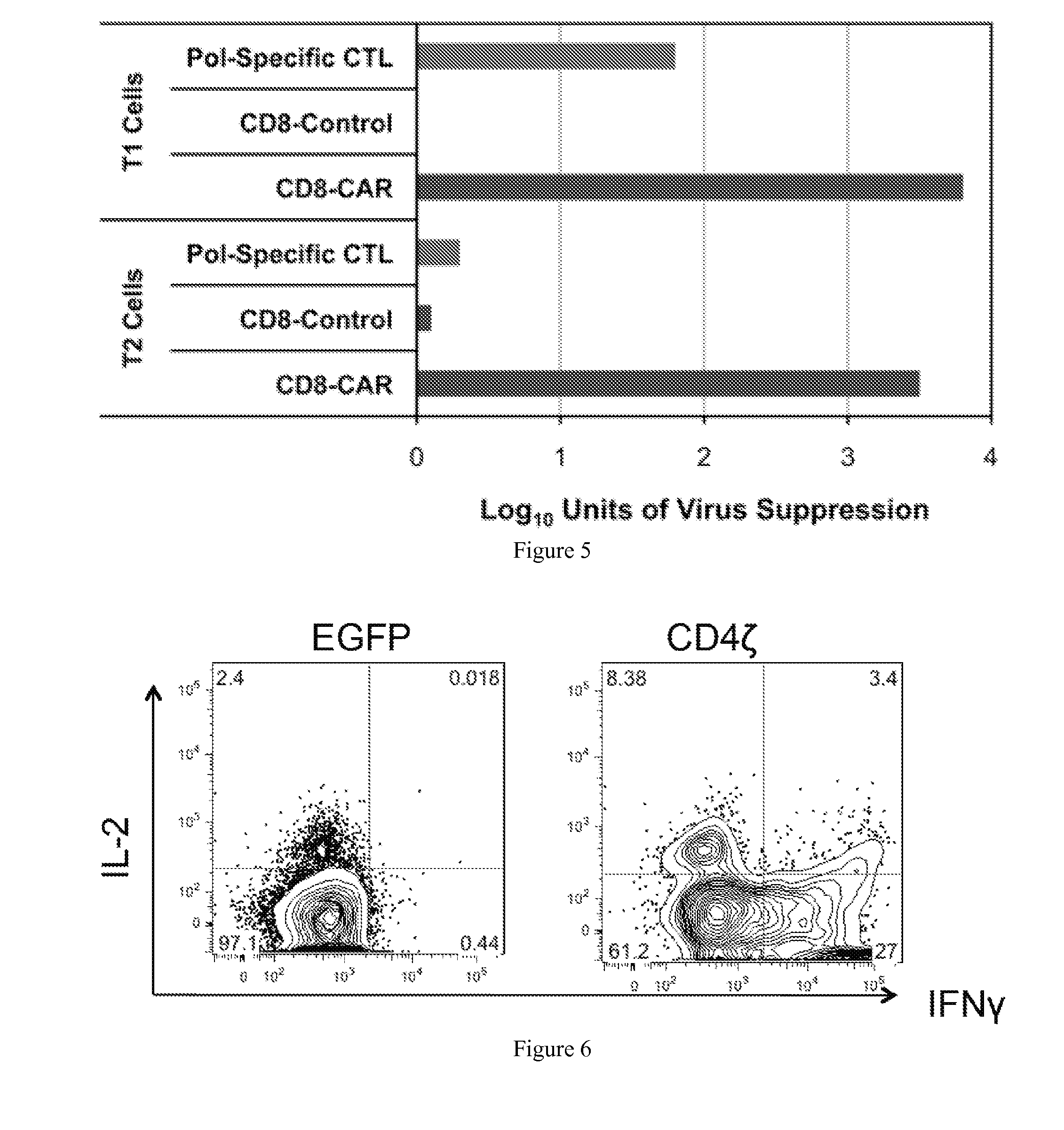
Engineering Antiviral T Cell Immunity through Stem Cells and Chimeric Antigen Receptors
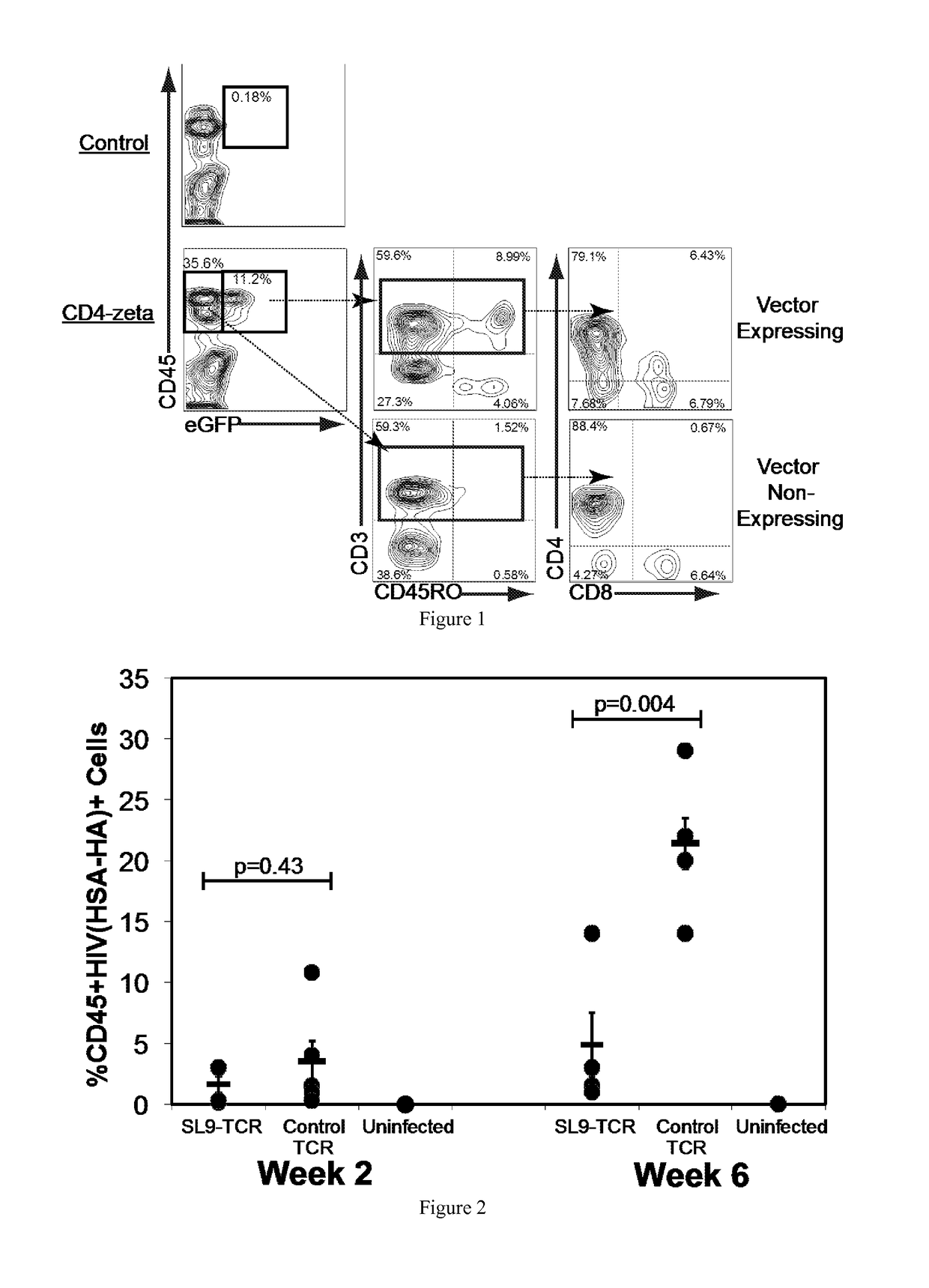
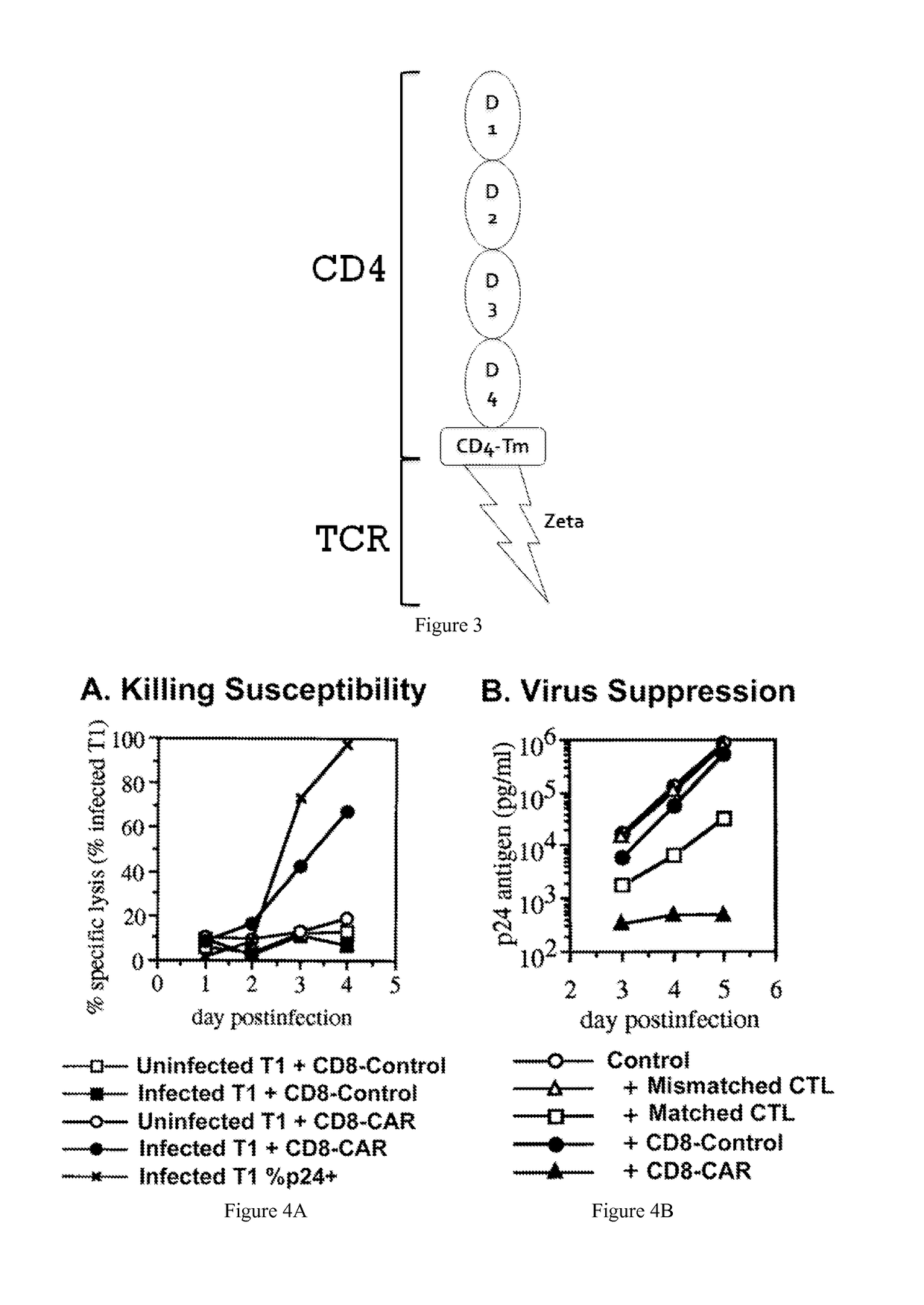
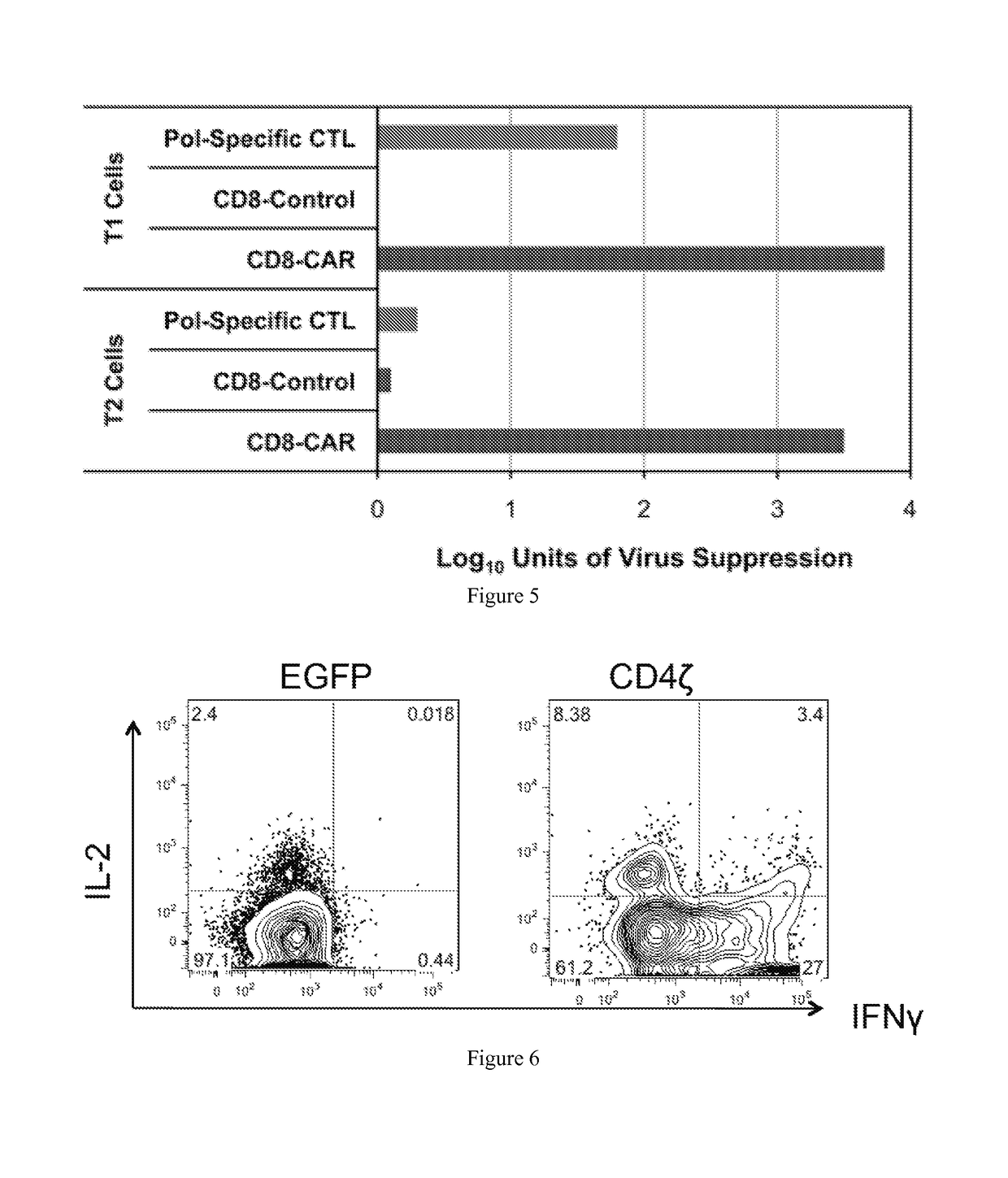
The HIV-specific cytotoxic T lymphocyte (CTL) response is a critical component in controlling HIV replication and is an important part of the ultimate failure to eradicate the virus. Disclosed herein are methods for genetically enhancing the HIV-specific CTL response to allow long-term viral suppression or viral clearance. Human hematopoietic stem cells (HSCs) were genetically modified such that they differentiate into mature CTLs that will kill HIV infected cells. As disclosed herein, the functional effector cells are not human leukocyte antigen (HLA)-restricted. As disclosed herein, stem cells are transduced with non HLA-restricted chimeric antigen receptors (CARs) that allow the recognition of HIV or HIV infected cells when expressed by a CTL.
Owner:RGT UNIV OF CALIFORNIA
Anti-cancer vaccines
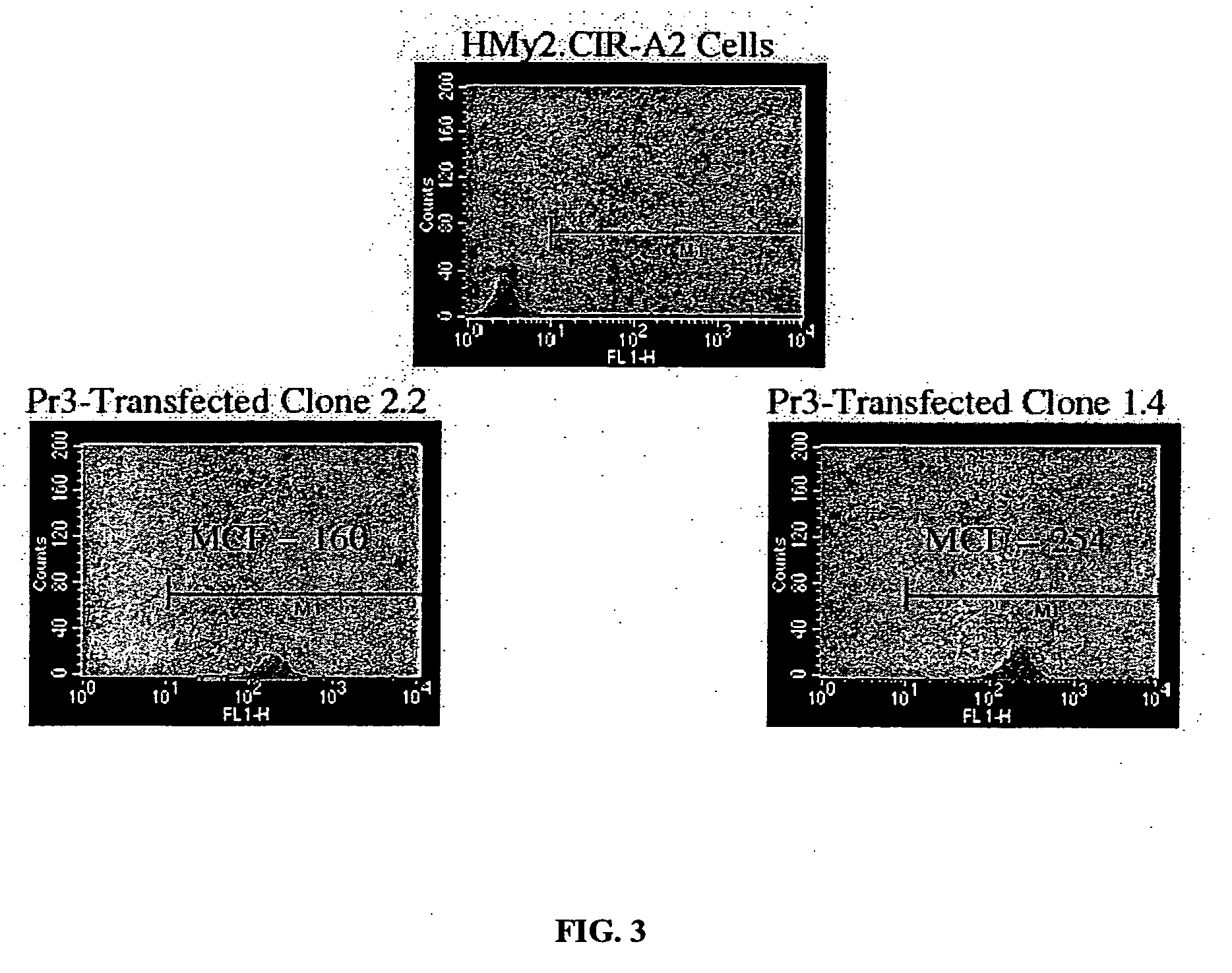
The present provides tumor-associated HLA-restricted antigens, and in particular HLA-A2 restricted antigens, as vaccines for treating or preventing cancers in a patient. In specific aspects, there is proteinase 3 peptides are provided. Such peptides can be used to elicit specific CTLs that preferentially attack myeloid leukemia based on overexpression of the target protein cells.
Owner:THE GOVERMENT OF THE UNITED STATES OF AMERICA REPRESENTED BY THE SEC DEPT OF HEALTH & HUMAN SERVICES (SEE PF37) +1
Combination/adjuvant therapy for wt-1-positive disease
InactiveUS20140271644A1Early inhibitionEnhance anti-tumor responseOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsCancer cellTyrosine-kinase inhibitor
In an attempt to improve primary disease responsiveness and / or to overcome resistant disease, the present disclosure provides a method for treating or inhibiting the proliferation of a WT-1-dependent cancer comprising providing to a subject in need thereof a therapeutically effective amount of a tyrosine kinase inhibitor along with an anti-WT-1 / HLA antibody, that is, an antibody that specifically binds to a peptide of Wilms' tumor protein (WT-1) presented on the surface of the cancer cells in an HLA-restricted fashion.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Human cytomegalovirus (HCMV) cytotoxic T cell epitopes, polyepitopes compositions comprising same and diagnostic and prophylactic and therapeutic uses therefor
InactiveUS7524503B2Minimize difficultyReduce in quantityPeptide/protein ingredientsVirus peptidesCtl epitopeVaccination
The present invention provides CTL epitope peptides and polyepitope peptides from 14 distinct antigens of human cytomegalovirus (HCMV) that are restricted through HLA the most commonly prevalent class I alleles in different ethnic populations of the world. These epitopes provide an important platform for CTL epitope-based vaccines against HCMV. The present invention further provides vaccine compositions comprising the subject epitope and polyepitope peptides and methods for vaccination of humans and for the adoptive transfer of HCMV-specific T cells to human subjects. The present invention further provides reagents and methods for determining the HCMV status or level of HCMV-specific immunity of a subject.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
Peptides for inducing cytotoxic T lymphocyte responses to hepatitis B virus
InactiveUS7744898B2Immunoglobulin superfamilyPeptide/protein ingredientsHepatitis B Virus AntigenHBV polymerase
Peptides are used to define epitopes that stimulate HLA-restricted cytotoxic T lymphocyte activity against hepatitis B virus antigens. The peptides are derived from regions of HBV polymerase, and are particularly useful in treating or preventing HBV infection, including methods for stimulating the immune response of chronically infected individuals to respond to HBV antigens.
Owner:THE SCRIPPS RES INST
CD8 T cell epitopes in HPV 16 E6 and E7 proteins and uses thereof
The present invention is directed to the examination of the pattern of immunodominant CD8 T cell epitopes in the E6 and E7 protein of Human Papillomavirus (HPV) and its further characterization in terms of its amino acid sequence and HLA restriction. These epitopes are identified based on their ability to induce strong CD8 T cell response and therefore, are important as sources of antigens for dendritic cell immunotherapy to treat cervical cancer. The present invention contemplates identifying a number of similar epitopes restricted by a wide variety of HLA types so that they can be used in concert to develop a preventative vaccine, which can be used for general population.
Owner:BIOVENTURES LLC
Vaccines and methods for prevention and treatment of drug-resistant hiv-1 and hepatitis b virus
ActiveUS20090317418A1Reduce viral loadLower viral loadSsRNA viruses positive-sensePeptide/protein ingredientsAntiviral drugResistant virus
The present invention provides methods for lowering a viral load of a virus resistant to an antiviral drug by inducing cytotoxic T lymphocytes (CTL) to recognize a predetermined mutated epitope within a viral protein of the drug-resistant virus. CTLs are induced by immunizing a host with a peptide comprising the predetermined mutation. The immunostimulating peptide may be further improved by epitope-enhancement for inducing specific CTLs. The antiviral protection against drug-resistant virus shown by compositions of the present invention and mediated by human HLA-restricted CTL has not been previously achieved.
Owner:HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US SEC THE DEPT OF
ELISA fleck diagnosis kit for tubercle bacillus infect and method for preparing specific antigen
The invention relates to a reagent kit for diagnosing enzyme linked immune spots infected by tubercle bacillus and a method for preparing a specific antigen. The reagent kit comprises a reagent kit body and a detection reagent arranged in the kit body; the detection reagent comprises a positive standard solution, a chromogenic agent, a concentrated detergent and a diluent; the detection reagent also comprises a specific antigen of mycobacterium tuberculosis, a coated INF-gamma resistant antibody and an enzyme label secondary antibody of a vector protein conjugate; the specific antigen of mycobacterium tuberculosis is fusion protein expressed by an ESAT-6 gene and an EIS gene of the mycobacterium tuberculosis; and the INF-gamma resistant antibody is a murine IgG antibody. The method for preparing the specific antigen comprises the following steps: proper tubercle bacillus special antigen ESAT-6 and EIS are combined; and the prepared recombining fusion protein contains main antigenic determinants of two antigens. The recombining fusion protein definitely contains a T cell epitope suitable for the limitation of different HLA, and does not influence results by different groups and different HLA distribution. Clinical tests on tuberculosis patients and healthy people of different groups show that the reagent kit has more excellent specificity and sensitivity than a current similar product.
Owner:THE THIRD PEOPLES HOSPITAL OF SHENZHEN
Anti-cancer vaccines
The present provides tumor-associated HLA-restricted antigens, and in particular HLA-A2 restricted antigens, as immunogenic compositions for treating and / or preventing breast cancer in an individual. In specific aspects, PR1 peptide or a derivative thereof, or a myeloperoxidase peptide, or a cyclin E1 or E2 peptide is provided in methods and compositions for breast cancer treatment and / or prevention. Such peptides can be used to elicit specific CTLs that preferentially attack breast cancer based on overexpression of the target protein cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
T cell receptor like antibodies having fine specificity
InactiveUS20180179283A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsPeptide antigenMajor histocompatibility
An antibody capable of binding, with a human major histocompatibility complex (MHC)-restricted specificity, a MHC being complexed with an HLA-restricted peptide antigen is provided. The antibody having a binding specificity dictated by at least 4 amino acid residues in said HLA-restricted peptide such that at least 70% reduction in binding of said antibody to said complex is observed when each of said at least 4 amino acid residues is substituted as determined by FACS of cells loaded with said HLA-restricted peptide comprising said substitution, said at least 4 amino acid residues not being anchor residues.
Owner:ADICET THERAPEUTICS INC
Method for identification and development of therapeutic agents
InactiveUS20060257865A1Slow onsetExcellent characteristicsHydrolasesMicrobiological testing/measurementCell selectionAmino acid
The present invention relates generally to the field of identification and determination of bioactive amino acid sequences. In particular, the present invention provides method(s) for determining the influence of variation in host genes on selection of microorganisms with particular amino acid variants for the purpose of therapeutic drug or vaccine design or individualisation of such treatment. The invention also provides methods for identifying HLA allele-specific microorganism sequence polymorphisms that result from HLA restriction of antigen-specific cellular immune responses. It also provides diagnostic and therapeutic methodologies that may be used to measure or treat infection by a microorganism or to prevent infection by the microorganism.
Owner:EPIPOP
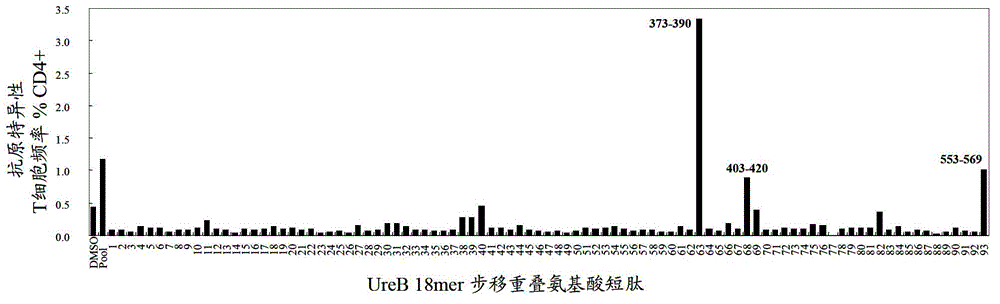
Helicobacter pylori antigen HLA restrictive immunodominance epitope peptide and preparation method and application thereof
ActiveCN102746381AImproving immunogenicityReduce the risk of useAntibacterial agentsBacterial antigen ingredientsImmunodominant EpitopesImmunodominance
The invention relates to Helicobacter pylori antigen HLA restrictive immunodominance epitope peptide as well as a preparation method and application thereof. The dominance epitope peptide has the amino acid sequences shown in SEQ ID NO:63, 74, 95 and 105. The invention also provides a preparation method of the epitope peptide, and further provides application of the epitope peptide to preparation for preventing or treating Helicobacter pylori infection.
Owner:ARMY MEDICAL UNIV
Novel human cytomegalovirus (HCMV) cytotoxic T cell epitopes, polyepitopes, compositions comprising same and diagnostic and prophylactic and therapeutic uses therefor
InactiveUS20100183647A1Minimize difficultyReduce in quantityBiocidePeptide/protein ingredientsCtl epitopeVaccination
The present invention provides CTL epitope peptides and polyepitope peptides from 14 distinct antigens of human cytomegalovirus (HCMV) that are restricted through HLA the must commonly prevalent class I alleles in different ethnic populations of the world. These epitopes provide an important platform for CTL epitope-based vaccines against HCMV. The present invention further provides vaccine compositions comprising the subject epitope and polyepitope peptides and methods for vaccination of humans and for the adoptive transfer of HCMV-specific T cells to human subjects. The present invention further provides reagents and methods for determining the HCMV status or level of HCMV-specific immunity of a subject.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
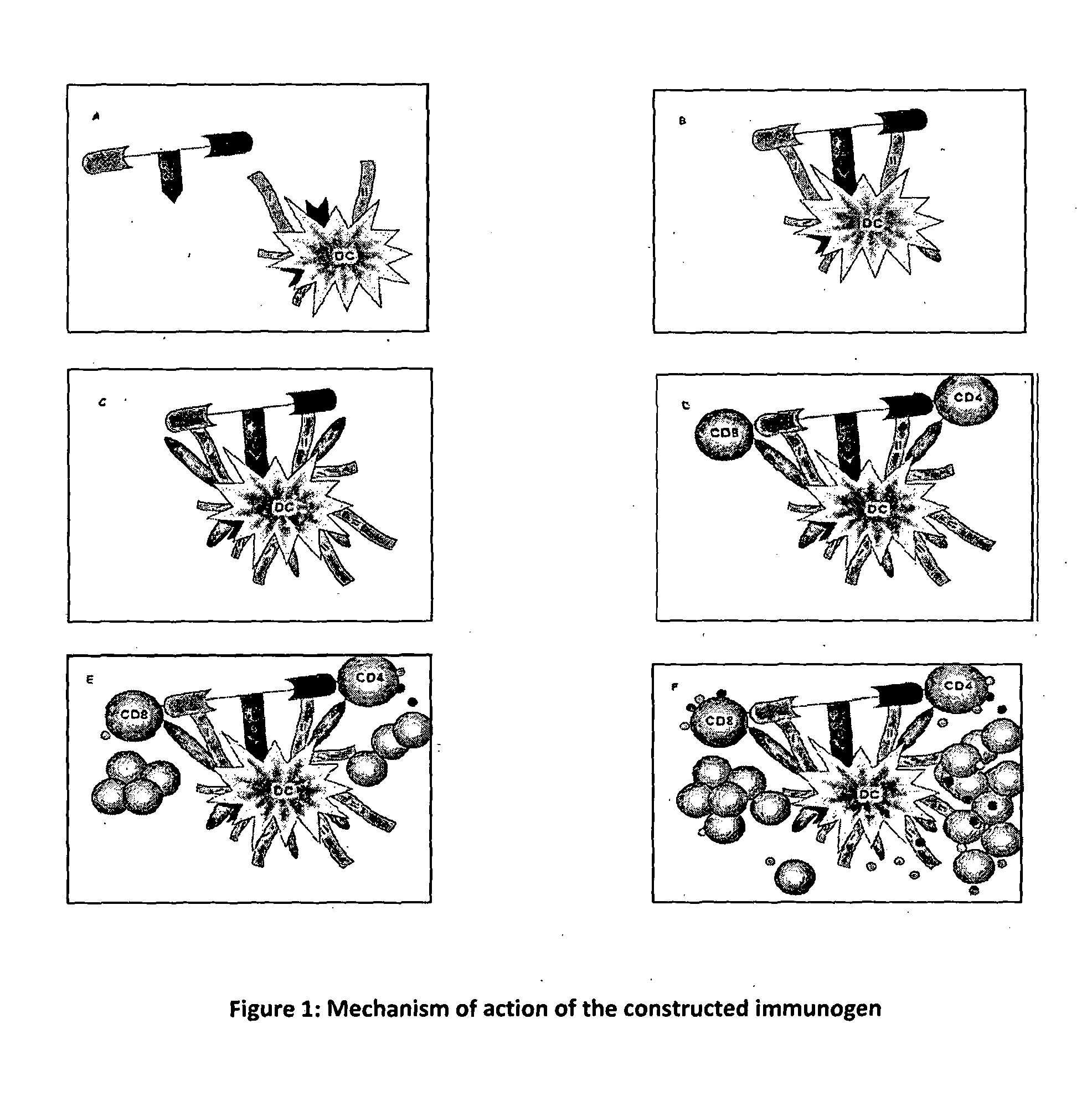
Synthetic immunogen useful for generating long lasting immunity and protection against pathogens
InactiveUS20130183377A1Induced proliferationGenerating long lasting protective immunityAntibacterial agentsPowder deliverySynthetic ImmunogensTrypanosomiasis
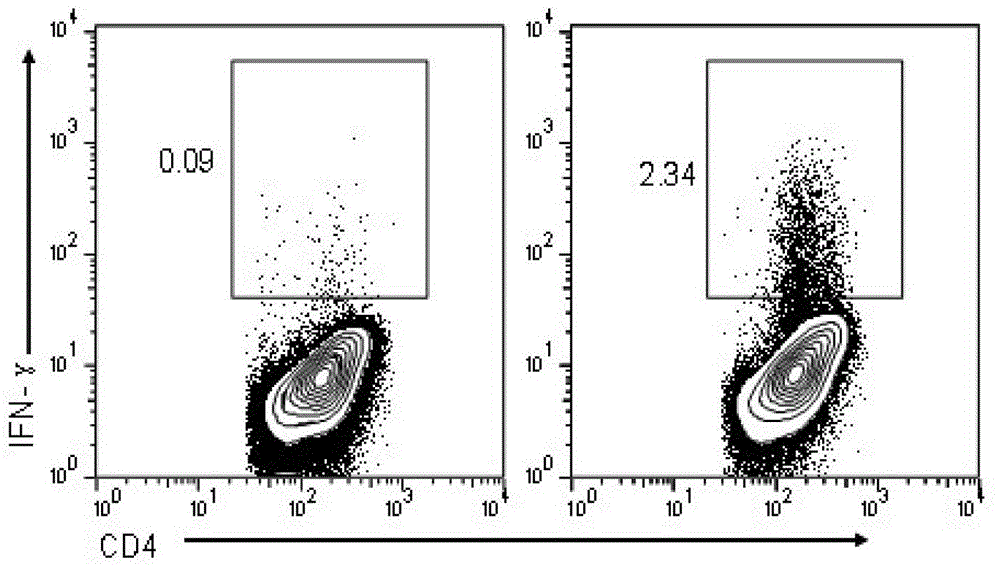
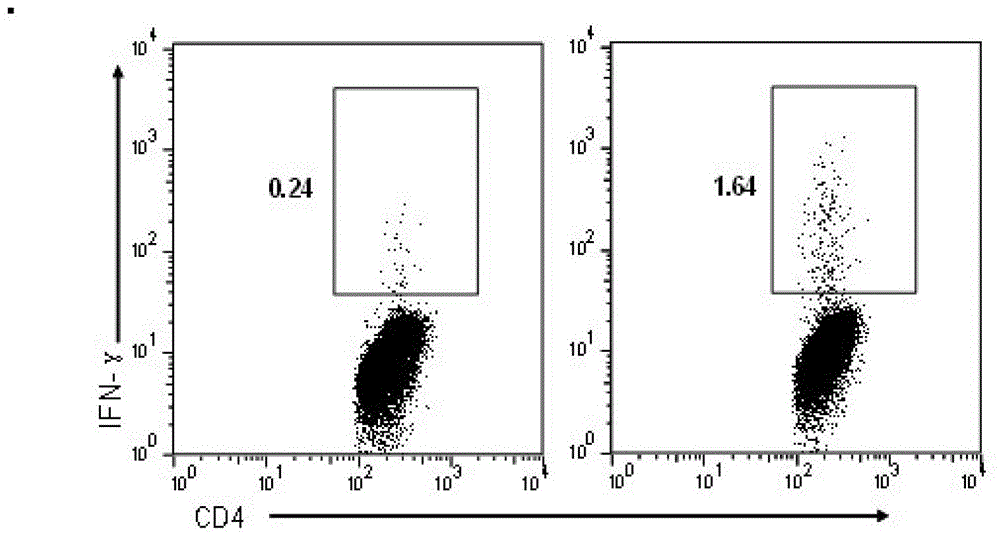
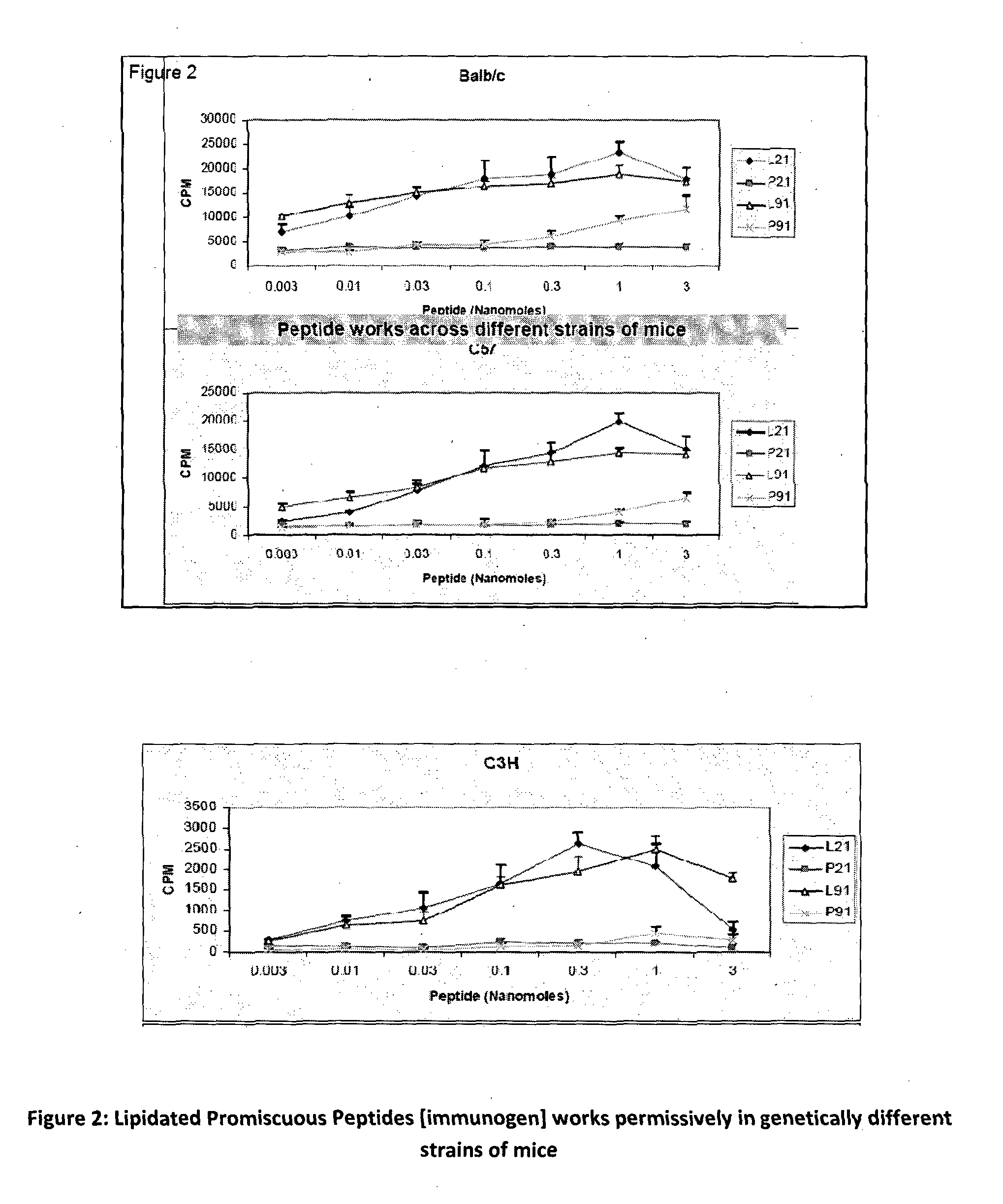
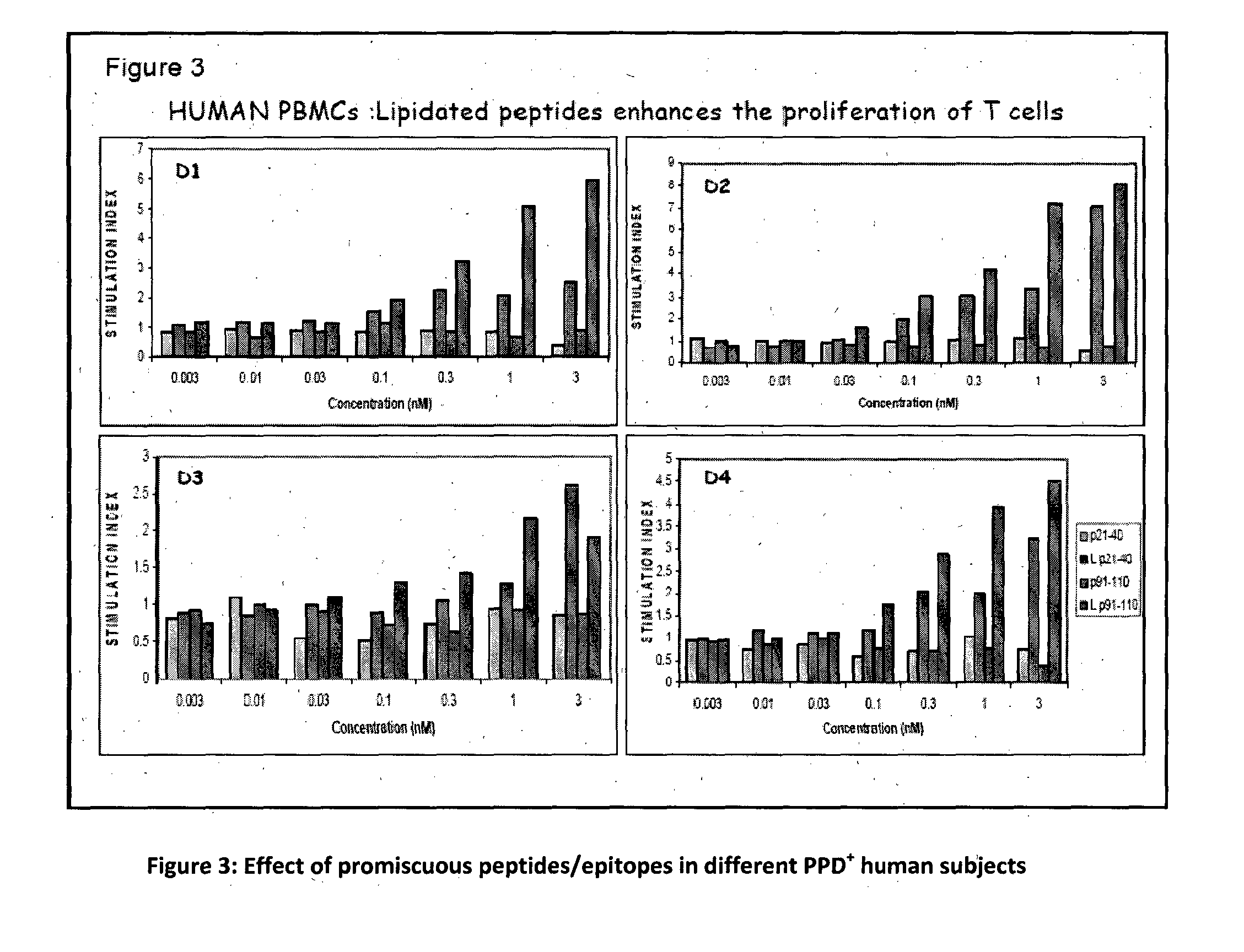
The present invention relates to a synthetic immunogen represented by the general formula 1, useful for generating long lasting protective immunity against various intracellular pathogens which are the causative agents of tuberculosis, leishmaniasis, AIDS, trypanosomiasis, malaria and also allergy, cancer and a process for the preparation thereof. The developed immunogen is able to circumvent HLA restriction in humans and livestock. The invention further relates to a vaccine comprising the said immunogen for generating enduring protective immunity against various diseases. The said vaccine is targeted against intracellular pathogens, more particularly the pathogen M. tuberculosis in this case. In the present invention, promiscuous peptides of M. tuberculosis are conjugated to TLR ligands especially; Pam2Cys to target them mainly to dendritic cells and therefore elicit long-lasting protective immunity. (The formula (I) should be inserted here) General formula (I) wherein, X1=a promiscuous CD4 T helper epitope selected from SEQ ID No. 1 to 98 OR nil; X2=a promiscuous CD8 T cytotoxic epitope selected from SEQ ID No. 99 to 103 OR nil; when X1=nil; X2=SEQ ID No. 99 to 103 and when X2=nil; X1=SEQ ID No. 1 to 98; Y=Lysine; and S=Serine.
Owner:COUNCIL OF SCI & IND RES +1
Peptides for inducing cytotoxic T lymphocyte responses to hepatitis B virus
InactiveUS6919203B2Peptide/protein ingredientsGenetic material ingredientsHepatitis B Virus AntigenT lymphocyte
Peptides are used to define epitopes that stimulate HLA-restricted cytotoxic T lymphocyte activity against hepatitis B virus antigens. The peptides are derived from regions of HBV envelope, and are particularly useful in treating or preventing HBV infection, including methods for stimulating the immune response of chronically infected individuals to respond to HBV antigens.
Owner:THE SCRIPPS RES INST
Method for Identification and Development of Therapeutic Agents
InactiveUS20100088037A1Excellent characteristicsIncreased longevityHydrolasesVirus peptidesImmunity responseTGE VACCINE
The present invention relates generally to the field of identification and determination of bioactive amino acid sequences. In particular, the present invention provides method(s) for determining the influence of variation in host genes on selection of microorganisms with particular amino acid variants for the purpose of therapeutic drug or vaccine design or individualisation of such treatment. The invention also provides methods for identifying HLA allele-specific microorganism sequence polymorphisms that result from HLA restriction of antigen-specific cellular immune responses. It also provides diagnostic and therapeutic methodologies that may be used to measure or treat infection by a microorganism or to prevent infection by the microorganism.
Owner:EPIPOP
Avian influenza vaccine
ActiveUS20100136098A1Effectively prevent infectionPeptide/protein ingredientsVirus peptidesAcyl groupT lymphocyte
The present invention provides an avian influenza vaccine containing a peptide-bound liposome wherein;the peptide contains:(1) an amino acid sequence shown by any one of SEQ ID NO:1 to 9, or(2) an amino acid sequence shown by any one of SEQ ID NO:1 to 9 wherein one or two amino acids are substituted,has a length of 9 to 11 amino acids, andis capable of inducing HLA-restricted cytotoxic T lymphocytes; wherein the liposome contains a phospholipid having an acyl group with 14 to 24 carbon atoms and one unsaturated bond or a hydrocarbon group with 14 to 24 carbon atoms and one unsaturated bond, and a liposome stabilizer; and wherein the peptide is bound to the surface of the liposome.
Owner:JAPAN AS REPRESENTED BY DIRECTOR GENERAL OF NAT INST OF INFECT IOUS DISEASES +3
Engineering antiviral T cell immunity through stem cells and chimeric antigen receptors
The HIV-specific cytotoxic T lymphocyte (CTL) response is a critical component in controlling HIV replication and is an important part of the ultimate failure to eradicate the virus. Disclosed herein are methods for genetically enhancing the HIV-specific CTL response to allow long-term viral suppression or viral clearance. Human hematopoietic stem cells (HSCs) were genetically modified such that they differentiate into mature CTLs that will kill HIV infected cells. As disclosed herein, the functional effector cells are not human leukocyte antigen (HLA)-restricted. As disclosed herein, stem cells are transduced with non-HLA restricted chimeric antigen receptors (CARs) that allow the recognition of HIV or HIV-infected cells when expressed by a CTL. These CARs are hybrid molecules that contain an extracellular HIV recognition domain and an intracellular TCR-zeta signaling domain. The CTL response may be enhanced through the targeting of T cell inhibitory receptors. The methods and compositions disclosed herein may be used to engineer antiviral immunity and HIV-specific CTL responses in vivo. Also disclosed herein are methods and compositions for the treatment of chronic viral infections such as HIV.
Owner:RGT UNIV OF CALIFORNIA
DNA-based plasmid formulations and vaccines and prophylactics containing the same
The invention is a general method for improving the performance of the DNA-based vaccines. The method utilizes a complex DNA-generated profile of antigens to extend the effects of DNA-based vaccines and to broaden the immune response. This broadened immune response in turn improves the protection of the recipient from divergent (but related) strains of a pathogen. In addition, it effectively improves the efficacy of DNA-based vaccines used for treatment of viral diseases, including acquired immunity disorder (AIDS). One embodiment, where the target viral pathogen is HIV (the causative agent for aids), the method identifies an orderly set of plasmids of related sequences that may be used to prime a broad and strong immune response to HLA-restricted viral antigens. This mixture of plasmids is thus capable of priming an appropriate immune response to reduce the viral burden in HIV infected patients or to protect uninfected patients from HIV infection.
Owner:LASHER ALFRED W +2
Combination/adjuvant therapy for WT-1-positive disease
InactiveCN106170297AOrganic active ingredientsAntibody ingredientsCancer cellTyrosine-kinase inhibitor
In an attempt to improve primary disease responsiveness and / or to overcome resistant disease, the present disclosure provides a method for treating or inhibiting the proliferation of a WT-1 -dependent cancer comprising providing to a subject in need thereof a therapeutically effective amount of a tyrosine kinase inhibitor along with an anti-WT-1 / HLA antibody, that is, an antibody that specifically binds to a peptide of Wilms' tumor protein (WT-1 ) presented on the surface of the cancer cells in an HLA-restricted fashion.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
HLA Epitope Identification
The present invention describes ways to identify HLA allele-specific epitopes that result from HLA restriction of antigen-specific cellular immune responses. The invention employs a combination of bioinformatics and functional assays to systematically identify and classify CTL mutations to determine correlates of virus-host interactions Peptides representing HLA allele-specific epitopes are provided as well as methods for validating HLA-restricted epitopes and methods for measuring T cell responses.
Owner:LUO MA +6
Hla-a2-restricted t-cell epitopes of the respiratory syncytial virus fusion protein as peptide-based vaccines
InactiveUS20100172925A1Reduce loadFast recoverySsRNA viruses negative-sensePeptide/protein ingredientsF proteinCD8
Respiratory syncytial virus (RSV) fusion protein-specific T-cell epitopes as peptide-based vaccines are disclosed. The isolated peptide contains a human HLA restricted CD8+ T-cell epitope that is specific to RSV F protein. The length of the peptide is no more than 9 or 10 amino acid residues. The peptide may be employed as an immunogen to stimulate cytotoxic T cells, indirectly activate helper T cells type 1, and cause release of cytokines from T cells.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH
Universal cell therapy product for expressing antigen recognition regions, and preparation method and application thereof
ActiveCN110331134APlay a role in the treatment of leukemiaInhibit or promote growthGenetically modified cellsSkeletal/connective tissue cellsAfter treatmentNeurotoxicity
The present invention relates to a universal cell therapy product for expressing antigen recognition regions, and a preparation method and an application thereof. The antigen recognition regions aiming at tumor cell antigens are carried on universal cells without an HLA restriction, the antigens are degraded after treatment, and tumor cells are changed in series until death, thereby playing a rolein treatment. A provided new strategy for treating tumors is expected to replace individualized CART, remarkably reduces preparation costs of CART cells, achieves an aim of treating the tumors, doesnot generate cytokine storm and neurotoxicity, reduces threshold of CART treatment, and benefits more patients.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
HLA-restricted cytotoxic T-Lymphocytevaccine for prevention of HIV infection
InactiveUS20020172688A1SsRNA viruses positive-senseViral antigen ingredientsHIV ProteinsDendritic cell
An improved method of inducing protective immunity to the Human Immunodeficiency Virus through isolating a person's antigen-presenting cells from blood, then pulsing these cells with short peptides that bind the person's Class I Major Histocompatibility Complex Types and correspond to conserved segments of the HIV structural and functional genes. These pulsed dendritic cells are then injected intravenously, where they will travel to lymph tissue and prime HIV-specific Cytotoxic T Lymphocytes. The immune system is then activated by injecting the person with a weakened arbovirus, Chikungunya. The interferons and other immune-activating chemicals induced by the virus will stimulate creation of billions of killer cells that recognize HIV proteins, allowing for rapid mobilization of memory immune cells to contain and eliminate any subsequent infection with the HIV virus. The vaccine is custom-designed to each person and uses no HIV virus for safety purposes. It is understood that the examples an embodiments described herein are for illustrative purposes only and various changes or modifications in light thereof will be suggested to persons skilled in the art and are to be included within the spirit and purview of this application and scope of the appended claims.
Owner:LYDAY BRUCE
Synthetic immunogen useful for generating long lasting immunity and protection against pathogens
InactiveUS9340622B2Generating long lasting protective immunityInduced proliferationAntibacterial agentsPowder deliverySynthetic ImmunogensDisease
The present invention relates to a synthetic immunogen represented by the general formula 1, useful for generating long lasting protective immunity against various intracellular pathogens which are the causative agents of tuberculosis, leishmaniasis, AIDS, trypanosomiasis, malaria and also allergy, cancer and a process for the preparation thereof. The developed immunogen is able to circumvent HLA restriction in humans and livestock. The invention further relates to a vaccine comprising the said immunogen for generating enduring protective immunity against various diseases. The said vaccine is targeted against intracellular pathogens, more particularly the pathogen M. tuberculosis in this case. In the present invention, promiscuous peptides of M. tuberculosis are conjugated to TLR ligands especially; Pam2Cys to target them mainly to dendritic cells and therefore elicit long-lasting protective immunity.wherein, X1=a promiscuous CD4 T helper epitope selected from SEQ ID No. 1 to 98 OR nil;X2=a promiscuous CD8 T cytotoxic epitope selected from SEQ ID No. 99 to 103 OR nil;when X1=nil; X2=SEQ ID No. 99 to 103 and when X2=nil; X1=SEQ ID No. 1 to 98;Y=Lysine; andS=Serine.
Owner:COUNCIL OF SCI & IND RES +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com