Novel branched sialo-sugar molecules and antiviral agents using the same
A sialic sugar, branched chain technology, applied in the direction of antiviral agents, medical preparations containing active ingredients, oligosaccharides, etc., can solve the problems of not developing influenza virus antibody anti-influenza agents, limiting the effectiveness of compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
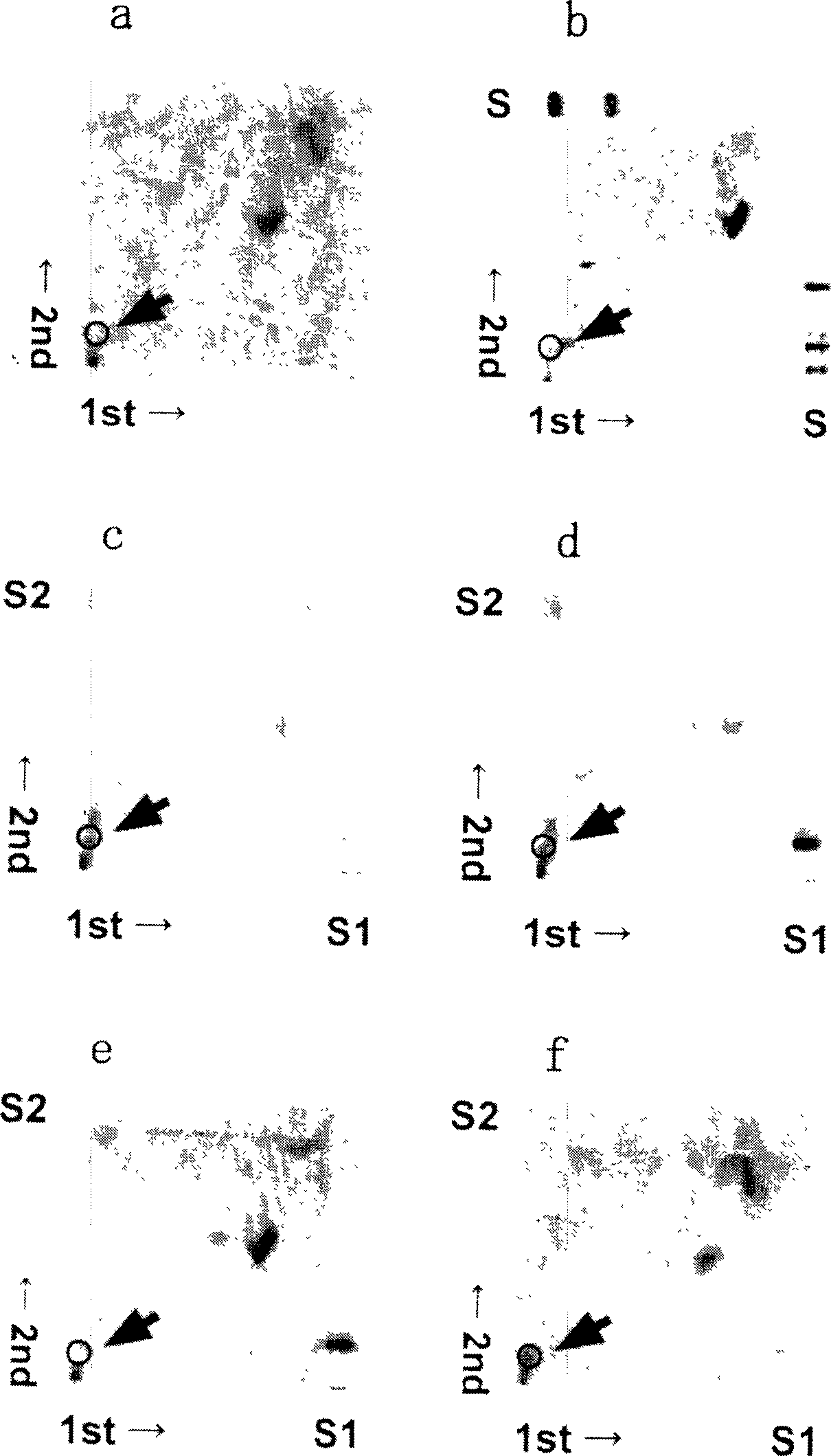
[0053] Example 1 Research on Influenza Virus Receptor Candidate Molecules
[0054] In order to study and identify the molecules that become influenza virus receptors, for the isolation of type A and type B from the plasma urine membrane of hatched eggs used for virus propagation and dog renal tubular cells (MDCK cells) used for virus isolation or infection experiments The lipid molecules to which the influenza virus responds were tested.
[0055] I. Experimental method
[0056] (1) Material
[0057] As influenza virus strains, those shown in Table 1 were used.
[0058] virus species
Binding specificity for NeuAc
A / PR / 8 / 34
H1N1
α2-6<<α2-3
A / Aichi / 2 / 68
H3N2
α2-6>α2-3
A / Memphis / 1 / 71
H3N2
α2-6>>α2-3
A / Duck / 313 / 4
H5N3
α2-6α2-3
A / Duck / 92 / 1 / 76
H8N2
α2-6α2-3
B / Lee / 40
α2-6>>α2-3
B / Gifu / 2 / 73
α2-6α2-3
[0059] In addition, the bindi...
Embodiment 2
[0104] From Example 1, it was confirmed that the specific lipid molecules contained in the plasma urine membrane of hatched eggs and MDCK cells reacted with type A and type B influenza viruses. In order to study the structure and binding specificity of the lipid molecule, individual isolation and purification are also carried out on a large scale. Finally, the binding specificity of the isolated lipid molecule to influenza virus was compared with that of sialyl pseudoerythroside, which is the standard sugar ester substance reported to bind to virus in the past.
[0105] I. Experimental method
[0106] (1) Material
[0107] As influenza virus strains, the virus species shown in Table 3 were used.
[0108] virus species
Binding specificity for NeuAc
A / PR / 8 / 34
H1N1
α2-6<<α2-3
A / Aichi / 2 / 68
H3N2
α2-6>α2-3
A / Memphis / 1 / 71
H3N2
α2-6>>α2-3
B / Lee / 40
α2-6>>α2-3
B / Gifu / 2 / 73
...
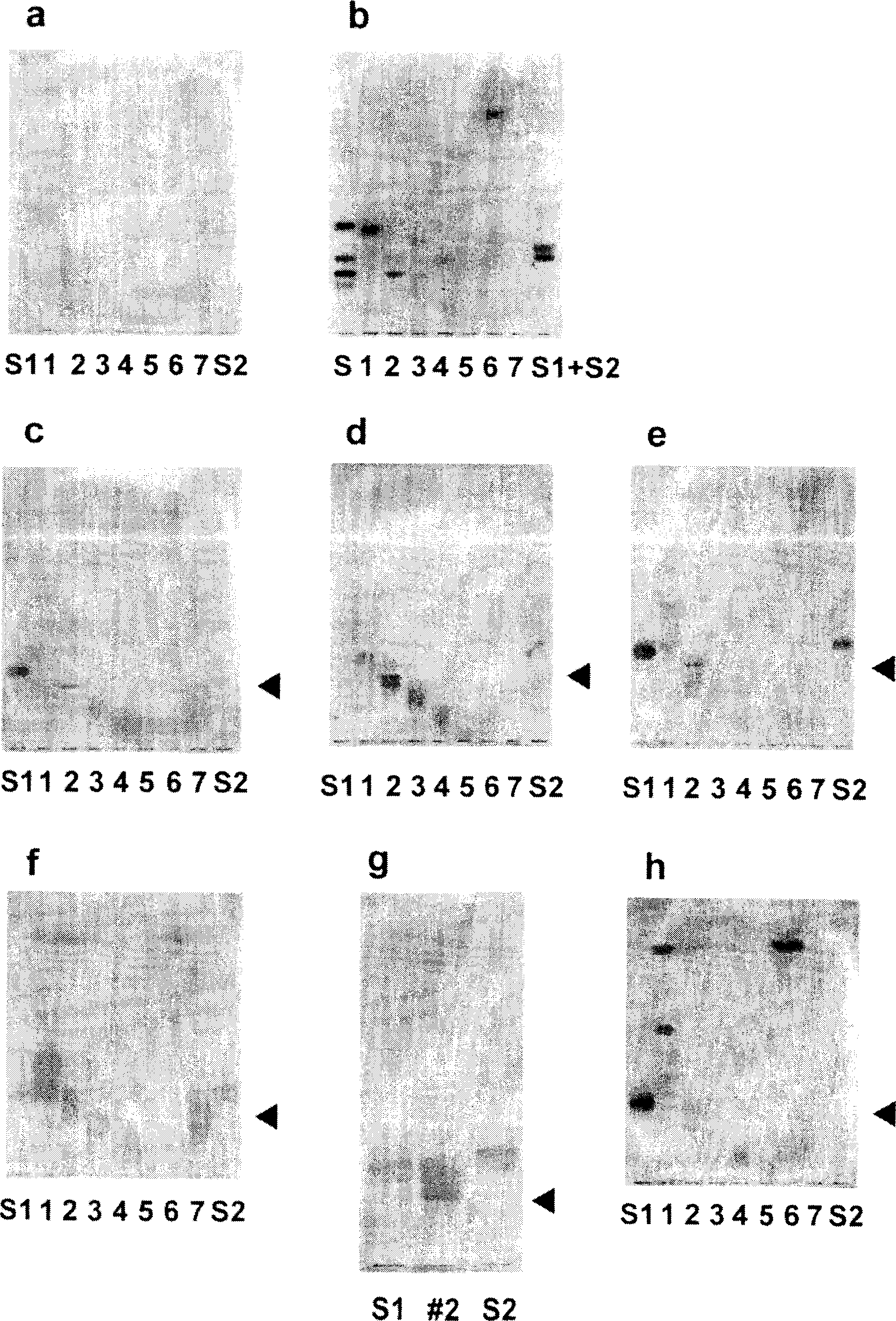
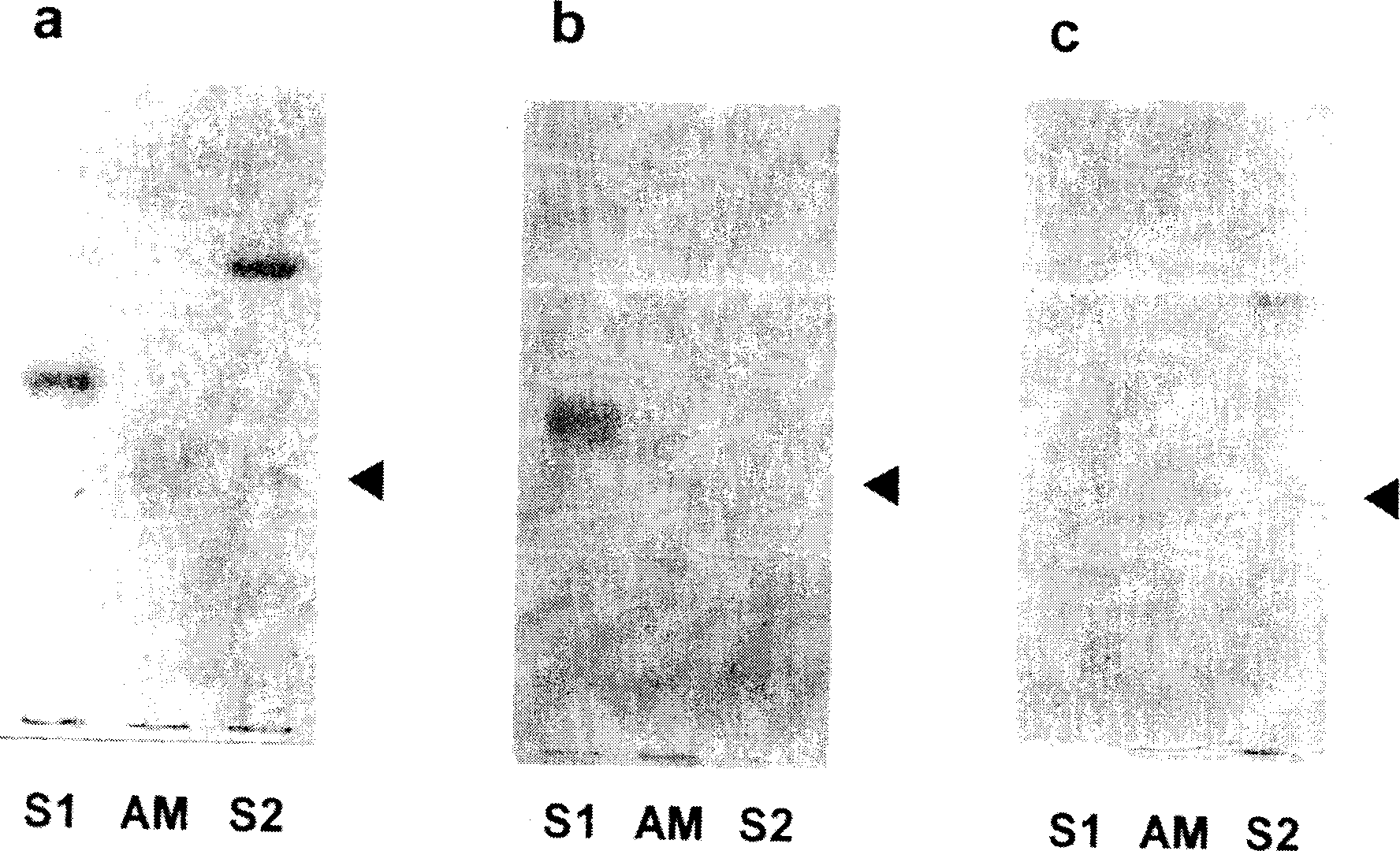
Embodiment 3
[0132] Example 3 Analysis of the structure of AM1, 3, and 4 by mass analysis
[0133] In order to resolve the structures of AM1, 3, and 4 that can be mass-analyzed among AM1-5, ESI (eletrospray ionization)-FTMS (Fourier transform ion resonance mass spectrometer) measurement and SIMS (Secondary ion mass spectroscopy)-FTMS measurement (both by BioAPEX ( Bruker instruments).
[0134] I. Experiment
[0135] (1)ESI-FIMS
[0136] Each sample of AM1, 3, and 4 was dissolved in methanol (3 pmol / µl), and sprayed at a flow rate of 1 µl / min using a microsyringe pump. After adjusting the ESI high-voltage controller or nozzle of the cylinder, inner plate, capillary, etc., the anion type is used for measurement.
[0137] (2) SIMS-FTMS
[0138] Dissolve the sample in CHCl 3 In / MeOH (2 / 1, v / v), a certain amount (300pmol) was taken out and mixed with the substrate (triethanolamine), and then measured with an accelerating voltage of 10kv and anion type.
[0139] II. Results
[0140] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com