Combination/adjuvant therapy for WT-1-positive disease
One-WT-1, composition technology, applied in the field of combined/adjuvant therapy for WT‑1‑positive diseases, capable of solving problems such as limited and refractory diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
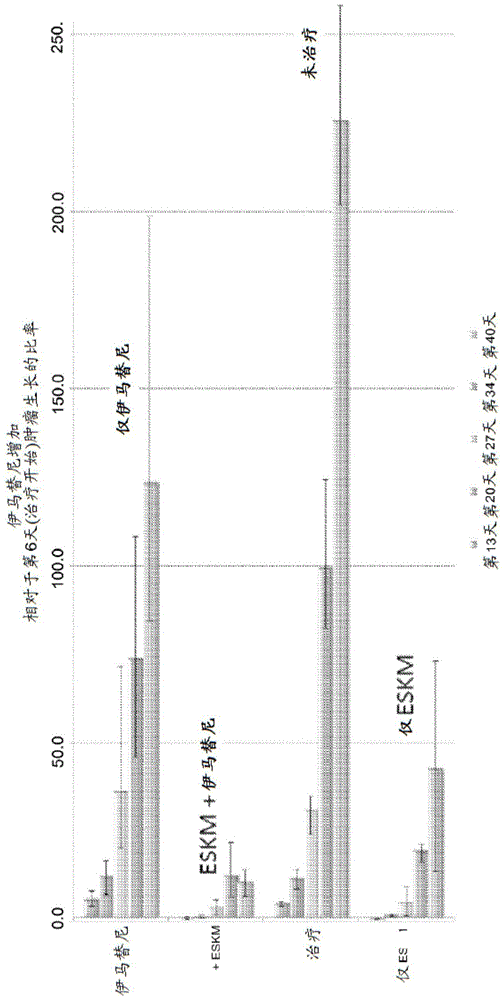
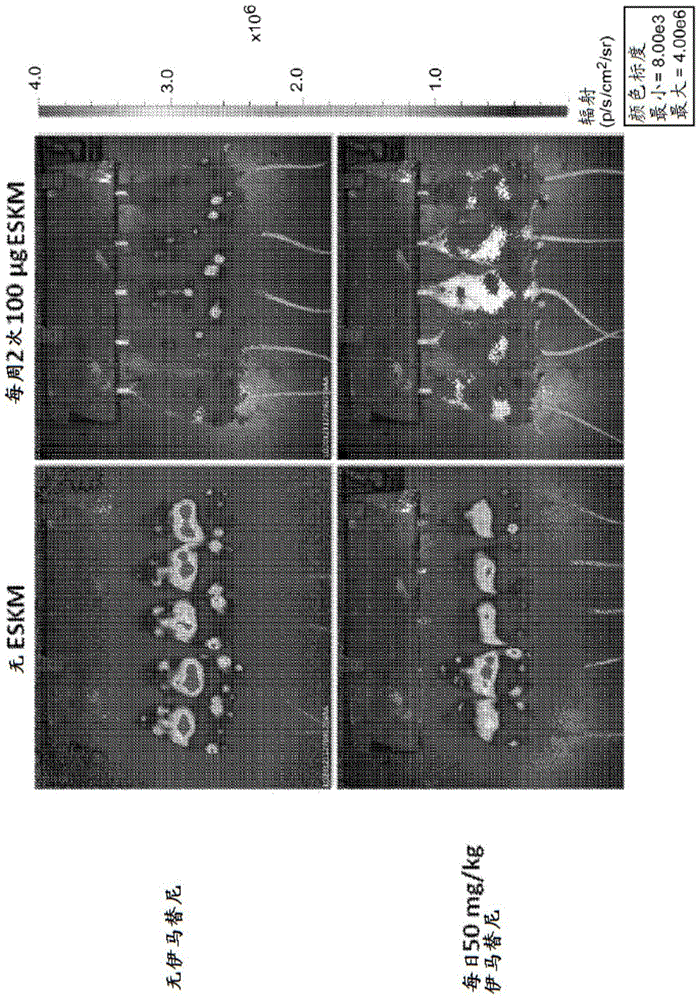
Examples
Embodiment approach
[0162] 1. A pharmaceutical composition comprising a tyrosine kinase inhibitor (TKI); and
[0163] (A) an antibody comprising a heavy chain (HC) variable region and a light chain (LC) variable region, the heavy chain (HC) variable region comprising HC-CDR1, HC-CDR2, and HC-CDR3, and the The light chain (LC) variable region comprises LC-CDR1, LC-CDR2 and LC-CDR3, and the heavy chain (HC) variable region and light chain (LC) variable region comprise Tables 1-14 and Figure 7-10 the amino acid sequence shown in; or
[0164] (B) contains V H and V L antibody, the V H and V L comprising a first amino acid sequence and a second amino acid sequence from Tables 1-12; or
[0165] (C) Antibodies comprising scFv comprising amino acid sequences from Tables 1-12.
[0166] 2. The pharmaceutical composition of embodiment 1, wherein the tyrosine kinase inhibitor is selected from the group consisting of imatinib, dasatinib, nilotinib, bosutinib, Natinib, bafetinib, erlotinib, gefitinib, l...
Embodiment 1
[0190] Materials and methods
[0191] Cell samples, cell lines and antibodies. Peripheral blood sheets were obtained from HLA-type healthy donors and patients by Ficoll density gradient centrifugation after signed informed consent on a protocol approved by the Sloan-Kettering Cancer Center Institutional Review Board nuclear cells (PBMC). All cells were HLA-typed by the Cellular Immunology Department of Memorial Sloan-Kettering Cancer Center. The leukemia cell line BV173 (WT-1+, A0201+) was a gift from Dr. H.J. Stauss (University College London, London, United Kingdom). at 37°C / 5% CO 2 Next, the cell lines were cultured in RPMI 1640 supplemented with 5% FCS, penicillin, streptomycin, 2mmol / L glutamine and 2-mercaptoethanol.
[0192] animal. Six- to eight-week-old male NOD.Cg-Prkdc scid IL2rgtm1Wjl / SzJ mice, designated NOD / SCIDγ (NSG), were purchased from the Jackson Laboratory (Bar Harbor, ME) or obtained from the MSKCC animal husbandry facility.
[0193] Transduction and...
Embodiment 2
[0195] Antibody-dependent cellular cytotoxicity (ADCC)
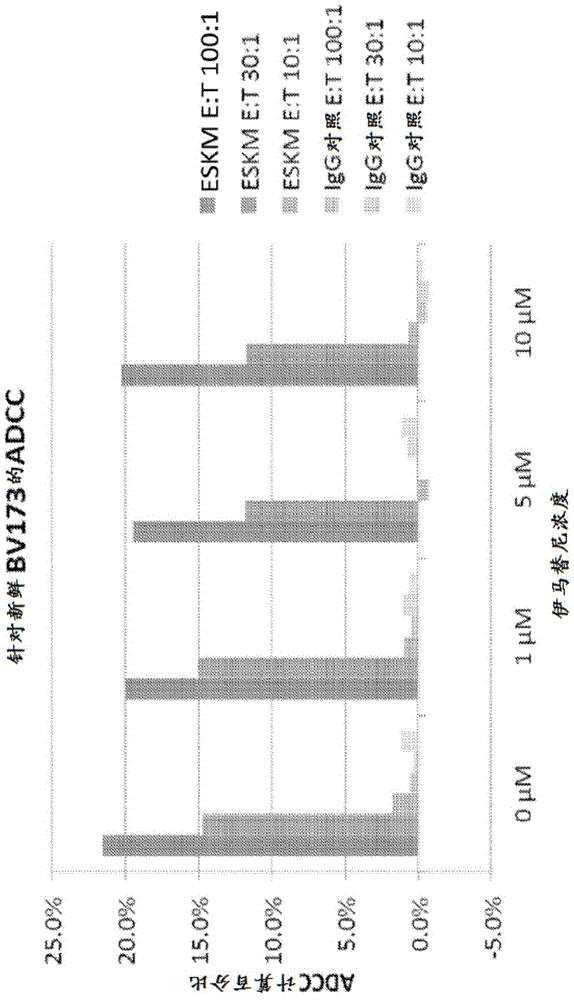
[0196] ADCC is considered to be one of the major effector mechanisms of therapeutic human mAbs. Therefore, the evaluation of efficacy began with an in vitro assay measuring ADCC against the BV173 cell line derived from CML at risk. Fresh BV173 cells were used for ADCC target cells. WT-1 antibody or its isotype control human IgG1 were incubated with target cells and fresh PBMCs at 750 ng / ml at different effector:target (E:T) ratios for 6 hours. Imatinib was added at concentrations of 0, 1, 5 and 10 μM. Supernatants were harvested and cytotoxicity was measured by standard Chromium 51 release assay.
[0197] In the presence of human PBMCs, WT-1 antibody mediates dose-dependent PBMCADCC against naturally presented RMF epitopes by HLA-A0201 molecules on tumor cells (leukemic cell line BV173). Importantly, WT-1 antibody was able to mediate ADCC in the presence of different doses of imatinib. Using PBMC from multiple healt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com