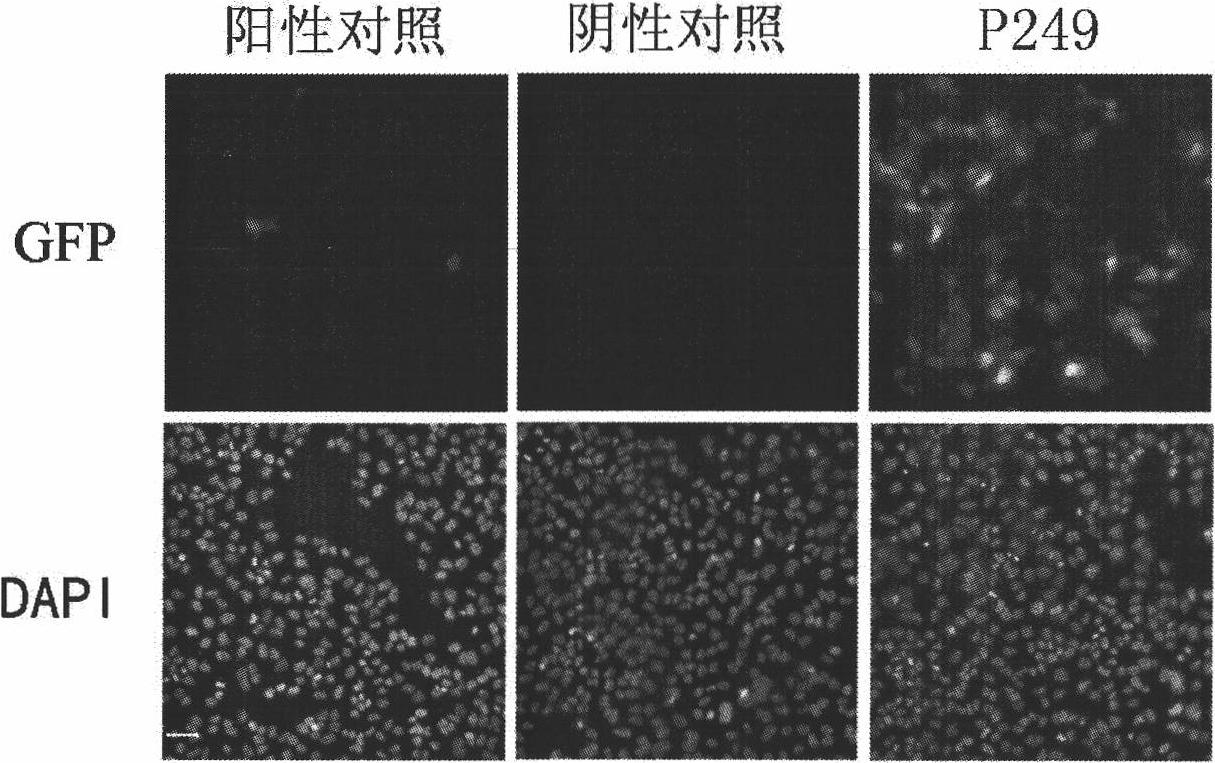
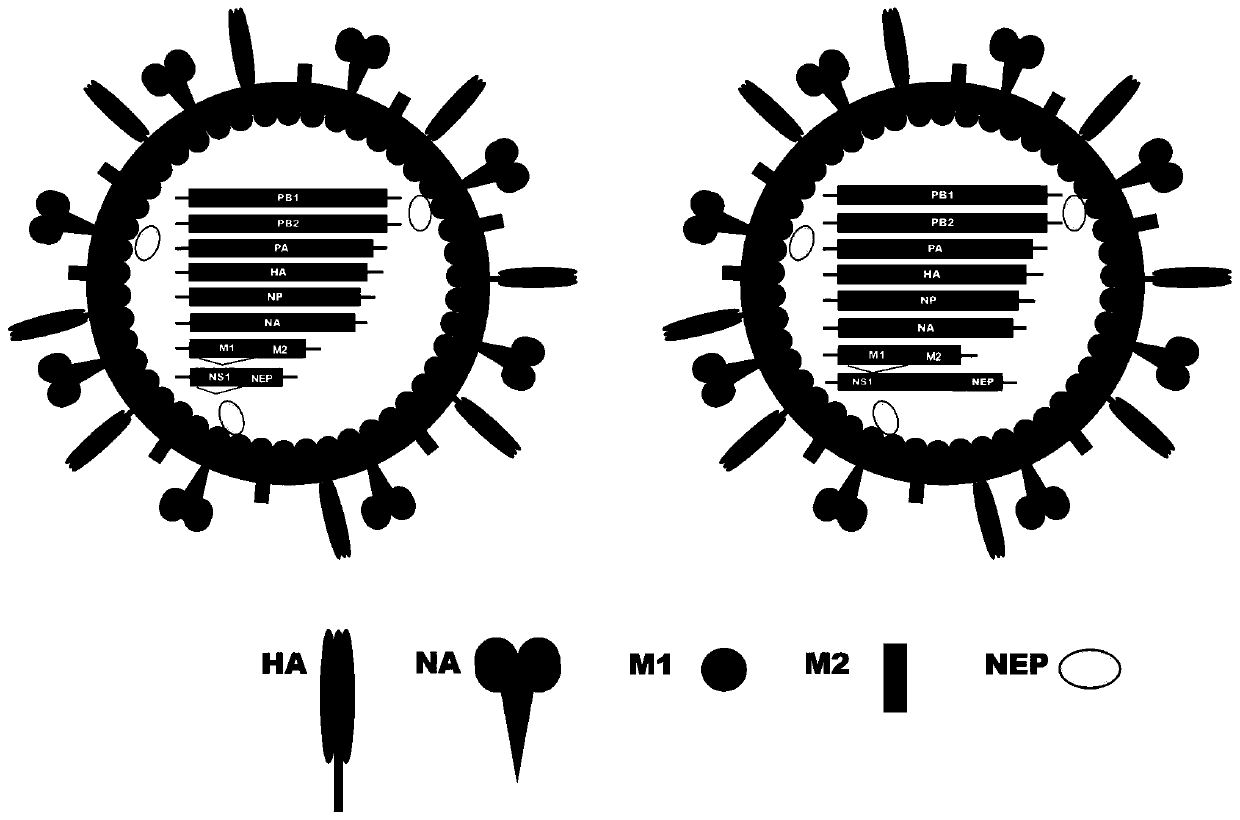
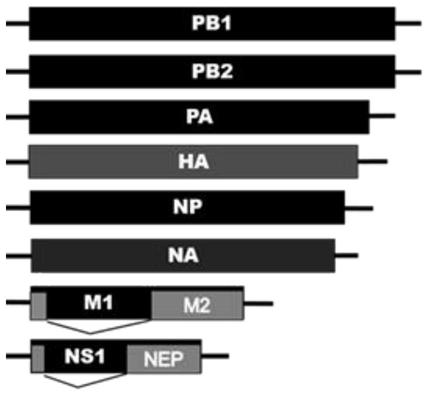
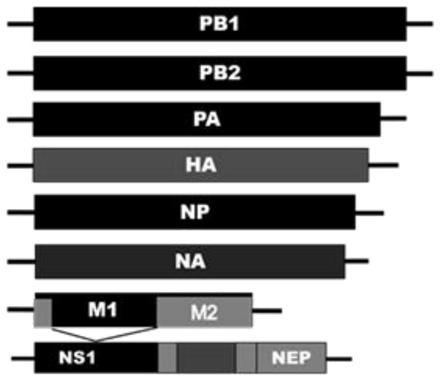
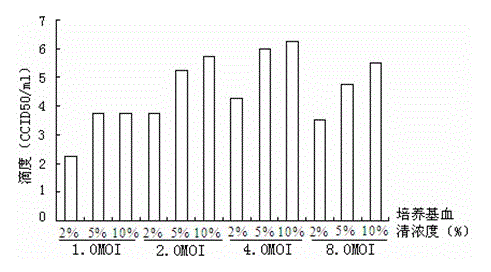
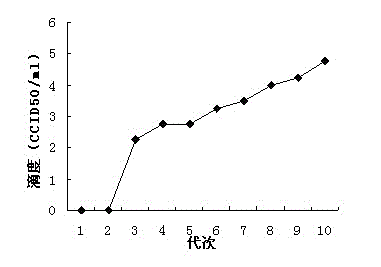
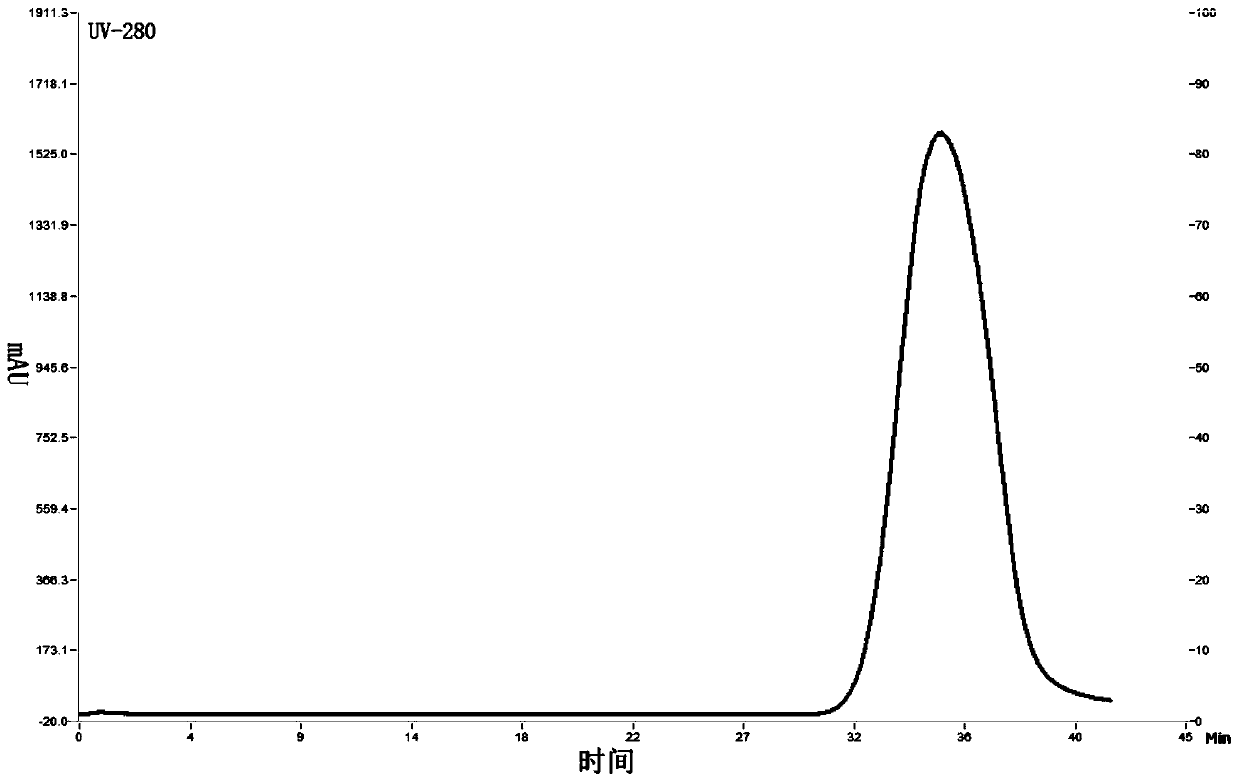
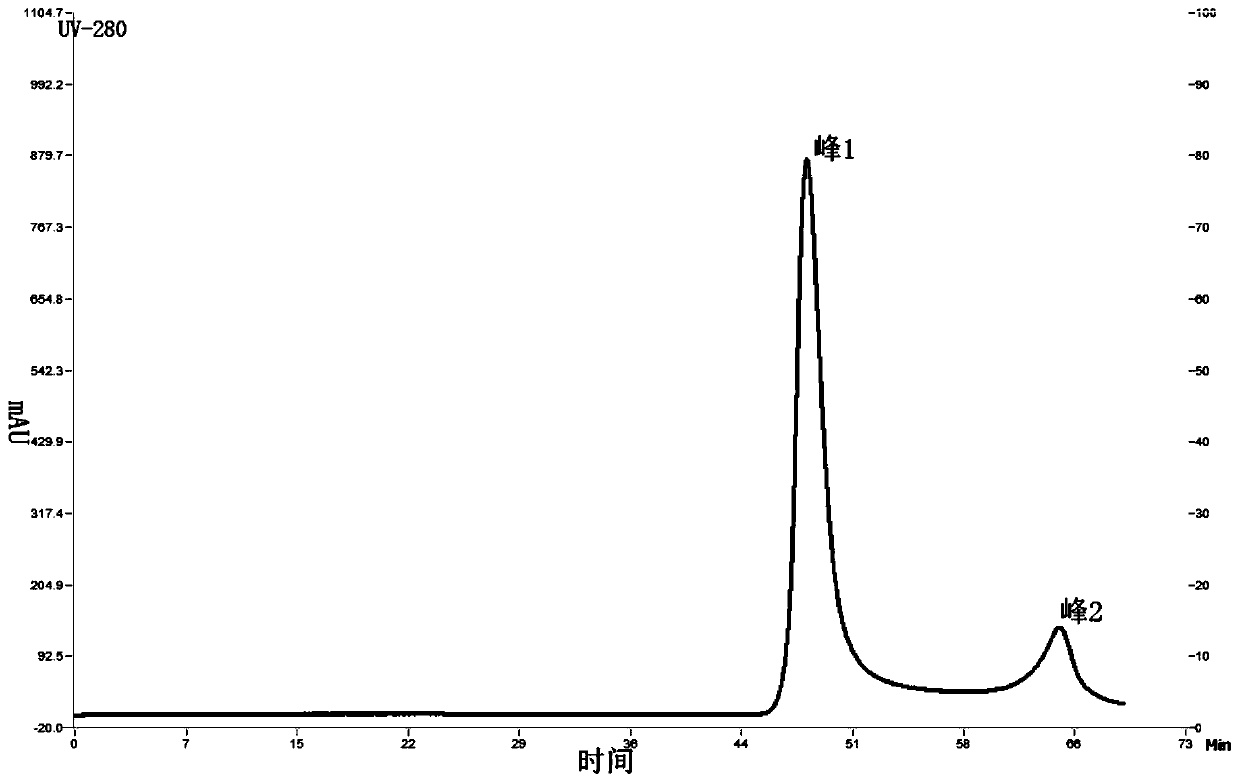
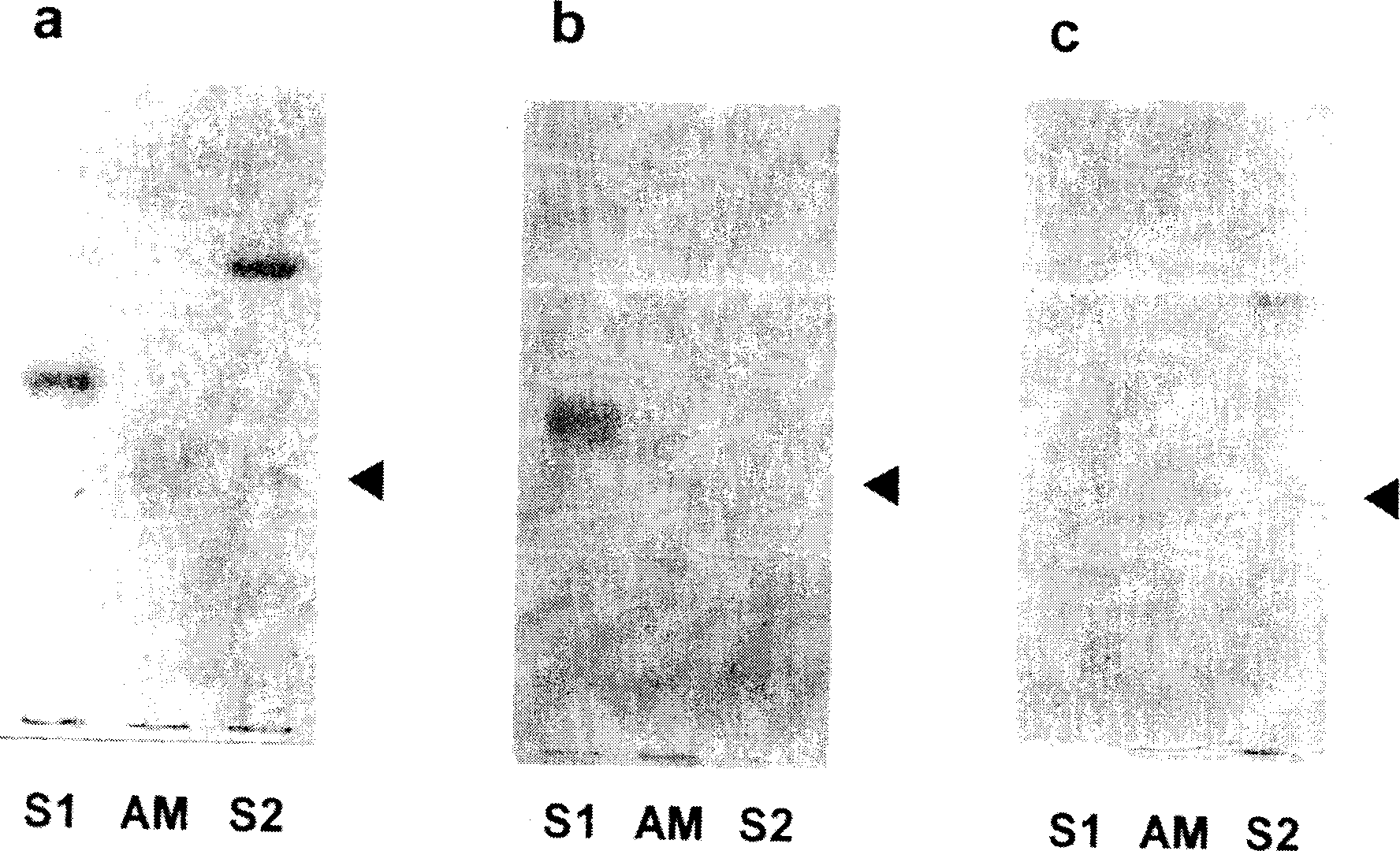
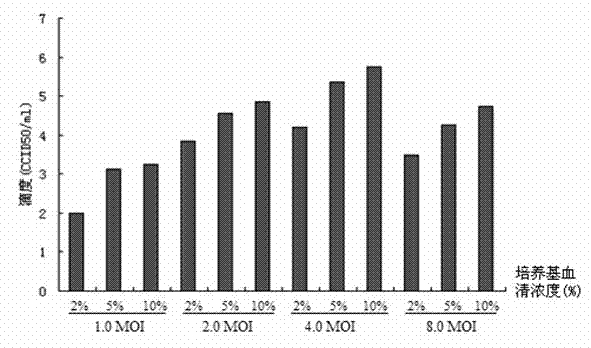
Patents
Literature
57 results about "Virus host" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A virus being an inert particle outside its hosts, the virion has neither metabolism, nor replication capability, nor autonomous evolution. A virus is a living organism only if we consider it associated with its host. Viruses of the same family can infect a wide range of hosts.
Zooblast culture medium dry powder composition, culture medium composition and preparation method thereof
The invention provides a dry powder composition for a zooblast culture medium. Compared with the prior dry powder composition for the zooblast culture medium, the dry powder composition provided by the invention reduces the serum dosage of animals by introduction of recombinant protein or animal origin compositions, the dry powder composition for the zooblast culture medium can be matched with low-content animal serum for use without additional introduction of protein compositions (such as the recombinant protein, plant protein or the animal origin compositions including cytokines and so on), and has the same or better function on promoting the growth of zooblast compared with high-content animal serum, so that the dry powder composition for the zooblast culture medium does not generate side effects along with the protein compositions, and has good safety and low cost. Cells cultured by the dry powder composition for the zooblast culture medium have small difference for different batches, low cost and good safety, are favorable for separating downstream products of the cells, and are suitable to be used for virus hosts, expression vectors and so on of biological products and other biological products.
Owner:BEIJING SKYWING TECH CO LTD
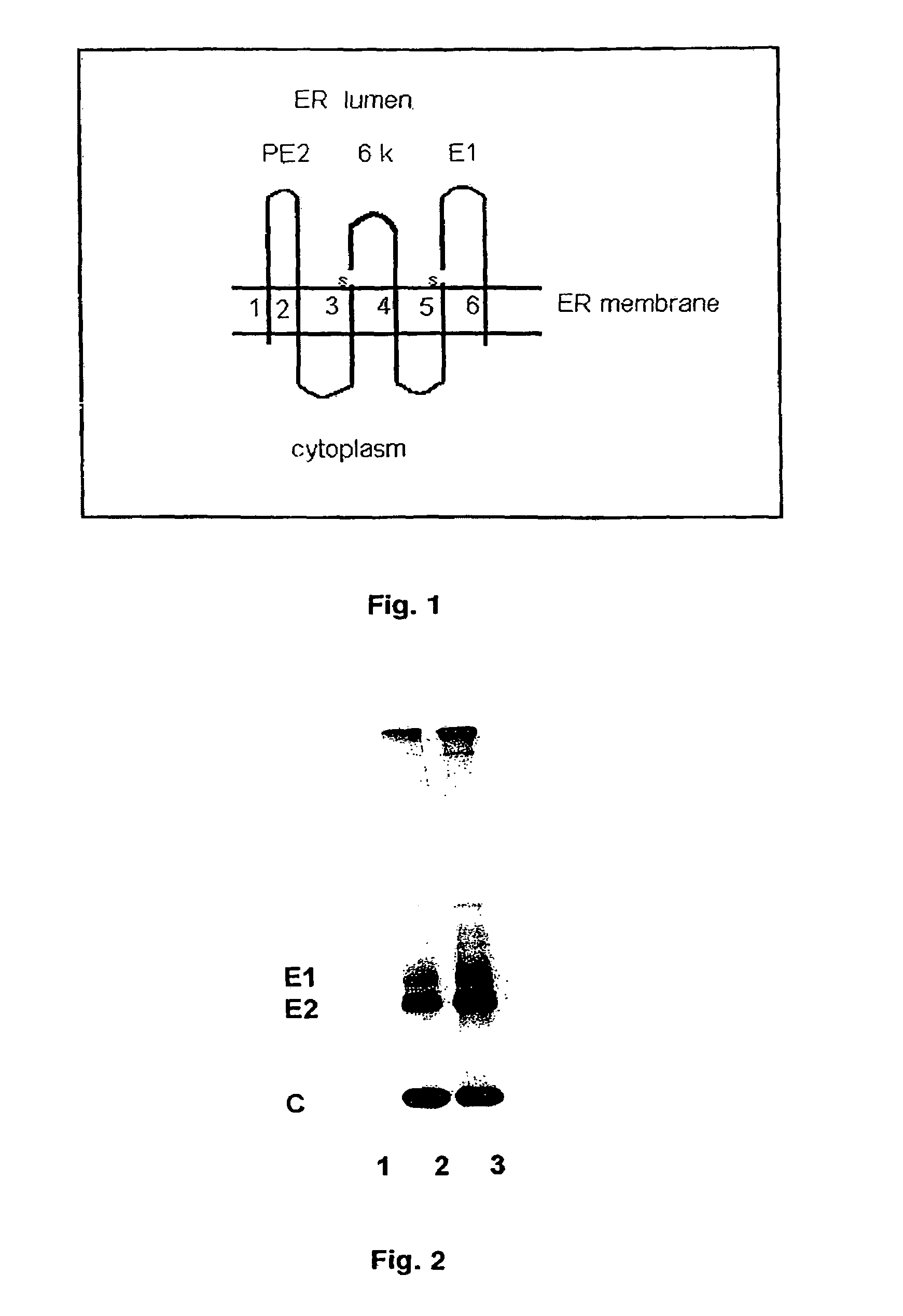
Membrane virus host range mutations and their uses as vaccine substrates
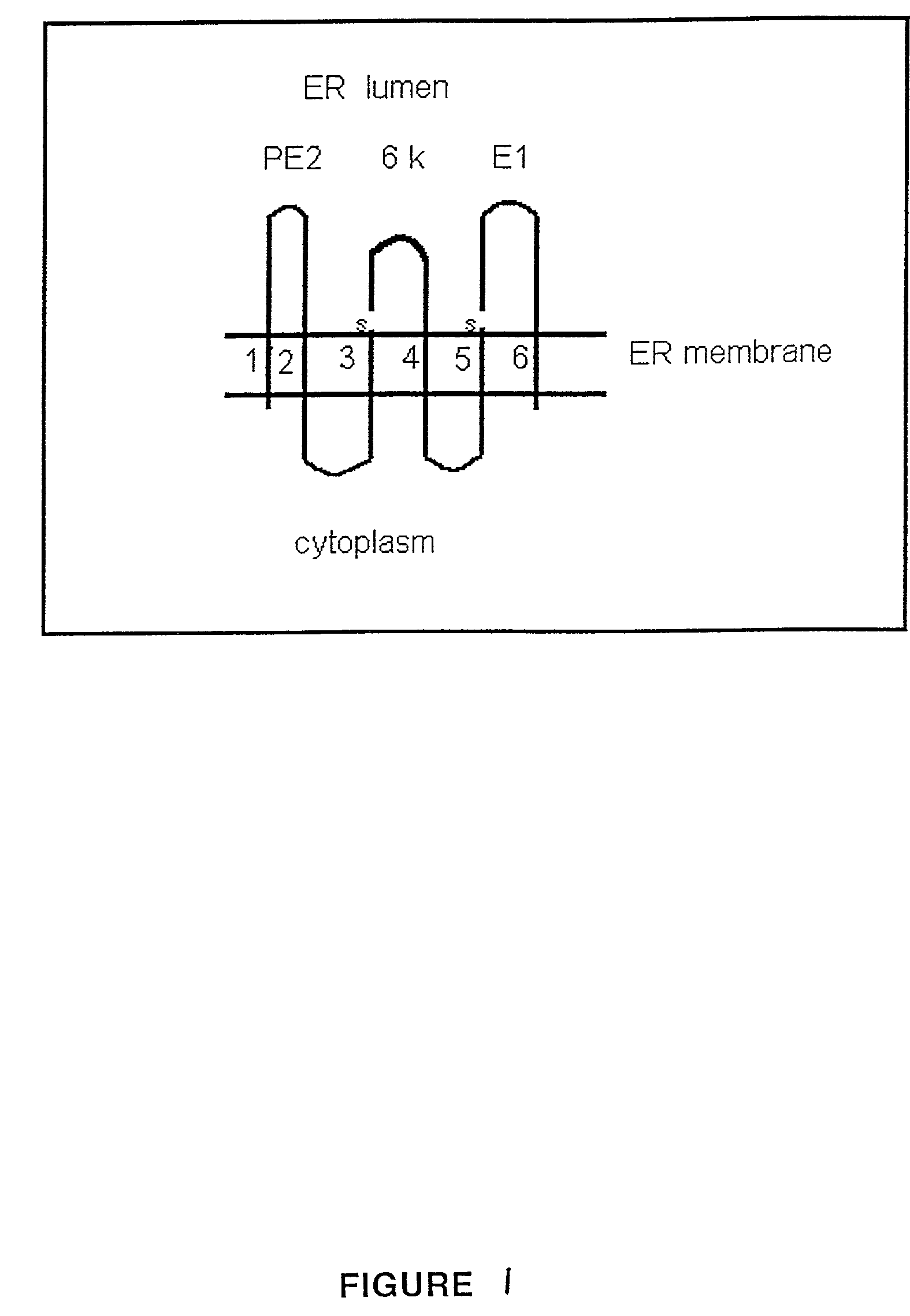
InactiveUS7128915B2Lower levelSsRNA viruses positive-senseSugar derivativesViral VaccineTransmembrane domain
The present invention is directed to genetically engineered, membrane-enveloped viruses with deletion mutations in the protein transmembrane domains. Also provided are viral vaccines based on the engineered viruses, methods of producing and using such vaccines.
Owner:RES DEVMENT FOUND
Method for culturing baby hamster kidney (BHK) 21 cell in serum-free way, and vaccine preparation method
InactiveCN102115729AIncrease culture densityIncrease productivityMicroorganism based processesAntiviralsBiotechnologyHamster
The invention provides a method for culturing baby hamster kidney (BHK) 21 cell in a serum-free way, which leads the BHK 21 cell to be inoculated into a cell culture medium for culturing, wherein the cell culture medium comprises a basic culture medium and further comprises 100-200g / 100L of soy protein, 100-200g / 100L of pea protein, 100-200g / 100L of broad bean protein, 0-100g / 100L of potato protein, 0-200g / 100L of wheat gluten protein and 50-100g / 100L of rice protein by taking the volume of solvent of the culture medium as reference. In addition, the invention also provides a vaccine (such asrabies vaccine and foot-and-mouth disease vaccine) preparation method comprising the method for culturing the BHK 21 cell. The BHK 21 cell cultured by the culture medium containing the vegetable protein is high in culture density, beneficial to separating down-stream products of the cells, low in cost, small in batch difference, good in safety and suitable for producing virus host, expression vector and the like of biological products such as vaccine and the like.
Owner:BEIJING SKYWING TECH CO LTD
Membrane virus host range mutations and their uses as vaccine substrates
InactiveUS7335363B2SsRNA viruses negative-senseSsRNA viruses positive-senseViral VaccineEngineered genetic
The present invention is directed to genetically engineered, membrane-enveloped viruses with deletion mutations in the protein transmembrane domains. Also provided are viral vaccines based on the engineered viruses, methods of producing and using such vaccines.
Owner:RES DEVMENT FOUND
Zooblast cultivation method
The invention provides a method for culturing an animal cell. The method does not need to introduce extra protein component outside animal serum (such as recombinant protein, vegetable protein or animal source component like cytokine); because using low content animal serum can achieve the same or even better effect as using high content animal serum in the aspect of promoting growth of the animal cell, the method for culturing the animal cell does not produce side effects accompanied by the protein component outside the animal serum, and has the advantages of good security and low cost. In addition, the method for culturing the animal cell uses less animal serum, therefore cells cultured by the method have little difference between different batches, low cost, good security, are good for separating cell downstream products, and are suitable to be virus hosts, expression vectors of biological products like vaccines.
Owner:BEIJING SKYWING TECH CO LTD
Serum-free animal cell culture medium dry powder, liquid culture medium and preparation method thereof
InactiveCN102115728AIncrease culture densityEasy to separateAnimal cellsCell culture mediaPea protein
The invention provides a serum-free animal cell culture medium dry powder and a preparation method thereof; the serum-free animal cell culture medium dry powder comprises basic culture medium dry powder and further comprises 100-200g / 100L of soy protein, 100-200g / 100L of pea protein, 100-200g / 100L of broad bean protein, 0-100g / 100L of potato protein, 0-200g / 100L of wheat gluten protein and 50-100g / 100L of rice protein by taking the volume of solvent of the culture medium as reference. The invention also provides a liquid serum-free animal cell culture medium containing the serum-free animal cell culture medium dry powder and a preparation method thereof. The cells cultured by the culture medium containing the vegetable protein are high in culture density, beneficial to separating down-stream products of the cells, low in cost, small in batch difference, good in safety and suitable for producing virus host, expression vector and the like of biological products such as vaccine and the like.
Owner:BEIJING SKYWING TECH CO LTD
Membrane virus host range mutations and their uses as vaccine substrates
InactiveUS20080026004A1SsRNA viruses negative-senseSsRNA viruses positive-senseViral VaccineTransmembrane domain
The present invention is directed to genetically engineered, membrane-enveloped viruses with deletion mutations in the protein transmembrane domains. Also provided are viral vaccines based on the engineered viruses, methods of producing and using such vaccines.
Owner:RES DEVMENT FOUND
Isolated avian cell that expresses Vaccinia virus host range genes
InactiveUS7473536B2Particularly safe in human beingsEasy to getGenetic material ingredientsVirus peptidesFowlVaccinia
The invention concerns an Avipoxvirus comprising in the viral genome a Vaccinia virus host range gene or a homologue of said host range gene. The invention further relates to cells, preferably avian cells, comprising a Vaccinia virus host range gene or a homologue of said host range gene. Moreover the invention concerns the use of a Vaccinia virus host range gene or an homologue thereof to increase the titer of avipoxviruses produced from cells after infection of said cells with the avipoxvirus, wherein the host range gene is expressed in said cells.
Owner:BAVARIAN NORDIC AS
Herpes simplex virus mutant and uses therefore
InactiveUS20100008944A1Enhance immune responseReduce frequencySugar derivativesViral antigen ingredientsDefective mutantVirology
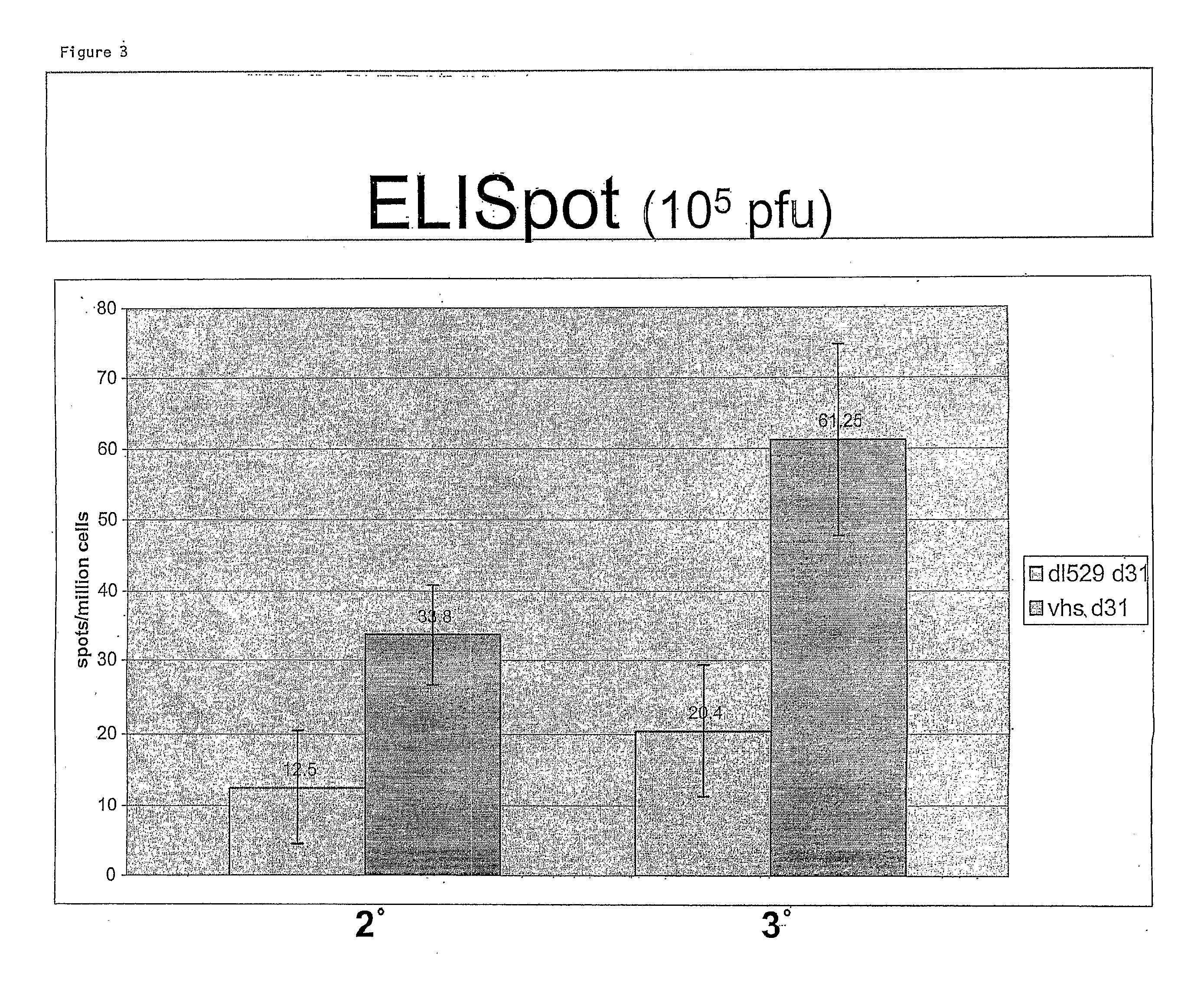
The invention generally provides therapeutic and prophylactic compositions that include a replication defective mutant HSV-2 virus having a mutation in a viral host shut-off protein, a herpes simplex virus having a mutation in a viral host shut-off protein and two additional mutations that render the virus replication defective, and related methods.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Process for clone gene of virus host factor
A method for cloning the virus host factor gene by use of dual-chain RNA low-poison virus (chesnut blight bacterium system) includes such steps as light irradiating to induce the generation of conidia of chestnut phytophthora disease bacteria, inducing to obtain mutant, purifying, discriminating the virus carried by said mutant, testing the activity of its mRNA precursor, transforming and complementing of said mutant, discriminating, sequentially the exogenous DN'A fragment of the transformed plasmid with complem entary power, and configuring gene map.
Owner:GUANGXI UNIV
Specific TT virus sequences and chimeric TT virus host cell DNA molecules for use in diagnosis, prevention and treatment of cancer and autoimmunity
InactiveUS20110318363A1Reduce riskReduce molecular weightOrganic active ingredientsDigital data processing detailsDiseaseSingle strand
Described are single-stranded new sequences of TT viruses, rearranged TTV sequences and hybrid molecules of a specific TT virus sequence and host cell DNA that are capable of replicating autonomously for use in diagnosis, prevention and treatment of diseases like cancer and autoimmunity. In addition, it relates to the use of such molecules as gene vectors and artificial chromosomes.
Owner:DEUTES KREBSFORSCHUNGSZENT STIFTUNG DES OFFENTLICHEN RECHTS
Universal strong promoter among species derived from white spot syndrome virus and application thereof
InactiveCN101818149AImprove efficiencyImprove general performanceVector-based foreign material introductionForeign genetic material cellsProtein targetWhite spot syndrome
The invention relates to a universal strong promoter among species derived from white spot syndrome virus and an application thereof. The invention provides the universal strong promoter among the species derived from the white spot syndrome virus, two primers of the strong promoter, an expression vector of the strong promoter and recombinant host cells of the expression vector. The promoter is an immediate early gene promoter of the white spot syndrome virus and can high-efficiently start the expression of downstream genes in insect cells, mammalian cells and the white spot syndrome virus host cells (including cells of crayfish and shrimp). The recombinant host cells are obtained by transfection of the expression vector containing the universal strong promoter among the species derived from the white spot syndrome virus in the host cells, and the recombinant host cells can express target protein genes controlled by the universal strong promoter among the species derived from the white spot syndrome virus.
Owner:THIRD INST OF OCEANOGRAPHY STATE OCEANIC ADMINISTATION
Recombinant influenza virus carrying Helicobacter pylori, host cells, and preparation method and application of recombinant influenza virus
ActiveCN111363727AImmunization method is convenientSimple immunizationSsRNA viruses negative-senseAntibacterial agentsEpitopeHelicobacter
The invention discloses a recombinant influenza virus carrying Helicobacter pylori, host cells, and a preparation method and application of the recombinant influenza virus. The recombinant influenza virus is obtained by integrating a Helicobacter pylori antigen or antigen-dominant epitope into a NS fragment of the influenza virus genome. The recombinant influenza virus carrying Helicobacter pyloriof the invention can be stably passaged in the host cells or chicken embryos, and can be used for the development of Helicobacter pylori vaccines, the development of related drugs, and the productionof Helicobacter pylori proteins by using chicken embryos or cells as a bioreactor.
Owner:WUHAN UNIV
European eel kidney cell line EK and application thereof
ActiveCN109207422AUniform shapeStable formMicroorganism based processesArtificial cell constructsResuscitationIndividual animal
The invention provides a European eel kidney cell line EK and application thereof, belonging to the technical field of animal cell culture. The kidney cell line of Anguilla anguilla is deposited as CCTCC NO: C2018160. The cell line is a fibroblast type cell, which is primary cultured by tissue block adherence method and passaged by trypsin digestion method. The kidney cell line EK in L-15 containing 8% of fetal bovine serum has the optimum state during culture at 26 DEG C.. The typical morphology of fibroblasts can be maintained after 65 passages in vitro. The karyotype of fibroblasts is aneuploid. After cryopreservation and resuscitation, the survival rate of fibroblasts is 85%, and the fibroblasts grew rapidly. The cell line EK can be used to construct the expression model of exogenous gene, can also be used as a host cell for viral isolation and other etiological and immunological research.
Owner:BIOLOGICAL TECH INST OF FUJIAN ACADEMY OF AGRI SCI
KMB17 cell-adapted strain for II type dengue virus and preparation method thereof
InactiveCN102911920AHigh purityToxicMicroorganism based processesViruses/bacteriophagesEmbryoTGE VACCINE
The invention discloses a KMB17 cell-adapted strain for the II type dengue virus and a preparation method thereof. The epidemic II type dengue virus strain can be adapted to the human embryo lung diploid cell KMB17 to obtain the cell-adapted strain with stable passage and high virus amplification capacity, and purified strains with strong virulence and purity are obtained through plague purification; after identification, the virus purified strains maintain basic biological characteristics of original strains, are provided with good antigenicity, and are capable of performing II type dengue virus amplification with the human embryo lung diploid cell KMB17 as the matrix, limitation of host cells of the II type dengue virus is broken, a new thinking with China's regional characteristics is opened up for research of the vaccine for preventing the dengue virus, the function is laid for research of inactivation of the II type dengue virus and attenuated live vaccines, feasibility of mass production is provided simultaneously, and the research and development of the vaccine can produce great economic value and social benefits.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Purification method for rabies virus inactivated vaccine
PendingCN111249456AEfficient removalImprove fluencySsRNA viruses negative-senseViral antigen ingredientsVirus hostMolecular sieve
The invention relates to a purification method for a rabies virus inactivated vaccine. The method includes the following steps: (1) inoculating and cultivating rabies vaccine strains by using Vero cells as virus hosts, and harvesting a virus concentrate; (2) performing virus inactivation; and (3) preparing the purified inactivated rabies vaccine by using molecular sieve chromatography and anion exchange chromatography. The method provided by the invention does not add any chemical purification agent to the vaccine, the operation is simple, the process consistency is good, and the virus antigencan be prevented from being diluted in the purification process.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
Chimeric viral receptor polypeptides, human viral receptor polypeptides and uses thereof
InactiveUS7071301B1Overcome deficienciesPeptide/protein ingredientsAntibody mimetics/scaffoldsViral ReceptorCell specific
Target cell specificity of delivery vectors is provided by incorporation of a target cell specific binding domain by the use of any binding domain, which binds specifically to a binding site on the target cell. The binding site may be endogenous to the target cell, provided by engineering the target cell, or a suitable binding site may be associated with the target cell. Target cell may also be associated with a CVR polypeptide to provide specificity for the delivery vector. The association of the CVR polypeptide confers target cell specificity for a second virus host cell range, which specificity differs from the viral host cell range of the endogeneous target cell or animal host cell viral receptors. The CVR polypeptide may thus comprise a chimeric virus binding site which binds a second virus env binding domain specific for a second virus host cell range, selected from at least one of the group consisting of amphotropic, polytropic, xenotropic, ecotropic and tissue specific.
Owner:NEW YORK UNIV
HLA Epitope Identification
The present invention describes ways to identify HLA allele-specific epitopes that result from HLA restriction of antigen-specific cellular immune responses. The invention employs a combination of bioinformatics and functional assays to systematically identify and classify CTL mutations to determine correlates of virus-host interactions Peptides representing HLA allele-specific epitopes are provided as well as methods for validating HLA-restricted epitopes and methods for measuring T cell responses.
Owner:LUO MA +6
Prawn white spot syndrome virus host distinguishing method
InactiveCN1876841AReduce degradationEarly screeningMicrobiological testing/measurementPrawnTotal rna
A method for distinguishing the host of prawn-hickie comprehensive viruses includes the following steps: first, under an atmospheric temperature, using Trizol agent to extract the central RNA of the tested animal tissue and making the ratio of ultraviolet spectrophotometer absorbance A260 / A280 of the tested RNA liquor arrive at 1.8-2.0, using available software Primer5.0 to design a pair of primers P1 and P2, and its size of augmentation-aim fragment of is 580bp, then using M-MLV reverse transcriptase to reverse transcription to compound cDNA, the using cDNA and the designed specificity primers P1 and P2 to do PCR reaction and making electrophoresis appraise for the product of PCR reaction. When it comes up a tested animal which has 580bp augmentation fragment, the animal is the host of prawn-hickie comprehensive viruses. The said pair of primers are the upstream primer of P1(5'-GTG GTT TCA CGA GGT TGT-3') and the downstream primer of P2(5'-AAG GAG GAG GTG TTG GAG-3'). This invention is simple and direct, of high sensibility; the method for extracting the central RNA of tissue is rapid and the quality is high, meanwhile, it reduces the degradation of RNA; furthermore, it can distinguish the tested animals earlier than presexisting protein immune crossing technology.
Owner:OCEAN UNIV OF CHINA
Sialyloligosaccharide-gold nano particle and preparation method and applications thereof
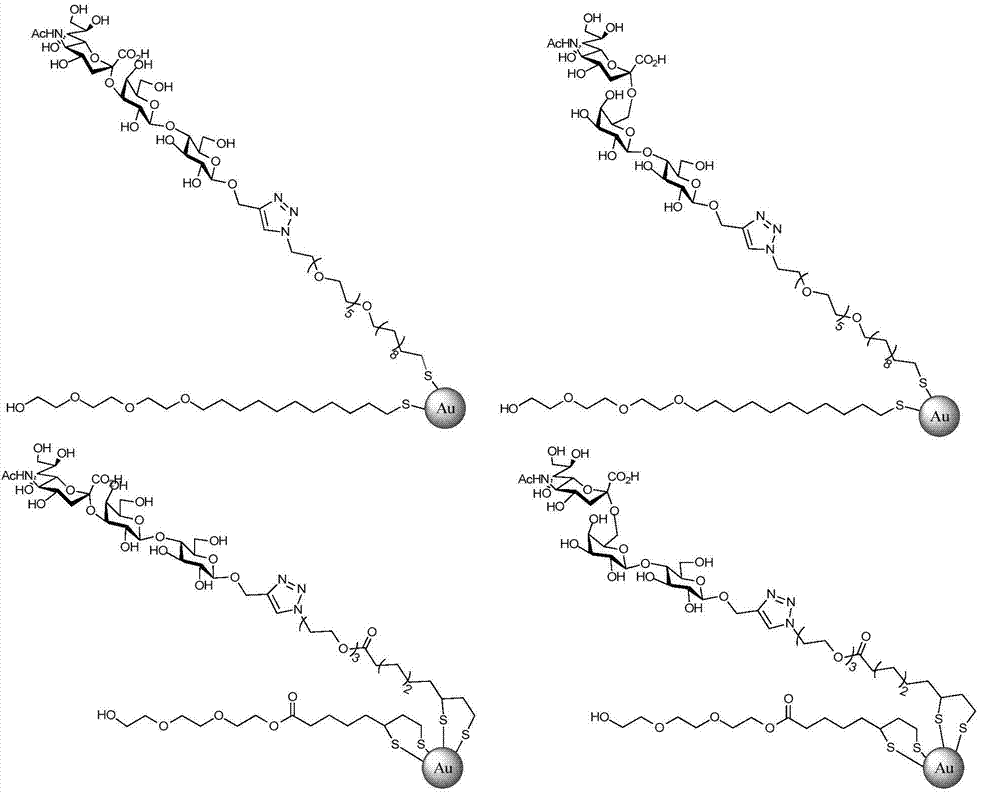
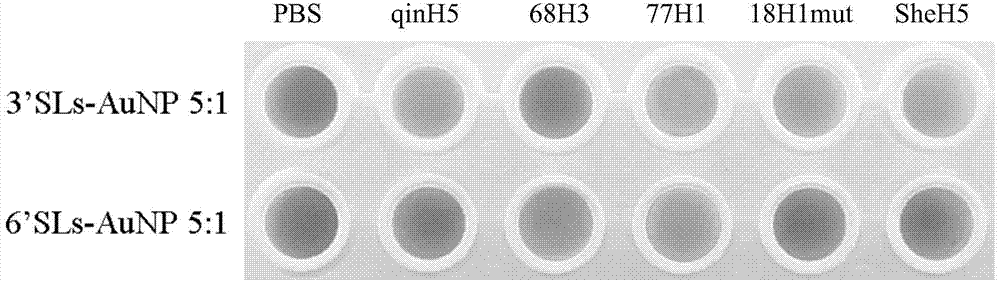
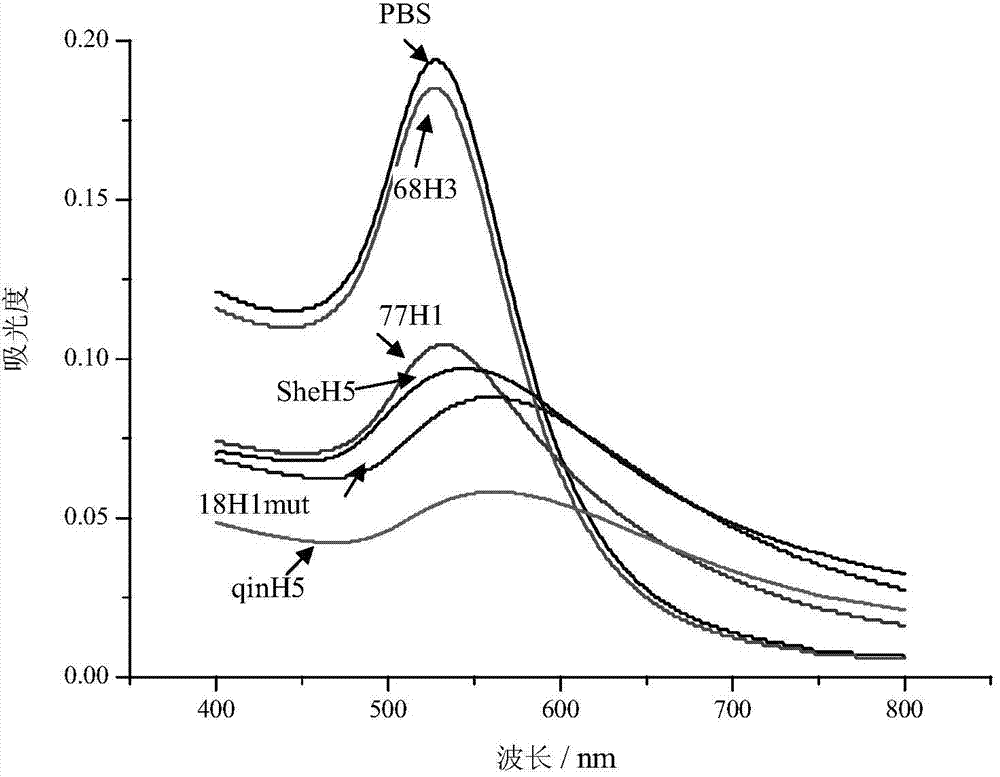
ActiveCN103551562BRapid access to receptor specificityImprove practicalityMicrobiological testing/measurementMicroorganism based processesHemagglutininSialic acid
The invention discloses a sialyloligosaccharide-gold nano particle and a preparation method and applications thereof. The sialyloligosaccharide-gold nano particle which is used for detecting influenza virus host specificity is a gold nano particle which connects sialic acid Alpha 2, 3 or Alpha 2, 6 oligose-O(CH2CH2O)m(CO)n(CH2)k(CH)pSR and HO(CH2CH2O)m(CO)n(CH2)k(CH)pSR through an S-Au key on the surface. When the sialyloligosaccharide-gold nano particle is used for detecting influenza virus strain or HA (Hemagglutinin) protein, receptor specificity of a to-be-detected influenza virus is rapidly obtained through naked eye observation, sensitivity of detecting the influenza virus HA protein can achieve 2.5 nm. The preparation method of the sialyloligosaccharide-gold nano particle enables interaction information between the influenza virus and host cells to be conveniently and rapidly obtained and has significance to influenza virus prevention and control.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Novel branched sialo-sugar molecules and antiviral agents using the same
The present invention provides a novel branched-chain sialose molecule represented by the following formula (I), which is capable of preventing type A from all humans and animals as a host-wide variation or antigenic variation against influenza virus Substances used for adsorbents such as pharmaceuticals infected with influenza virus and influenza B virus, and filters for virus removal. (In the formula, NeuAc represents N-acetylneuraminic acid in which the hydroxyl group, carboxyl group and amide group of the NeuAc may be chemically modified by halogen, alkyl group or acyl group in the same or different ways, Hex represents hexose, HexNAc represents acetylhexosamine , R is a matrix selected from hydrogen atoms, hydrocarbon chains, sugar chains, lipids, proteins, and synthetic polymers, and R may also have substituents. In addition, the combination of N-acetylneuraminic acid and hexose may be naturally occurring The ortho-glycosidic bond may be a chemically transformed bond such as S-glycosidic or Se-glycosidic bond.)
Owner:JAPAN SCI & TECH CORP
Cultivating and preparing method of I-type dengue virus KMB17 cell adapted strain
InactiveCN103695377AHigh purityToxicMicroorganism based processesRecovery/purificationVirus inactivationCellular adaptation
The invention discloses an I-type dengue virus KMB17 cell adapted strain and a preparation method thereof. A popular I-type dengue virus strain can be adapted onto a human embryo lung cell KMB17 for growing to obtain the cell adapted strain which stably goes down to posterity on the KMB17 cell and is strong in virus replication capacity, and the purified adapted strain with high purity is obtained by virtue of a method of purifying bacteriophage plaque by many times. According to a series of identifications on the virus, the I-type dengue virus keeps good antigenicity and basic biological characteristics of the original virus strain. According to the invention, the I-type dengue virus KMB17 cell adapted strain can carry out I-type dengue virus amplification by taking the human embryo lung cell KMB17 as substrate, breaks through limitation of an I-type dengue virus host cell, and lays a foundation for quickening developing a novel idea for researching a dengue virus prophylactic vaccine, and developing I-type dengue virus inactivated and attenuated live vaccines; meanwhile, large-scale production feasibility is achieved.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Zooblast culture medium dry powder composition, culture medium composition and preparation method thereof
The invention provides a dry powder composition for a zooblast culture medium. Compared with the prior dry powder composition for the zooblast culture medium, the dry powder composition provided by the invention reduces the serum dosage of animals by introduction of recombinant protein or animal origin compositions, the dry powder composition for the zooblast culture medium can be matched with low-content animal serum for use without additional introduction of protein compositions (such as the recombinant protein, plant protein or the animal origin compositions including cytokines and so on),and has the same or better function on promoting the growth of zooblast compared with high-content animal serum, so that the dry powder composition for the zooblast culture medium does not generate side effects along with the protein compositions, and has good safety and low cost. Cells cultured by the dry powder composition for the zooblast culture medium have small difference for different batches, low cost and good safety, are favorable for separating downstream products of the cells, and are suitable to be used for virus hosts, expression vectors and so on of biological products and otherbiological products.
Owner:BEIJING SKYWING TECH CO LTD
Immunogenic peptide conjugate and method for inducing an anti-influenza therapeutic antibody response therewith
Immunogenic influenza hemagglutinin-derived peptide conjugates described herein induce a specific therapeutic antibody response against influenza virus. The immunogenic peptide conjugates comprise a segment from the fusion initiation region (FIR) domain of an influenza hemagglutinin protein conjugated to an immunogenic carrier protein, such as keyhole limpet hemocyanin (KLH), bovine serum albumin (BSA), an influenza hemagglutinin (HA) protein (i.e., full length HA), and the like. The immunogenic peptide conjugates described herein can be utilized to treat or prevent influenza infection and to prepare influenza-specific therapeutic antibodies that interfere with influenza virus-host cell membrane fusion. The peptide conjugates can be formulated in pharmaceutical compositions useful for broad spectrum treatment or prevention of influenza infections.
Owner:THE ADMINISTRATORS OF THE TULANE EDUCATIONAL FUND +1
Cell labeling method and application thereof in MRI imaging of rare cells
InactiveCN111671921AAchieve positioningAchieve therapeutic effectPolypeptide with localisation/targeting motifLuminescence/biological staining preparationTherapeutic effectCancer research
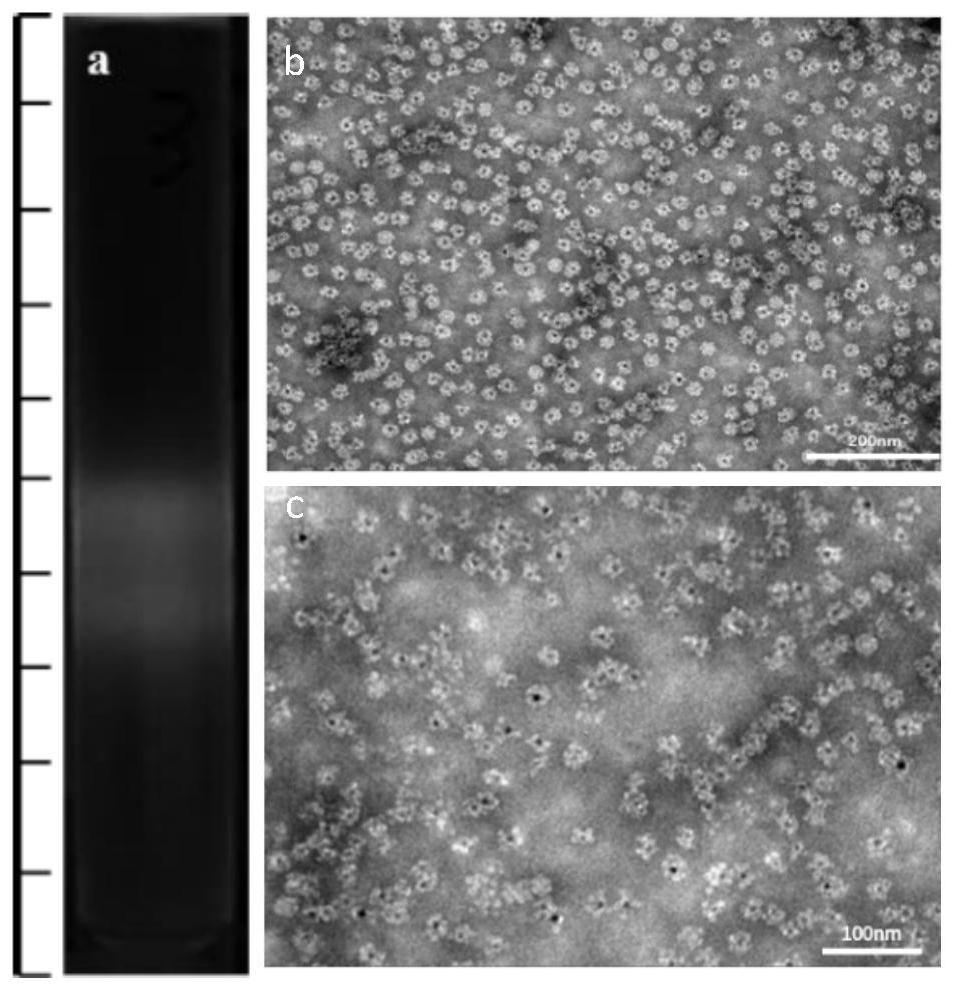
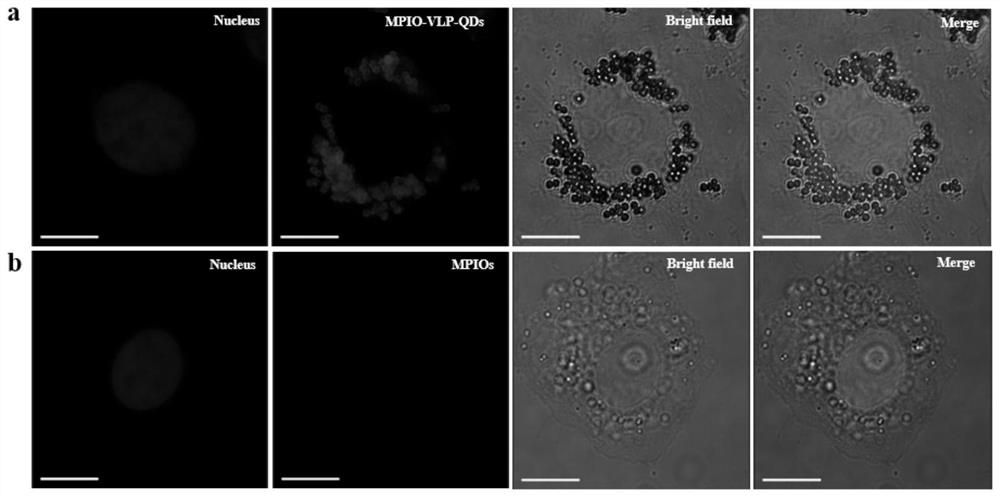
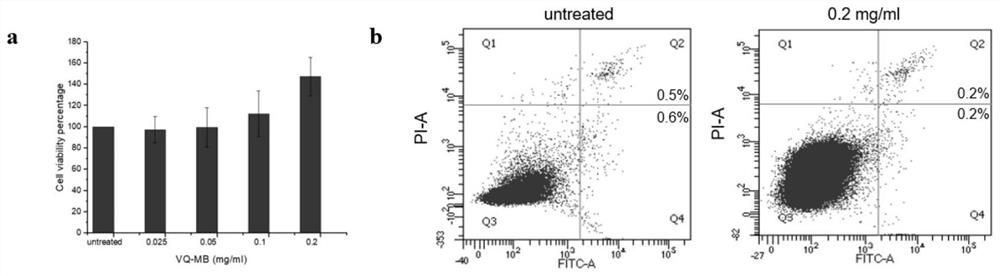
The present invention discloses a cell labeling method and an application thereof in MRI imaging of rare cells. The cell labeling method uses a magnetic micron particle marked by a carrier containingquantum dots to infect cells for labeling and the carrier is a virus-like particle or a targeting group; the virus-like particle is self-assembled by virus capsid proteins fused with cell-penetratingpeptide fragments; and cell-penetrating peptides are connected to the targeting group. The fluorescent magnetic micron particle used in the labeling method contains the cell penetrating peptides, canintroduce MPIO into a variety of cells in a low-toxicity and high-efficiency manner, realizes targeted positioning and precise treatment of virus host cells or tumors, realizes a treatment mode integrating multi-modal diagnosis and treatment, and thus improves a treatment effect of the tumors. Long-term in vivo tracking of the rare cells in vivo is realized, MRI imaging is conducted, the efficientand reliable method for studying a behavior mechanism of the rare cells in body is provided, and the method provides reliable technical means for in vivo imaging for studying cancer invasion and metastasis mechanisms.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Application of CCND1 in preparation of avian retrovirus production enhancer
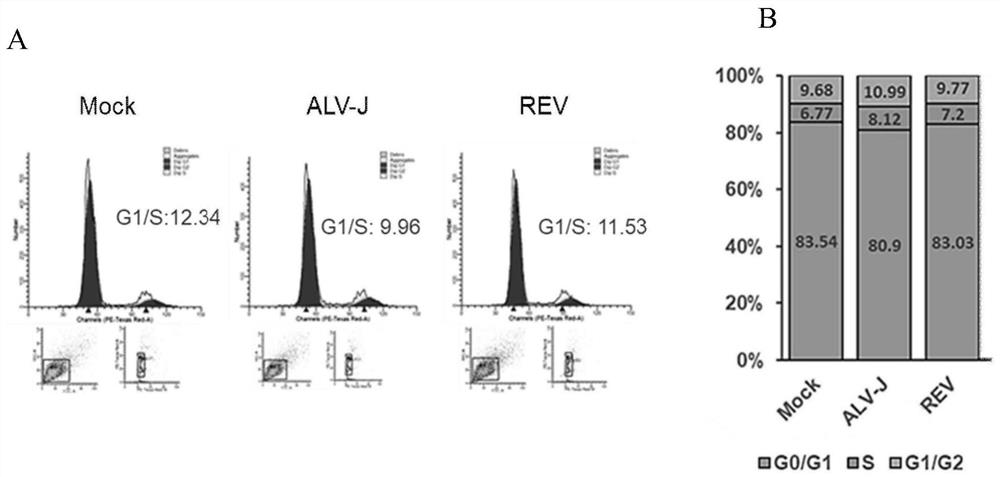
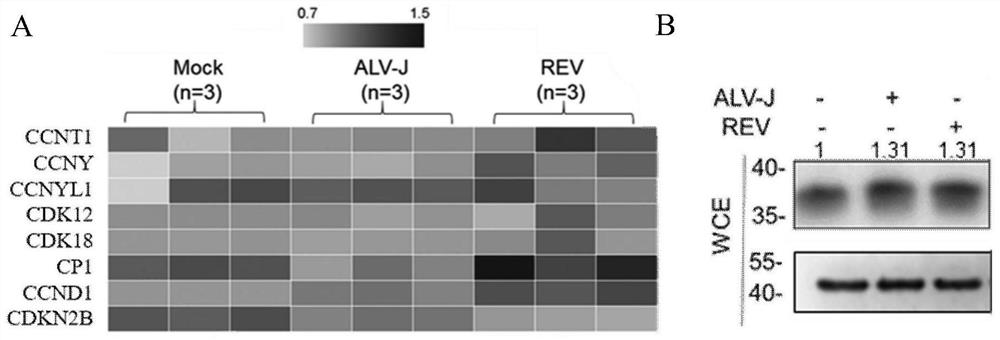
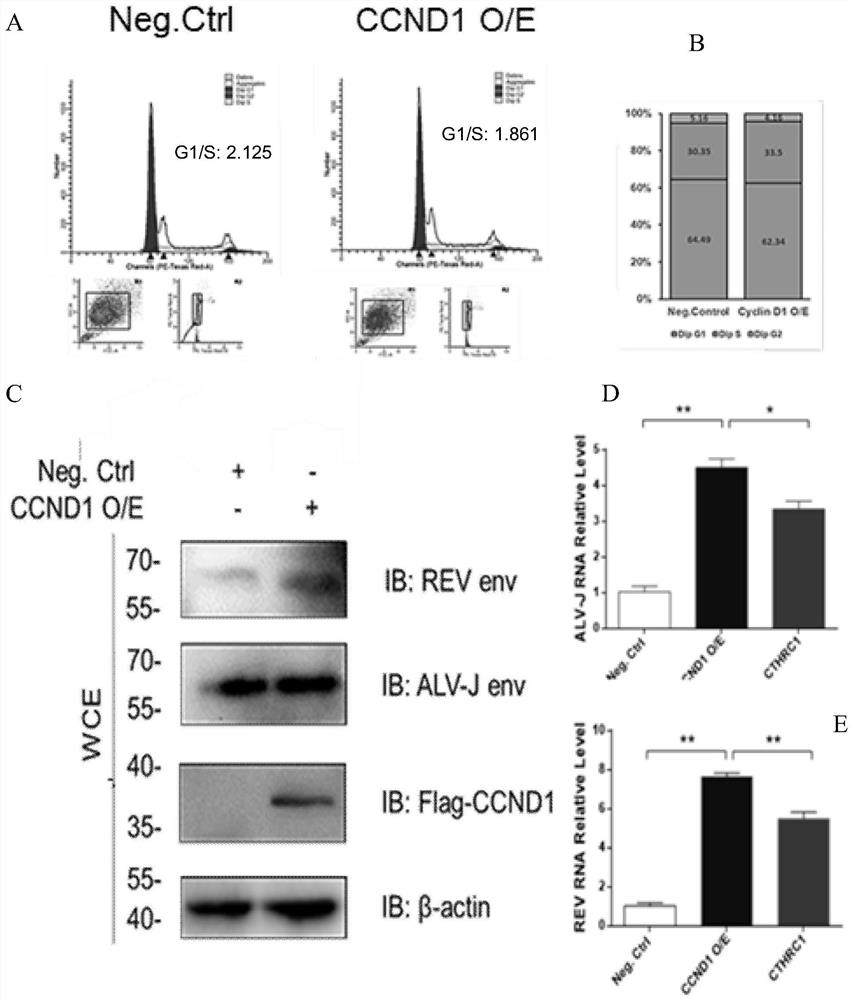
ActiveCN113698466AEasy to copyExtended S phase timeNucleic acid vectorFermentationCyclin D1Nucleotide
The invention relates to the field of virology, and particularly provides application of CCND1 in preparation of an avian retrovirus production enhancer; the CCND1 is cyclin D1 (G1 / S-specific cyclin-D1, CCND1); the nucleotide sequence of the CCND1 is as shown in SEQ ID NO.1; the coded amino acid sequence of the CCND1 is as shown in SEQ ID NO.2; the inventor establishes a preparation and use method of the CCND1 as the avian retrovirus production enhancer for the first time; the mechanism of the CCND1 for promoting replication of the avian retrovirus is defined for the first time: the CCND1 regulates and controls the period of a virus host cell to be converted from a G1 period to an S period; and the S period is prolonged, so that replication of the retrovirus is promoted.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Specific TT Virus Sequences and Chimeric TT Virus Host Cell DNA Molecules for use in Diagnosis, Prevention and Treatment of Cancer and Autoimmunity
ActiveUS20150247165A1Reduce riskReduce molecular weightMicrobiological testing/measurementVirus peptidesDiseaseSingle strand
Described are single-stranded new sequences of TT viruses, rearranged TTV sequences and hybrid molecules of a specific TT virus sequence and host cell DNA that are capable of replicating autonomously for use in diagnosis, prevention and treatment of diseases like cancer and autoimmunity. In addition, it relates to the use of such molecules as gene vectors and artificial chromosomes.
Owner:DEUTES KREBSFORSCHUNGSZENT STIFTUNG DES OFFENTLICHEN RECHTS
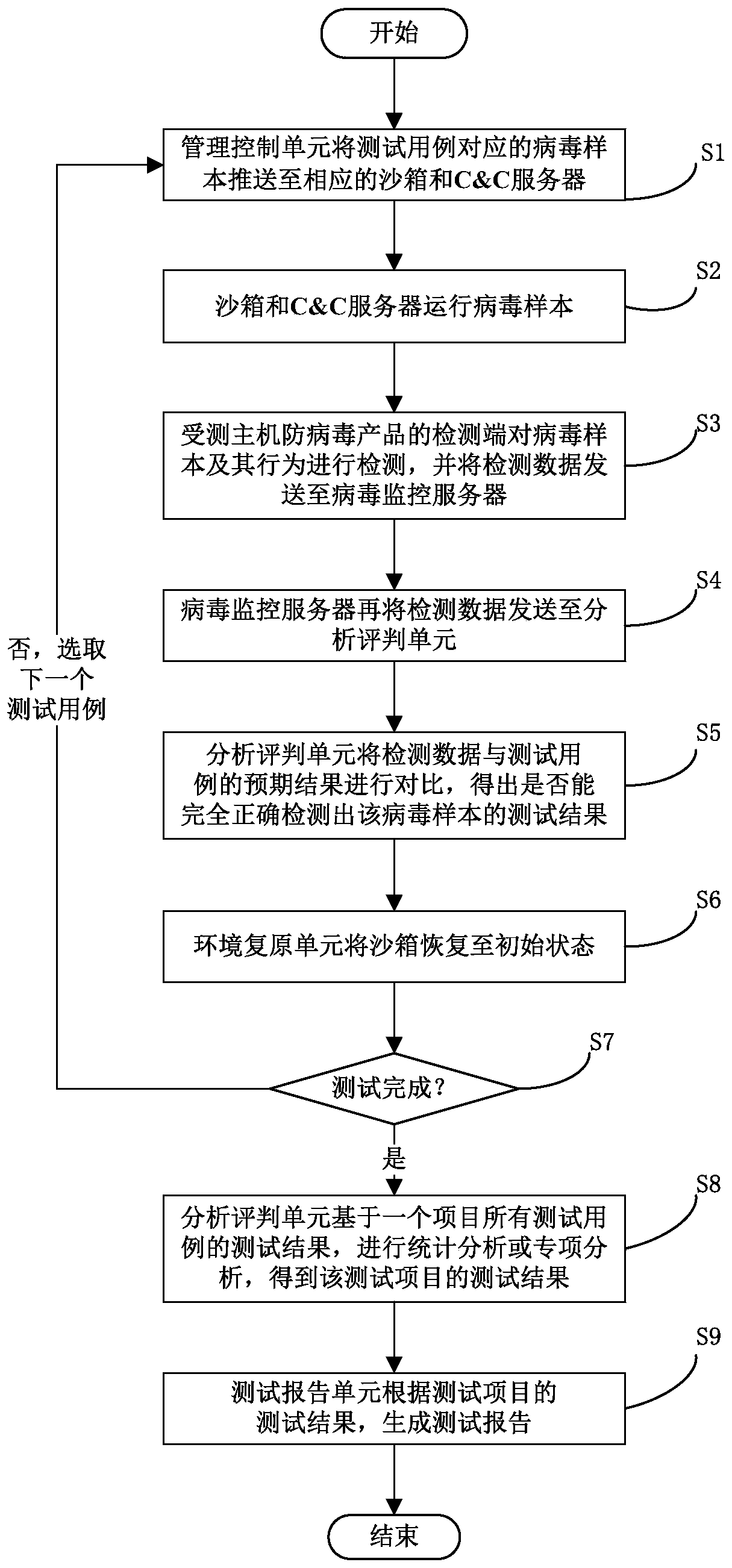
A test system and test method for host antivirus products
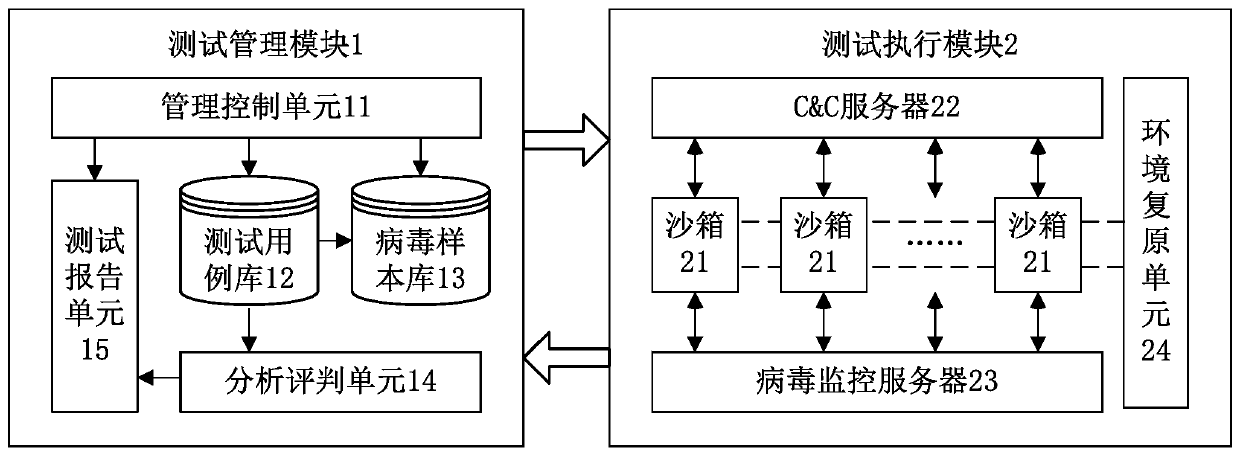
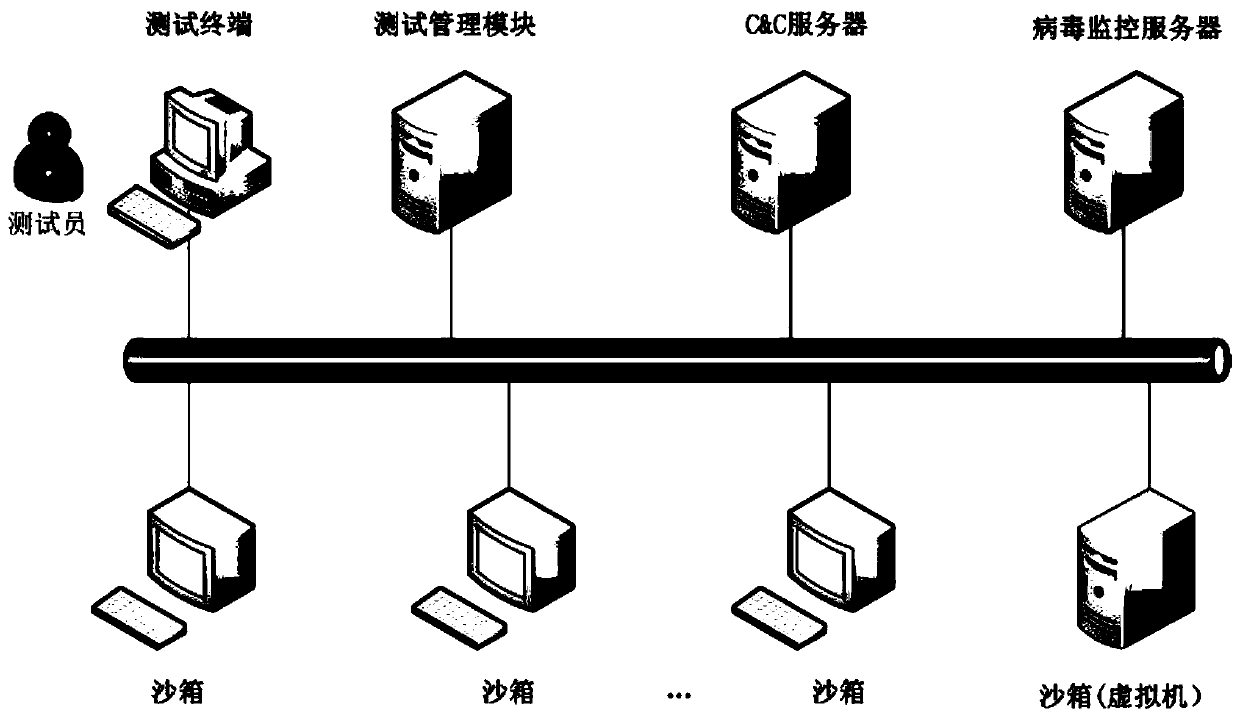
ActiveCN107463493BImprove dynamic testing capabilitiesImprove test resultsSoftware testing/debuggingPlatform integrity maintainanceTest managementTest execution
The invention discloses an anti-virus host product-oriented test system. The system includes a test management module and a test execution module. The test management module includes a management and control unit, a test case library, a virus sample library, an analysis and judgment unit and a test report unit, and is used for managing test processes, test cases and virus samples, analyzing and judging test results and generating test reports. The test execution module includes a C&C server (command and control server), sandboxes, a virus monitoring server and an environment restoration unit, and is used for executing test tasks and returning test data. The invention also discloses an anti-virus host product-oriented test method at the same time. Through the system and the method, problems of low efficiency, insufficient accuracy, nonstandard processes, incomplete test contents and the like which are ubiquitous in current tests can be solved, and the test capability and efficiency are greatly improved.
Owner:BEIJING VRV SOFTWARE CO LTD
Method for culturing baby hamster kidney (BHK) 21 cell in serum-free way, and vaccine preparation method
InactiveCN102115729BIncrease culture densityIncrease productivityMicroorganism based processesArtificial cell constructsRabiesCell culture media
The invention provides a method for culturing baby hamster kidney (BHK) 21 cell in a serum-free way, which leads the BHK 21 cell to be inoculated into a cell culture medium for culturing, wherein the cell culture medium comprises a basic culture medium and further comprises 100-200g / 100L of soy protein, 100-200g / 100L of pea protein, 100-200g / 100L of broad bean protein, 0-100g / 100L of potato protein, 0-200g / 100L of wheat gluten protein and 50-100g / 100L of rice protein by taking the volume of solvent of the culture medium as reference. In addition, the invention also provides a vaccine (such asrabies vaccine and foot-and-mouth disease vaccine) preparation method comprising the method for culturing the BHK 21 cell. The BHK 21 cell cultured by the culture medium containing the vegetable protein is high in culture density, beneficial to separating down-stream products of the cells, low in cost, small in batch difference, good in safety and suitable for producing virus host, expression vector and the like of biological products such as vaccine and the like.
Owner:BEIJING SKYWING TECH CO LTD
Method for extracting and purifying adeno-associated virus and adenovirus from host cells, components and kit thereof
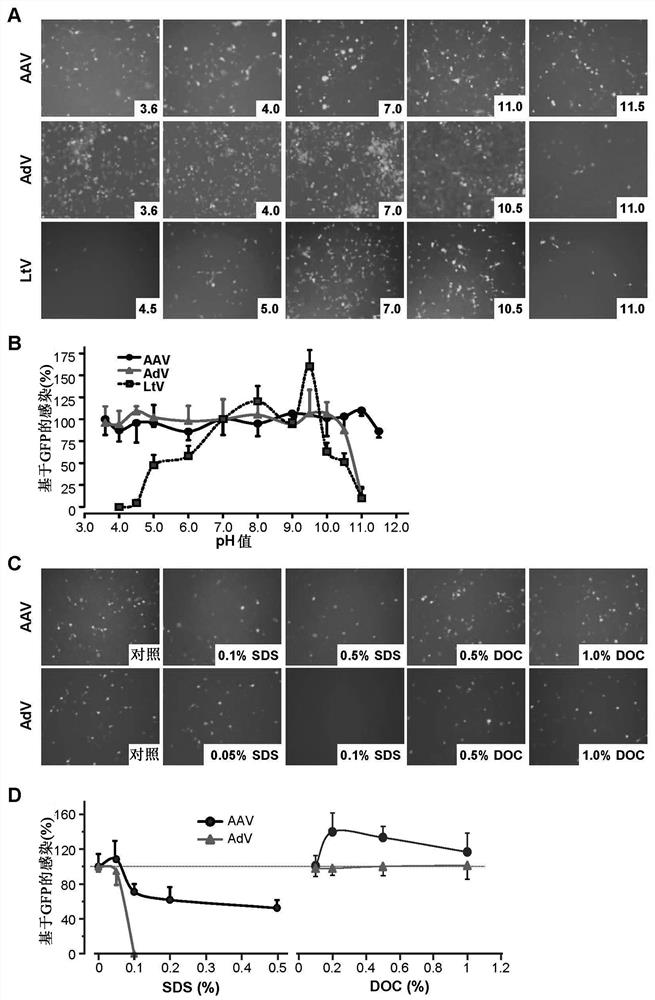
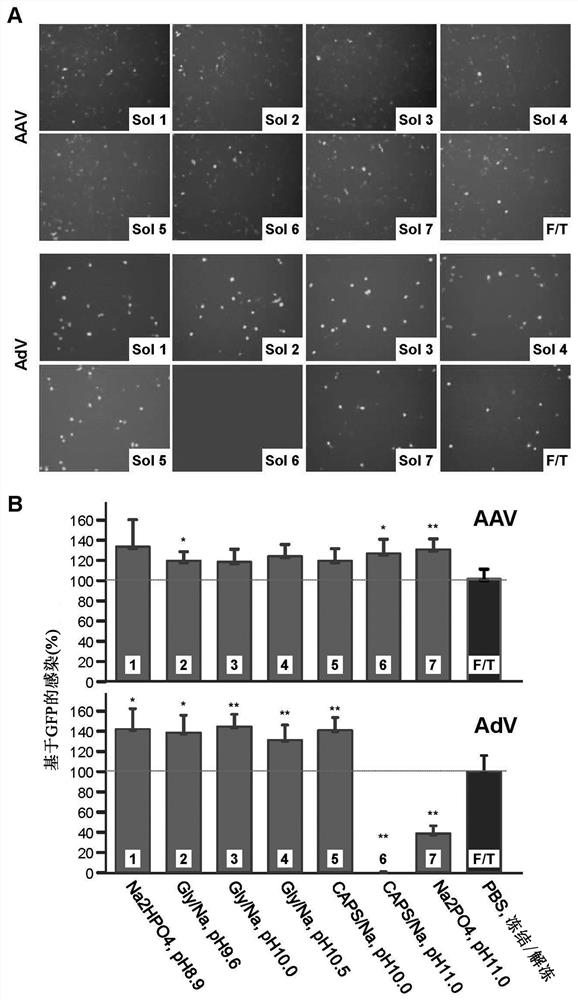
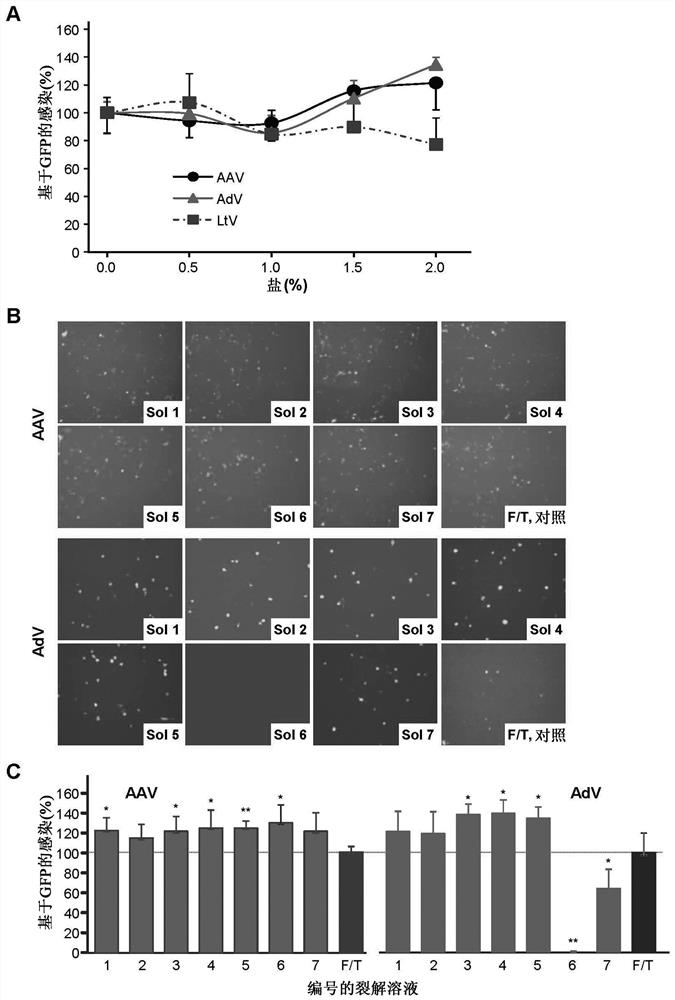
The present invention provides a method for tertiary purification of virus particles from a sample containing host cells packaging virus particles. The three-stage purification method includes: using the first fractionation solution to precipitate and remove most of the impurity proteins; using the second fractionation solution to precipitate and collect virus particles to remove some remaining impurities; the collected virus particles are further purified by column layer The remaining impurities were removed by analysis to obtain an ultra-pure virus solution. The method also includes the steps of collecting virus host cells, cell suspending and cell lysing. This purification method is easy to operate, convenient to obtain ultra-pure virus solution with high recovery rate, suitable for large-scale and small-scale preparation, and the solution and operation steps used do not contain toxic substances to cells. Therefore, this method represents a highly desirable purification technique for vector viruses, including AAVs and adenoviruses. The present invention also includes kits or packages designed according to the above techniques.
Owner:贺道耀
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com